Abstract
The life cycle of Vibrio parahaemolyticus has been conventionally associated with estuarine areas characterized by moderate salinity and warm seawater temperatures. Recent evidence suggests that the distribution and population dynamics of V. parahaemolyticus may be shaped by the existence of an oceanic transport of communities of this organism mediated by zooplankton. To evaluate this possibility, the presence of V. parahaemolyticus in the water column of offshore areas of Galicia was investigated by PCR monthly over an 18-month period. Analysis of zooplankton and seawater showed that the occurrence of V. parahaemolyticus in offshore areas was almost exclusively associated with zooplankton and was present in 80% of the samples. The influence of environmental factors assessed by generalized additive models revealed that the abundance and seasonality of V. parahaemolyticus in zooplankton was favoured by the concurrence of downwelling periods that promoted the zooplankton patchiness. These results confirm that offshore waters may be common habitats for V. parahaemolyticus, including strains with virulent traits. Additionally, genetically related populations were found in offshore zooplankton and in estuaries dispersed along 1500 km. This finding suggests that zooplankton may operate as a vehicle for oceanic dispersal of V. parahaemolyticus populations, connecting distant regions and habitats, and thereby producing impacts on the local community demography and the spread of Vibrio-related diseases.
Keywords: zooplankton, Vibrio ecology, Vibrio diseases, population structure
Introduction
The genus Vibrio comprises more than 60 recognized species that occupy a wide range of aquatic habitats. Among these species, Vibrio parahaemolyticus inhabits natural environments with moderate salinity and warm temperature conditions, and its life cycle has consequently been associated with estuarine systems (Kaneko and Colwell, 1973, 1975, 1978; Joseph et al., 1982). Moreover, V. parahaemolyticus has been regularly isolated from the microbiome of different marine organisms, such as corals (Chimetto et al., 2008), fish (Cabrera-Garcia et al., 2004; Herrera et al., 2006; Terzi et al., 2009), molluscs (Blackstone et al., 2003; Martinez-Urtaza et al., 2008b), sponges (Hoffmann et al., 2010), shrimp (Cabanillas-Beltrán et al., 2006) and zooplankton (Kaneko and Colwell, 1973; Baffone et al., 2006). The interaction with planktonic organisms has a central role in the pelagic ecology of Vibrio populations. Copepods represent the largest natural reservoir of vibrios in estuarine waters (Colwell, 1996), and the chitin present in copepods provides a number of resources that facilitate enhanced survival in the environment of these organisms, such as food availability, tolerance to stress and protection (Dawson et al., 1981; Huq et al., 1983; Amako et al., 1987; Dumontet et al., 1996; Pruzzo et al., 2008).
Given the ecological affinity of V. parahaemolyticus for brackish environments, this organism has been presumed to occur outside of estuarine waters very rarely because of the low temperatures, high salinities and low nutrient concentrations prevailing in these areas (Joseph et al., 1982). Nevertheless, ocean currents have often been proposed as a vehicle for spread of human Vibrio diseases through dispersal of pathogenic specimens attached to zooplankton (Colwell, 1996; Lipp et al., 2002; Halpern et al., 2008; Pruzzo et al., 2008). In recent years, increasing evidence has related major epidemic outbreaks of V. parahaemolyticus to the incursion of oceanic waters of subtropical origin in coastal areas. The emergence and spread of infections in Peru, Spain, Chile and Alaska occurred concurrently with the arrival and movement of warm oceanic waters along the coast (Martinez-Urtaza et al., 2008a, 2010; Ansede-Bermejo et al., 2010; Baker-Austin et al., 2010), and with an increase in the occurrence of atypical warm-water zooplankton (Baker-Austin et al., 2010; Martinez-Urtaza et al., 2010). The existence of a mechanism for the oceanic migration of V. parahaemolyticus populations implies that V. parahaemolyticus can persist in the open sea long enough to allow transport across the oceans under adverse ecological conditions for the presence of these bacteria. Zooplankton communities may provide a platform for the long-distance displacement of estuarine Vibrio populations. The association of Vibrio with zooplankton may provide protection from the stresses associated with these cold saline environments and may represent a food source that ensures survival during prolonged journeys (Martinez-Urtaza et al., 2008a). Furthermore, owing to the characteristic patchy distribution of zooplankton (Olson et al., 1994; Genin et al., 2005), Vibrio transport mediated by zooplankton may represent a mechanism for the aggregation of Vibrio specimens in the open sea at high densities, an ecological aspect that may be critical for achieving the level of pathogenic organisms required to cause infection (FDA, 2005).
In addition to these ecological aspects, the potential transport of V. parahaemolyticus populations across different regions raises the possibility that local Vibrio communities may be exposed to periodic invasions by foreign populations originated in distant areas. In the absence of dispersal barriers, resident V. parahaemolyticus would be exposed to a frequent admixture with zooplankton-associated populations. This mechanism can produce constraints on niche specialization, as recently suggested for Vibrio populations from different microbiomes (Hoffmann et al., 2010; Preheim et al., 2010).
In order to elucidate the potential contribution of offshore environments to the distribution and population dynamics of V. parahaemolyticus, we have conducted an investigation directed towards evaluating the presence and temporal trend of V. parahaemolyticus in offshore areas along the northwest coast of Spain. The influence of the abiotic and biotic factors on the seasonal pattern of V. parahaemolyticus was additionally assessed by means of generalized additive models to ascertain which factors have a major influence on the ecology of V. parahaemolyticus in these non-estuarine environments. Finally, the potential relationships of offshore populations of V. parahaemolyticus and resident communities in estuarine areas were investigated.
Materials and methods
Study design
The study was performed in offshore areas of the ria of Vigo, the southernmost ria in Galicia, located on the Atlantic west coast of Spain close to the Portuguese border (Supplementary Figure 1). Samples were collected monthly from June of 2005 to December of 2006 in the water column at two sites: Station-1 (42° 12.8′ N and 8° 51′ W, 39 m depth), located in the external area of the ria, and Station-15 (42° 13.3′ N and 8° 47.7′ W, 29 m depth), located in the mouth of the ria. Sample collection was performed in coordination with the monitoring program for zooplankton conducted by the National Institute of Oceanography (Instituto Español de Oceanografia, Vigo, Spain). Duplicates of the mesozooplankton samples were collected by two parallel 5-min oblique tows with a 200 μm-mesh net. Samples were washed, placed in sterile bottles and re-suspended to 1 l with sterile seawater for microbiological analysis. The second sample was fixed with formalin and stored for further analysis of zooplankton abundance and species identification. Samples of 150 ml of seawater were collected at 0, 5 and 10 m depths using Niskin bottles and transferred to sterile plastic containers. All samples were immediately transported in portable coolers at ambient temperature and analysed on arrival at the laboratory. Environmental variables were recorded on board in parallel with the sample collection using a CTD (Conductivity, Temperature and Depth) device providing sensors for conductivity, temperature, chlorophyll and depth. The biological productivity in the area was estimated by the daily values of upwelling for a point having coordinates 10°30_W, 42°30_N from the 6-h upwelling values of the Pacific Fisheries Environmental Laboratory (National Oceanic and Atmospheric Administration, Pacific Grove, CA, USA).
Analysis of V. parahaemolyticus
The presence of V. parahaemolyticus in seawater was investigated using the ISO 8914 standard method (ISO 8914:1990) modified according to Blanco-Abad et al. (2009). Briefly, 150 ml of each water sample was concentrated by filtration through a 0.45-μm-pore-size filter (Sartorius, Goettingen, Germany) with a vacuum pump, and filters were subsequently placed in 225 ml of alkaline peptone water (APW; 1% (wt/vol) peptone, 1% (wt/vol) sodium chloride; pH 8.2). After incubation at 37 °C for 16–18 h, 1-ml aliquots of enrichment broths showing growth were analysed by PCR or plated onto thiosulfate–citrate–bile salts–sucrose (TCBS) (Oxoid, Hampshire, UK) and CHROMagar Vibrio (CHROMagar Microbiology, Paris, France) agar plates, and incubated for 16–18 h at 37 °C.
Mesozooplankton was filtered through a sterile 200-μm sieve of stainless steel mesh and washed with sterile water. The retained zooplankton was weighed and divided into two fractions that were analysed independently. Samples were analysed by the three-tube most probable number (MPN) technique following the Bacteriological Analytical Manual procedure (Elliot et al., 1995), with the modifications described by Blanco-Abad et al. (2009). Each sample was diluted 1:10 in APW broth and homogenized for 60 s using a stomacher. A total of 10 ml of the 1:10 APW dilution (containing 1 g of the sample) or a total of 1 ml of the 1:10 or of the 1:100 dilutions was inoculated in the three tubes of three MPN series and incubated at 37 °C for 18–24 h. After incubation, all the APW tubes were analysed by PCR. All positive tubes were computed and MPN values were assigned accordingly using the MPN tables (Elliot et al., 1995).
Additionally, APW broths were plated onto TCBS and CHROMagar Vibrio plates, and incubated for 16–18 h at 37 °C. Between three and five presumptive colonies of V. parahaemolyticus from TCBS and CHROMagar plates were selected at random from each plate, cultured in trypticase soy agar (TSA) containing 2% NaCl (Difco , Franklin Lakes, NJ, USA) and incubated at 37 °C for 24 h prior to PCR analysis.
PCR analysis and strain characterization
Aliquots of APW broths and strains isolated during the study were used for DNA extraction. Genomic DNA extraction was performed by standard procedures using Chelex resin according to Blanco-Abad et al. (2009).
The presence of V. parahaemolyticus in broths and confirmation of presumptive colonies was investigated by use of the V. parahaemolyticus-specific gene Vp-toxR (Kim et al., 1999). The presence of the virulence-related genes tdh and trh in broth and strains was determined using independent PCR assays for each gene, according to Tada et al. (1992). The PCRs were performed using a PTC 200 thermocycler (MJ Research, South San Francisco, CA, USA). All PCR products were checked by electrophoresis on 1.6% agarose gels (Agarose type II; Sigma-Aldrich, St Louis, MO, USA) and photographed with an Alpha Innotech 2200 UV transilluminator (Alpha Innotech, San Leandro, CA, USA). Strains without amplification of the Vp-toxR gene were considered negative for V. parahaemolyticus and were not subjected to further analysis for tdh and trh genes. The reference strains AQ4037 (tdh−, trh+), ATCC43996 (tdh+, trh−) and VP81 (pandemic strain, tdh+, trh−) were used as positive controls in all the assays, whereas strains Vibrio vulnificus ATCC 33815 and Vibrio cholerae ATCC14035 were the negative controls.
All of the tdh+ and trh+ strains were subjected to serotyping by agglutination tests using specific antisera according to the manufacturer's instructions (Denka-Seiken, Tokyo, Japan) and to pulsed-field gel electrophoresis analysis, as described previously (Martinez-Urtaza et al., 2004). Offshore strains were compared with representative strains obtained from seawater and shellfish collected in estuarine areas located in the northwest coast of Spain over the course of previous studies. An unrooted pulsed-field gel electrophoresis dendrogram was generated by the UPGMA algorithm with the Dice coefficient of similarity using the BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium) and a circular tree was drawn using the online iTOL software package (Letunic and Bork, 2007).
Statistical analysis
MPN values for V. parahaemolyticus counts were log-transformed to approximate normality because the values showed a skewed distribution. One-way analysis of variance was used to evaluate the significance of differences in the log-transformed MPN across the sampling sites.
Generalized additive models (Hastie and Tibshirani, 1990; Wood, 2006) were used to examine (a) the seasonal trend in the abundance and presence of V. parahaemolyticus over the course of the study; (b) the seasonal trend of several biotic and abiotic factors over the course of the study; and (c) the association of these biotic and abiotic factors with the presence and abundance of V. parahaemolyticus.
The main advantage of GAMs over traditional regression methods is that they do not impose a parametric form on the effects of continuous covariates on the response of interest. Instead, they only assume that these effects are additive and reasonably smooth. More specifically, let Y be a response variable and X1, X2, …, Xp be a set of continuous predictors. A GAM can be expressed as
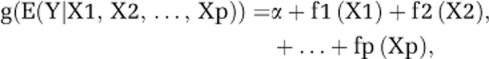 |
where fi (i=1, 2, …, p) are smooth and unknown functions of the continuous predictors, and g is a monotonic known function (the link function).
In this study, the identity link was used for GAMs with a continuous response and the logit link function was used for those with a binary response (presence/absence of V. parahaemolyticus). In all cases, thin plate regression splines (Wood, 2003) were used as smoothers, with optimal degrees of freedom chosen by means of restricted (or residual) maximum likelihood (Ruppert et al., 2003). In those situations where the estimated degrees of freedom associated with a covariate had the value 1 (indicating a linear relationship), the GAM regression model was then refitted assuming a linear relationship with the response. Finally, a Bayesian approach to uncertainty estimation was used to derive standard errors of predictions and obtain 95% credible confidence bands for the smooth effects.
Owing to the nature of the data (a time series), we also evaluated the possible presence of autocorrelation between sequential observations based on an analysis of the regression residuals. To this aim, we computed the autocorrelation function and the partial autocorrelation function. In none of the analyses, did the results suggest the presence of autocorrelation, probably owing to the short time series. For this reason, no residual correlation structure was incorporated into the GAMs.
All of the statistical analyses were performed using the gam function of the mgcv package (Wood, 2006) in the free R software, version 2.11 (R Development Core Team, 2010).
Results
Environmental factors
Changes in seawater temperature, salinity and upwelling were investigated over the study period. Environmental conditions were highly similar at the two sampling sites over the study period, and no statistical differences were found between the two sites in relation to the oceanographic variables investigated. Salinity showed a narrow range of variation, with values fluctuating from 35 to 36 p.p.t., with the exception of September 2006, when salinity began to decrease until it reached its lowest value of 32 p.p.t. in December. Seawater temperature ranged from 10.5 to 18.5 °C and showed a nonspecific seasonal pattern, with highs and lows throughout the study. Upwelling in the area was characterized by the existence of two independent peaks in summer and fall, with the highest levels of upwelling occurring in December 2005.
The seasonal trends for each environmental variable over time were investigated through independent GAMs for each variable (Supplementary Figure 2). Variations in salinity and upwelling during the period of study were statistically significant, and these seasonal trends explained 87.8% and 77.6% of total the deviance, respectively. By contrast, seawater temperature showed a non-significant change over time.
Analysis of zooplankton
The >200-μm plankton fraction was composed primarily of copepods. Copepods represented 52.2% (mean relative abundance) of the total zooplankton (Table 1). A total of 33 different copepod taxa were identified in the study. The number of species found at any given sampling time was highly variable and ranged from 9 to 23. The highest diversity was typically observed during months of lowest abundance (fall and winter). Acartia clausi, the dominant species, represented nearly half of all copepods, followed by Temora longicornis (8%), Calanus helgolandicus (6%) and Paracalanus parvus (5%). In addition to copepods, the most abundant groups in zooplankton were Cladocera (12%), Echinodermata larvae (8%), Appendicularia (8%) and Cirripeda larvae (7%).
Table 1. Composition of zooplankton and mean relative abundance of the different groups identified in all samples collected over the course of the study.
| Group | Mean relative abundance (%) | Class or order | Mean relative abundance (%) |
|---|---|---|---|
| Radiolaria | 0.96 | Lamellibranchia larvae | 2.00 |
| Acantharia | 0.01 | Cirripeda larvae | 6.83 |
| Foraminifera | 0.36 | Cirripeda cypris | 0.33 |
| Tintinnidae | 0.28 | zoea Pisidia longicornis | 0.24 |
| Cnidaria | 2.16 | Decapoda larvae | 0.14 |
| Nauplius cladocera | 0.27 | Braquiura zoea | 0.17 |
| Cladocera | 12.10 | Braquiura megalopa | 0.03 |
| Isopoda | 0.01 | Euphasiaceae larvae | 0.06 |
| Ostracoda | 0.01 | Echinodermata larvae | 8.11 |
| Eufasiaceos | 0.01 | Polychaeta larvae | 0.33 |
| Chaetognatha | 1.66 | Sardina pilchardus eggs | 0.03 |
| Appendicularia | 7.84 | Fishes larvae | 0.01 |
| Doliolila | 0.01 | Sardina pilchardus larvae | 0.02 |
| Siphonophora | 1.62 | Phoronida larvae | 0.01 |
| Briozoan larvae | 0.33 | Copepoda | 52.28 |
| Gastropoda larvae | 1.75 |
Total zooplankton abundance peaked at the beginning of the study and started to decline during the winter months, with a second peak of abundance in August 2006 (Supplementary Figure 3). This seasonal trend was similar for all groups of zooplankton analysed (Supplementary Figure 2).
Effects of temperature, salinity and upwelling on the abundance of zooplankton were also analysed using GAMs. Seawater temperature had a non-significant influence on the abundance of zooplankton. Salinity showed a significant positive linear effect (P<0.01), but lost its significance when the effect of the time effect was incorporated into the GAM regression model. By contrast, upwelling showed a significant slight decreasing effect on the total number of individuals (results not shown).
V. parahaemolyticus in zooplankton
V. parahaemolyticus was detected by PCR in 56 (80%) of 70 samples of mesozooplankton analysed. No significant difference in the presence of V. parahaemolyticus was observed between the two sites investigated. Abundance of V. parahaemolyticus in zooplankton ranged from undetected levels (<0.3 MPN per gram) to a maximum value of >110 MPN per gram. The highest levels of V. parahaemolyticus were detected during the summer and autumn months of both years, whereas undetectable levels or minimum values were recorded during the winter (Supplementary Figure 3). The estimated seasonal trend in the log-transformed levels of V. parahaemolyticus in zooplankton over the course of the study was investigated through GAM for continuous responses (Figure 1a). The abundance of V. parahaemolyticus peaked at the beginning of the study in June 2005, started to decline after this point and reached the lowest levels from December 2005 to February 2006. The trend observed in the abundance of V. parahaemolyticus in 2006 was delayed relative to the corresponding trend in 2005.
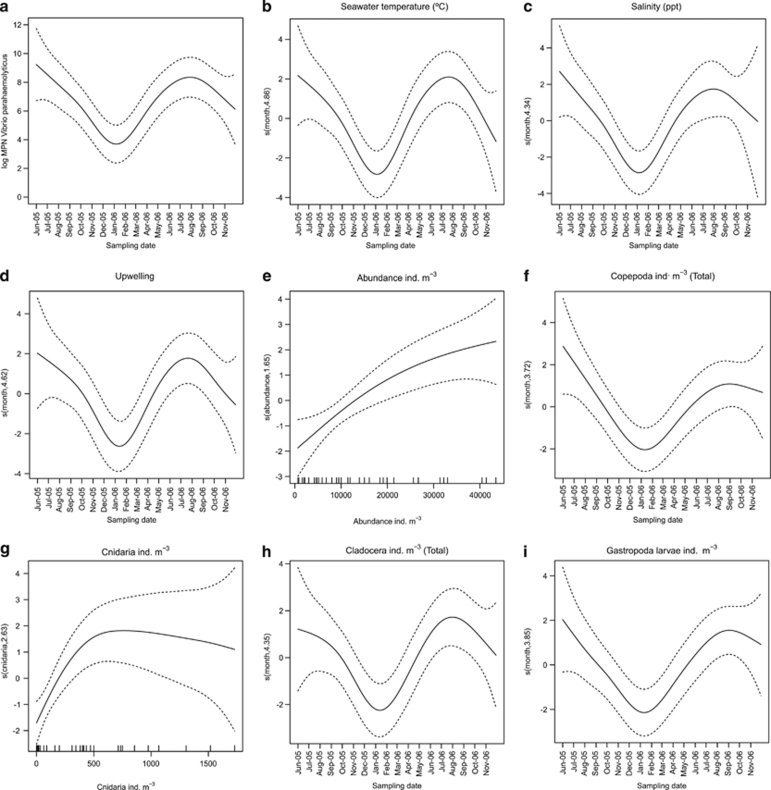
Figure 1.
Temporal variation in the predicted log-transformed levels of V. parahaemolyticus in zooplankton throughout the period of study (a) and estimated effects of different variables on the predicted abundance of V. parahaemolyticus (log MPN counts) when the effect of time is incorporated into the GAM regression models. Except for total abundance and Cnidaria, the effect of all environmental variables and zooplankton groups is linear when the time effect is included in the GAM regression models (not shown), and their non-linear effects are then associated with their specific seasonality as shared by these variables (b, c, d, f, h and i). Total abundance and Cnidaria abundance are the only variables retaining a non-linear effect on the response independent of the seasonal trend (e and g). The dashed lines indicate the 95% point-wise confidence bands for the predictions.
Influence of environmental factors
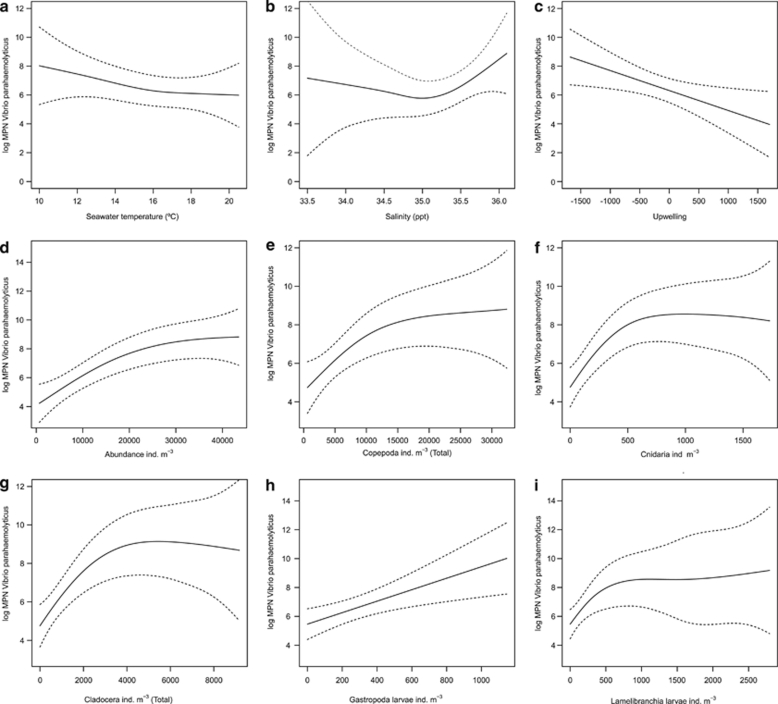
Seawater temperature and salinity had a non-significant effect on the modulation of the abundance of V. parahaemolyticus in zooplankton (Table 2 and Figure 2). Results from the GAM regression models showed a slight increasing effect for salinity at values above 35 p.p.t., whereas seawater temperature showed no influence for the entire period. By contrast, abundance of V. parahaemolyticus was significantly affected by negative values of upwelling (downwelling periods) in a linear relationship (df=1). The model explained 14.5% of the total deviance. Nevertheless, when the effect of the time effect was incorporated into the GAM regression model, the influence of upwelling lost its statistical significance and all of the changes previously found to be associated with this variable were instead found to be associated with its seasonal trend (Tables 2 and 3, and Figure 1).
Table 2. Estimated effects of the environmental variables and zooplankton abundance on the levels of V. parahaemolyticus detected in >200-μm zooplankton, as inferred from GAM analysis.
| Effectsa | Effective degrees of freedom (edf) | Coefficient (95% CI) | Deviance explained (%) | P-value |
|---|---|---|---|---|
| s (Seawater temperature) | 1.406 | — | 6.9 | 0.280 |
| s (Salinity) | 2.122 | — | 14.4 | 0.195 |
| Upwelling | 1.000 | −0.0013 (−0.0025, −0.0002) | 14.5 | 0.024 |
| s (Abundance (total)) | 1.938 | — | 40.7 | <0.001 |
| s (Copepoda (total)) | 1.982 | — | 29.6 | 0.011 |
| s (Cladocera (total)) | 2.322 | — | 39.6 | 0.001 |
| s (Cnidaria) | 2.465 | — | 42.0 | <0.001 |
| Gastropoda larvae | 1.000 | 0.0040 (0.0013, 0.0067) | 20.5 | 0.006 |
| s (Lamellibranchia larvae) | 2.409 | — | 27.3 | 0.037 |
Abbreviations: CI, confidence interval; GAM, generalized additive model.
s (predictor): Smooth (centred) effect of the predictor.
Figure 2.
Effects of the different variables on the abundance of V. parahaemolyticus (log MPN counts) in zooplankton obtained from GAMs: seawater temperature (a), salinity (b), upwelling (c), total abundance of zooplankton (d), Copepoda abundance (e), Cnidaria abundance (f), Cladocera abundance (g), Gastropoda larvae abundance (h) and Lamellibranchia larvae abundance (i). The dashed lines indicate the 95% point-wise confidence bands for the predictions.
Table 3. Estimated effects of the environmental variables and zooplankton abundance, adjusted by the time effect, on the levels of V. parahaemolyticus detected in >200-μm zooplankton, as inferred from GAM analysis.
| Model | Effectsa | Effective degrees of freedom (edf) | Coefficient (95% CI) | P-value | Deviance explained (%) |
|---|---|---|---|---|---|
| Seawater temperature | |||||
| s (Month) | 4.864 | — | 0.001 | 54.5% | |
| Seawater temperature | 1.000 | −0.2092 (−0.4786, 0.0603) | 0.145 | ||
| Salinity | |||||
| s (Month) | 4.337 | — | 0.002 | 52.0% | |
| Salinity | 1.000 | 0.1535 (−0.9431, 1.2502) | 0.829 | ||
| Upwelling | |||||
| s (Month) | 4.624 | — | 0.008 | 54.4% | |
| Upwelling | 1.000 | −0.0007 (−0.0018, 0.0004) | 0.289 | ||
| Abundance | |||||
| s (Month) | 2.552 | — | 0.084 | 53.7% | |
| s (Abundance) | 1.647 | — | 0.003 | ||
| Copepoda | |||||
| s (Month) | 3.225 | — | 0.012 | 55.2% | |
| Copepoda | 1.000 | 0.0001 (0.00004, 0.0002) | 0.007 | ||
| Cladocera | |||||
| s (Month) | 4.354 | — | 0.013 | 58.8% | |
| Cladocera | 1.000 | 0.0004 (0.0001, 0.0008) | 0.018 | ||
| Cnidaria | |||||
| s (Month) | 1.986 | — | 0.205 | 51.8% | |
| s (Cnidaria) | 2.628 | — | 0.003 | ||
| Gastropoda larvae | |||||
| s (Month) | 3.853 | — | 0.007 | 55.6% | |
| Gastropoda larvae | 1.000 | 0.0033 (0.0009, 0.0057) | 0.015 | ||
| Lamellibranchia larvae | |||||
| s (Month) | 4.184 | — | 0.003 | 55.3% | |
| Lamellibranchia larvae | 1.000 | 0.0013 (0.0001, 0.0025) | 0.043 | ||
Abbreviations: CI, confidence interval; GAM, generalized additive model.
s (predictor): Smooth (centred) effect of the predictor.
Association with zooplankton
The total abundance of zooplankton showed a significant (P<0.001) non-linear increasing effect on the abundance of V. parahaemolyticus. This effect explained 40.7% of the deviance (Table 2). All taxonomic groups analysed showed a similar pattern and different levels of explained deviance (Table 2 and Figure 2). Cnidaria showed the highest explained deviance (42%), whereas the effect of Copepoda only accounted for 29.6% of the explained deviance. However, when the influence of the time was evaluated by adding the time term to the GAM regression models, the effect of the different groups of zooplankton, including Copepoda, became linear. Their non-linear influence on the response was then associated with the specific time trend shared by all groups (Table 3 and Figure 1). This pattern was common for all groups but Cnidaria. Cnidaria showed a distinctive significant non-linear effect that accounted for 51.87% of the explained deviance in a model incorporating the time term. A similar pattern was observed for the total abundance of zooplankton when the time term was added. These factors then accounted for 53.7% of the explained deviance (Table 3 and Figure 1).
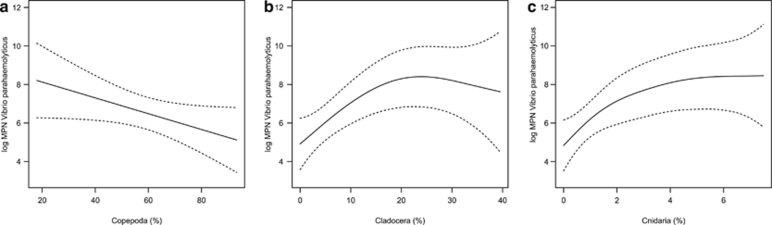
Finally, the effect of the relative abundance (%) of each zooplankton taxon on the level of V. parahaemolyticus was also assessed (Figure 3). A significant positive effect on the response was only found for the relative abundance of Cladocera and Cnidaria, and these variables represented 26.3% and 27.6% of the explained deviance, respectively. The relative abundance of Copepoda, the dominant taxon in the zooplankton (more than 50% of total zooplankton), showed a non-significant effect on V. parahaemolyticus levels.
Figure 3.
Effects of the relative abundance (%) of Copepoda (a), Cladocera (b) and Cnidaria (c) on the V. parahaemolyticus levels in zooplankton. The dashed lines indicate the 95% point-wise confidence bands for the predictions.
V. parahaemolyticus in seawater
V. parahaemolyticus was found in only 14 of 108 seawater samples (12%) and occurred mostly during the summer and autumn months. Results from the logistic GAM for the binary response showed that the occurrence of V. parahaemolyticus in seawater was not significantly influenced by variations in temperature, salinity and upwelling. Moreover, the abundance of zooplankton was not associated with the presence of V. parahaemolyticus in seawater.
Presence of virulence genes
The trh gene was detected in 22 (31%) samples of zooplankton. A higher occurrence of the trh gene was observed throughout summer and autumn, with four peaks of maximum abundance: August and October 2005, with values of >110 and 24 MPN per gram, respectively, and April and October 2006, which both had counts of 46 MPN per gram (Supplementary Figure 3). The abundance of trh-positive samples was only influenced by total abundance of zooplankton (P<0.01) according to the results of the GAM model. The presence of the trh gene in zooplankton was strongly associated with the total abundance of V. parahaemolyticus (P<0.005). By contrast, tdh-positive samples were found in 10 (14%) zooplankton samples. The levels of tdh-positive samples were particularly low for four sample dates (July 2005, and May, July and November 2006), whereas a single sample collected in August 2005 accounted for the highest MPN value of >110 MPN per gram.
A total of 1266 colonies from zooplankton and 143 colonies from seawater were selected from the TCBS and CHROMagar plates. Of these colonies, 267 originating from zooplankton and 21 originating from seawater were confirmed by PCR as V. parahaemolyticus. Investigation of the pathogenic marker genes trh and tdh allowed the identification of 41 trh+/tdh− strains in zooplankton, whereas only one isolate with this genotype was found in seawater. These strains were isolated throughout the entire study period with the exception of August and December 2005, and February, March and May 2006. The mean relative abundance of the trh+/tdh− strains in zooplankton was 15.3%. No tdh+ strains were identified.
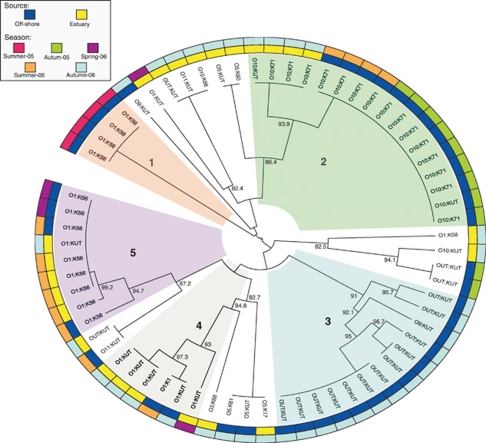
DNA fingerprints of the trh+ strains were used to evaluate the degree of similarity between strains isolated in different periods and habitats (Figure 4). Pulsed-field gel electrophoresis analysis of the 42 trh+ strains revealed the presence of nine major genetic clusters. More than 90% of the strains were assigned to the four main groups. The two largest clusters (2 and 3 in Figure 4), both with 13 strains from offshore areas, grouped strains with serotypes O10:K71, Cluster-2, and OUT:KUT, Cluster-3. Strains from clusters 1 and 5 shared the serotype O1:K56. However, they showed marked genetic differences. Three of the genetic clusters showed a clear seasonality and a specific period of appearance: Cluster-1 was exclusively detected in summer 2005; Cluster-3 in autumn 2006; and Cluster-4 in spring and summer 2006. Cluster-2 was the only group detected in the seasons of two different years, in autumn 2005 and summer 2006. Offshore trh+ strains were compared with a pool of genetically diverse trh+ strains representative of the different genetic variants isolated over the last 6 years from estuarine areas in dispersed locations along more than 1500 km of coast (Figure 4). Results of the comparative analysis revealed genetic clusters comprised exclusively of offshore strains (clusters 1 and 3) and groups including strains from both offshore and estuarine areas, and sharing indistinguishable genetic profiles (clusters 2, 4 and 5).
Figure 4.
Pulsed-field gel electrophoresis dendrogram generated by the BioNumerics software and drawn using the online iTOL software package, showing the genetic relationships among the 42 trh+/tdh− V. parahaemolyticus strains isolated in offshore waters in the present study and the 24 strains obtained from estuarine areas along 1500 km of the coast of Galicia in previous studies. Read from the inner to outer ring, the rings indicate the area of origin (offshore and estuary) and the season of isolation, respectively, for each strain, and the numbers at the dendrogram branches represent the percentage of similarity among the different groups.
Discussion
The life cycle of V. parahaemolyticus has conventionally been associated with estuarine areas where moderate levels of salinity, warm temperatures and nutrient availability provide suitable conditions for the survival of many Vibrio species (Joseph et al., 1982). However, the occurrence of V. parahaemolyticus in offshore waters has been found to be extremely limited, owing to the influence of adverse conditions prevailing throughout the year (Joseph et al., 1982). The results of the present study show that offshore zones are common habitats for V. parahaemolyticus, where this organism has been found to survive almost exclusively in close association with zooplankton. V. parahaemolyticus was detected in 80% of zooplankton samples from offshore areas over the entire study period, with the exception of December 2005. Previous studies conducted in internal areas of the rias in Galicia showed a V. parahaemolyticus occurrence of 12.5% in shellfish (Martinez-Urtaza et al., 2008b) and 35.3% in estuarine waters (Rodriguez-Castro et al., 2010). In estuaries, temperature and salinity represent critical factors influencing the dynamics of V. parahaemolyticus. However, these variables showed no effect on the prevalence and seasonality of V. parahaemolyticus in offshore areas.
The rias are subjected to seasonal upwelling–downwelling sequences that determine the patterns of circulation and exchange of water in the inner areas of the rias (Álvarez-Salgado et al., 2000). The cold and nutrient-rich Eastern North Atlantic Central Water is transported onto the shelf in the course of the upwelling events, in turn enhancing the primary production and the subsequent local production of zooplankton. Presence of V. parahaemolyticus has been found to be associated with primary production in estuaries and areas of moderate salinity in different regions of the world, such as Germany (Oberbeckmann et al., 2011), the Gulf of Mexico (Johnson et al., 2010) and France (Julie et al., 2010). Conversely, the offshore occurrence of V. parahaemolyticus in this study was favoured by reduction of primary production, which resulted in an enhanced abundance of zooplankton. During downwelling periods, warm and salty subtropical waters move poleward from the south of Portugal to the Armorican shelf in France (Álvarez-Salgado et al., 2000). The arrival of these downwelling fronts in Galicia allows oceanic waters to enter the interior of the rias (Álvarez-Salgado et al., 2000; Crespo et al., 2006). Moreover, zooplankton showed a behaviourally mediated concentration in density fronts, such as downwelling fronts (Olson et al., 1994), where population densities may be up to three times greater than in other waters (Genin et al., 2005). The displacement of the density fronts towards the interior of the rias during downwelling periods may represent an important supply of oceanic zooplankton and a parallel source of V. parahaemolyticus populations attached to the zooplankton. Coincidentally, one of the largest V. parahaemolyticus outbreaks detected in Europe occurred in Galicia in 1999 (Lozano-Leon et al., 2003) and was concurrent with the incursion in the rias of warm tropical waters and the presence of large patches of zooplankton (Baker-Austin et al., 2010). Similar oceanographic conditions have been observed to be related to large outbreaks of V. parahaemolyticus in Peru (Martinez-Urtaza et al., 2008a), Alaska (Martinez-Urtaza et al., 2010) and Chile (Ansede-Bermejo et al., 2010). Likewise, temperate Gulf Stream waters have been suggested as a vehicle for transport of subtropical strains of Vibrio to Barnegat Bay, USA (Thompson et al., 2004).
V. parahaemolyticus counts were observed in the present study to increase in parallel to increased zooplankton density, measured as the number of individuals per cubic metre of water. Because V. parahaemolyticus counts were always expressed on a 1-g basis, the detection of higher counts of V. parahaemolyticus could not be related directly to the analysis of a larger biomass of zooplankton, but rather to a higher density of V. parahaemolyticus cells per gram of zooplankton. This greater density of V. parahaemolyticus would then be associated with an increase in the number of the V. parahaemolyticus cells attached to 1 g of zooplankton. Higher V. parahaemolyticus densities may be the result of a rise in temperature, because warmer conditions are expected to promote the proliferation of V. parahaemolyticus cells on the zooplankton exoskeleton (Kaneko and Colwell, 1973, 1975, 1978; Baffone et al., 2006). However, no tendency of seawater temperature to produce higher observed loads of V. parahaemolyticus and zooplankton was observed in this study. Another possible explanation for the increase of V. parahaemolyticus densities in zooplankton may involve a distinctive influence of the different groups constituting the zooplankton. Temporal shifts in zooplankton composition and species dominance may promote the proliferation of groups carrying larger V. parahaemolyticus loads. In contrast to the importance of copepods in the ecology of Vibrio populations in estuarine areas (Colwell, 1996; Pruzzo et al., 2008), the results of the present study suggest only a small significance for copepods in the offshore occurrence of V. parahaemolyticus. This study also revealed that two different groups, Cnidaria and Cladocera, are relatively more influential on V. parahaemolyticus levels. Cladocerans have been identified as a reservoir of V. cholerae in Bangladesh (Huq et al., 1990) and in shelf waters in Brazil (Martinelli Filho et al., 2011). It has recently been reported that V. cholerae non-O1/non-O139 has a preference for growth on cladocerans rather than on copepods (Kirschner et al., 2010). A more explicit association has been found between V. parahaemolyticus and cnidarian zooplankton. The presence of cnidarians accounted for 51.87% of the V. parahaemolyticus variation, whereas cnidarians represented roughly 2% of the total zooplankton. This finding underscores the profound effect that rare members of the plankton community may exert on Vibrio populations, as indicated by the results of previous studies (Turner et al., 2009). Gelatinous zooplankton is typically oceanic and has been found to be concentrated in density fronts of subtropical waters (Graham et al., 2001). Associations of this particular group of zooplankton with Vibrio have seldom been investigated.
V. parahaemolyticus strains bearing virulence genes have not often been detected in environmental sources. However, this study has revealed that potential pathogenic strains are not rare in offshore zooplankton and that they represent a relevant fraction of the mesozooplankton microbiome. Although zooplankton has been previously proposed as a carrier and vehicle for the dispersal of cholera (Lipp et al., 2002) and pathogenic V. parahaemolyticus (Martinez-Urtaza et al., 2008a, 2010), the results of the present study represent one of the first empirical indications that virulent populations can survive in open sea conditions. The analysis of the population structure of trh-positive strains used to track the dispersal of Vibrio populations has also revealed a specific pattern of seasonal partitioning marked by distinctive populations that occur during different periods of the year. These findings may suggest the presence of community-specific populations associated with shifts in zooplankton composition. Differentiated communities of Vibrio isolated from cold- and warm-ocean waters have been also reported for the north Atlantic coast in the United States of America (Thompson et al., 2004). Simultaneously, a common genetic profile was detected among some groups of estuarine and offshore strains. Genetically indistinguishable strains were detected in zooplankton from offshore areas from southern Galicia and from estuaries isolated in the innermost parts of the rias. These localities are dispersed along more than 1500 km of the west and north coasts of the Iberian Peninsula. Their locations specifically mirror the poleward flow of warm and salty waters of downwelling fronts. According to this finding, it is reasonable to postulate that the dispersal of V. parahaemolyticus in this area is mediated by mechanisms associated with the migration of zooplankton trapped in ocean currents. The influx of zooplankton and the continuous recruitment of new genetic communities of V. parahaemolyticus to estuarine areas may stimulate a process of frequent population admixture at a local scale and may produce subsequent impacts on the bacterial population demography. The absence of barriers for dispersal prevents the operation of selection for speciation and niche specialization (Oakley et al., 2010; Preheim et al., 2010). Together with the high rates of recombination of Vibrio (Vos and Didelot, 2009), these processes may constitute the major forces shaping the genetic diversity of the Vibrio populations found in marine microbiomes (Hoffmann et al., 2010; Preheim et al., 2010).
Our results confirm that offshore waters may be common habitats for V. parahaemolyticus, including strains with virulence traits. The occurrence of V. parahaemolyticus in these natural systems has been found to be almost exclusively linked to the distribution and phenology of zooplankton, which likely provide a protective environment for the survival of V. parahaemolyticus under the adverse conditions of offshore areas. The interaction with zooplankton may also provide a platform for displacement in the open sea drifting along with the ocean currents. Furthermore, these findings unlock the possibility that, in the absence of geographical barriers, Vibrio may move freely across vast areas and habitats so as to connect different populations, thereby producing impacts on local bacterial community demography and on the epidemiology of the diseases caused by these organisms.
Acknowledgments
This work was partially supported by the Spanish Ministry of Science and Innovation Grant CGL2009-10488.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Álvarez-Salgado XA, Gago J, Míguez BM, Gilcoto M, Pérez FF. Surface waters of the NW Iberian margin: upwelling on the shelf versus outwelling of upwelled waters from the Rías Baixas. Estuarine Coastal Shelf Sci. 2000;51:821–837. [Google Scholar]
- Amako K, Shimodori S, Imoto T, Miake S, Umeda A. Effects of chitin and its soluble derivatives on survival of Vibrio cholerae O1 at low temperature. Appl Environ Microbiol. 1987;53:603–605. doi: 10.1128/aem.53.3.603-605.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansede-Bermejo J, Gavilan RG, Trinanes J, Espejo RT, Martinez-Urtaza J. Origins and colonization history of pandemic Vibrio parahaemolyticus in South America. Mol Ecol. 2010;19:3924–3937. doi: 10.1111/j.1365-294X.2010.04782.x. [DOI] [PubMed] [Google Scholar]
- Baffone W, Tarsi R, Pane L, Campana R, Repetto B, Mariottini GL, et al. Detection of free-living and plankton-bound vibrios in coastal waters of the Adriatic Sea (Italy) and study of their pathogenicity-associated properties. Environ Microbiol. 2006;8:1299–1305. doi: 10.1111/j.1462-2920.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C, Stockley L, Rangdale R, Martinez-Urtaza J. Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: a European perspective. Environ Microbiol Rep. 2010;2:7–18. doi: 10.1111/j.1758-2229.2009.00096.x. [DOI] [PubMed] [Google Scholar]
- Blackstone GM, Nordstrom JL, Vickery MCL, Bowen MD, Meyer RF, DePaola A. Detection of pathogenic Vibrio parahaemolyticus in oyster enrichments by real time PCR. J Microbiol Methods. 2003;53:149–155. doi: 10.1016/s0167-7012(03)00020-4. [DOI] [PubMed] [Google Scholar]
- Blanco-Abad V, Ansede-Bermejo J, Rodriguez-Castro A, Martinez-Urtaza J. Evaluation of different procedures for the optimized detection of Vibrio parahaemolyticus in mussels and environmental samples. Int J Food Microbiol. 2009;129:229–236. doi: 10.1016/j.ijfoodmicro.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Cabanillas-Beltrán H, Llausás-Magaña E, Romero R, Espinoza A, García-Gasca A, Nishibuchi M, et al. Outbreak of gastroenteritis caused by the pandemic Vibrio parahaemolyticus O3:K6 in Mexico. FEMS Microbiol Lett. 2006;265:76–80. doi: 10.1111/j.1574-6968.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- Cabrera-Garcia ME, Vazquez-Salinas C, Quinones-Ramirez EI. Serologic and molecular characterization of Vibrio parahaemolyticus strains isolated from seawater and fish products of the Gulf of Mexico. Appl Environ Microbiol. 2004;70:6401–6406. doi: 10.1128/AEM.70.11.6401-6406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Crespo BG, Figueiras FG, Porras P, Teixeira IG. Downwelling and dominance of autochthonous dinoflagellates in the NW Iberian margin: the example of the Ría de Vigo. Harmful Algae. 2006;5:770–781. [Google Scholar]
- Chimetto LA, Brocchi M, Thompson CC, Martins RCR, Ramos HR, Thompson FL. Vibrios dominate as culturable nitrogen-fixing bacteria of the Brazilian coral Mussismilia hispida. Syst Appl Microbiol. 2008;31:312–319. doi: 10.1016/j.syapm.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Dawson MP, Humphrey BA, Marshall KC. Adhesion: a tactic in the survival strategy of a marine vibrio during starvation. Curr Microbiol. 1981;6:195–199. [Google Scholar]
- Dumontet S, Krovacek K, Baloda SB, Grottoli R, Pasquale V, Vanucci S. Ecological relationship between Aeromonas and Vibrio spp. and planktonic copepods in the coastal marine environment in southern Italy. Comp Immunol Microbiol Infect Dis. 1996;19:245–254. doi: 10.1016/0147-9571(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Elliot EL, Kaysner CA, Jackson L, Tamplin ML.1995Vibrio cholerae, V. parahaemolyticus, V. vulnificus and other Vibrio spp FDA Bacteriological Analytical Manual8th ed. AOAC International: Gaithersburg; pp 9:9–9.27. [Google Scholar]
- FDA . Quantitative Risk Assessment on the Public Health Impact of Pathogenic Vibrio Parahaemolyticus in Raw Oysters. FDA: Washington, DC; 2005. [Google Scholar]
- Genin A, Jaffe JS, Reef R, Richter C, Franks PJS. Swimming against the flow: a mechanism of zooplankton aggregation. Science. 2005;308:860–862. doi: 10.1126/science.1107834. [DOI] [PubMed] [Google Scholar]
- Graham WM, Pagès F, Hammer WM. A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia. 2001;451:199–212. [Google Scholar]
- Halpern M, Senderovich Y, Izhaki I. Waterfowl: the missing link in epidemic and pandemic cholera dissemination. PLoS Pathog. 2008;4:e1000173. doi: 10.1371/journal.ppat.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Exploring the nature of covariate effects in the proportional hazards model. Biometrics. 1990;46:1005–1016. [PubMed] [Google Scholar]
- Herrera FC, Santos JA, Otero A, García-López ML. Occurrence of foodborne pathogenic bacteria in retail prepackaged portions of marine fish in Spain. J Appl Microbiol. 2006;100:527–536. doi: 10.1111/j.1365-2672.2005.02848.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Fischer M, Ottesen A, McCarthy PJ, Lopez JV, Brown EW, et al. Population dynamics of Vibrio spp. associated with marine sponge microcosms. ISME J. 2010;4:1608–1612. doi: 10.1038/ismej.2010.85. [DOI] [PubMed] [Google Scholar]
- Huq A, Colwell RR, Rahman R, Ali A, Chowdhury MA, Parveen S, et al. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl Environ Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 8914:1990 . International Organization for Standarization: Geneva; 1990. Microbiology—General guidance for the detection of Vibrio parahaemolyticus. [Google Scholar]
- Johnson CN, Flowers AR, Noriea NF, 3rd, Zimmerman AM, Bowers JC, DePaola A, et al. Relationships between environmental factors and pathogenic Vibrios in the Northern Gulf of Mexico. Appl Environ Microbiol. 2010;76:7076–7084. doi: 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SW, Colwell RR, Kaper JB. Vibrio parahaemolyticus and related halophilic Vibrios. Crit Rev Microbiol. 1982;10:77–124. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- Julie D, Solen L, Antoine V, Jaufrey C, Annick D, Dominique HH. Ecology of pathogenic and non-pathogenic Vibrio parahaemolyticus on the French Atlantic coast. Effects of temperature, salinity, turbidity and chlorophyll a. Environ Microbiol. 2010;12:929–937. doi: 10.1111/j.1462-2920.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Colwell RR. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J Bacteriol. 1973;113:24–32. doi: 10.1128/jb.113.1.24-32.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Colwell RR. Incidence of Vibrio parahaemolyticus in Chesapeake Bay. Appl Microbiol. 1975;30:251–257. doi: 10.1128/am.30.2.251-257.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Colwell RR. The annual cycle of Vibrio parahaemolyticus in Chesapeake Bay. Microb Ecol. 1978;4:135–155. doi: 10.1007/BF02014284. [DOI] [PubMed] [Google Scholar]
- Kim YB, Okuda J, Matsumoto C, Takahashi N, Hashimoto S, Nishibuchi M. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J Clin Microbiol. 1999;37:1173–1177. doi: 10.1128/jcm.37.4.1173-1177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner AK, Schauer S, Steinberger B, Wilhartitz I, Grim CJ, Huq A, et al. Interaction of Vibrio cholerae non-O1/non-O139 with Copepods, Cladocerans and competing bacteria in the large Alkaline Lake Neusiedler see, Austria. Microb Ecol. 2011;61:496–506. doi: 10.1007/s00248-010-9764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- Lipp EK, Huq A, Colwell RR. Effects of global climate on infectious disease: the cholera model. Clin Microbiol Rev. 2002;15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Leon A, Torres J, Osorio CR, Martinez-Urtaza J. Identification of tdh-positive Vibrio parahaemolyticus from an outbreak associated with raw oyster consumption in Spain. FEMS Microbiol Lett. 2003;226:281–284. doi: 10.1016/S0378-1097(03)00604-9. [DOI] [PubMed] [Google Scholar]
- Martinelli Filho JE, Lopes RM, Rivera ING, Colwell RR. Vibrio cholerae O1 detection in estuarine and coastal zooplankton. J Plankton Res. 2011;33:51–62. [Google Scholar]
- Martinez-Urtaza J, Bowers JC, Trinanes J, DePaola A. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780–1790. [Google Scholar]
- Martinez-Urtaza J, Huapaya B, Gavilan RG, Blanco-Abad V, Ansede-Bermejo J, Cadarso-Suarez C, et al. Emergence of Asiatic Vibrio diseases in South America in phase with El Nino. Epidemiology. 2008a;19:829–837. doi: 10.1097/EDE.0b013e3181883d43. [DOI] [PubMed] [Google Scholar]
- Martinez-Urtaza J, Lozano-Leon A, DePaola A, Ishibashi M, Shimada K, Nishibuchi M, et al. Characterization of pathogenic Vibrio parahaemolyticus isolates from clinical sources in Spain and comparison with Asian and North American pandemic isolates. J Clin Microbiol. 2004;42:4672–4678. doi: 10.1128/JCM.42.10.4672-4678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Urtaza J, Lozano-Leon A, Varela-Pet J, Trinanes J, Pazos Y, Garcia-Martin O. Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the rias of Galicia, Spain. Appl Environ Microbiol. 2008b;74:265–274. doi: 10.1128/AEM.01307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BB, Carbonero F, van der Gast CJ, Hawkins RJ, Purdy KJ. Evolutionary divergence and biogeography of sympatric niche-differentiated bacterial populations. ISME J. 2010;4:488–497. doi: 10.1038/ismej.2009.146. [DOI] [PubMed] [Google Scholar]
- Oberbeckmann S, Wichels A, Wiltshire KH, Gerdts G. Occurrence of Vibrio parahaemolyticus and Vibrio alginolyticus in the German Bight over a seasonal cycle. Antonie Van Leeuwenhoek. 2011;100:291–307. doi: 10.1007/s10482-011-9586-x. [DOI] [PubMed] [Google Scholar]
- Olson DB, Hitchcock GL, Mariano AJ, Ashjan CJ, Peng G, Nero RW, et al. Life on the edge: marine life and fronts. Oceanography. 1994;7:52–60. [Google Scholar]
- Preheim SP, Boucher Y, Wildschutte H, David LA, Veneziano D, Alm EJ, et al. Metapopulation structure of Vibrionaceae among coastal marine invertebrates. Environ Microbiol. 2010;13:265–275. doi: 10.1111/j.1462-2920.2010.02328.x. [DOI] [PubMed] [Google Scholar]
- Pruzzo C, Vezzulli L, Colwell RR. Global impact of Vibrio cholerae interactions with chitin. Environ Microbiol. 2008;10:1400–1410. doi: 10.1111/j.1462-2920.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria; 2010. [Google Scholar]
- Rodriguez-Castro A, Ansede-Bermejo J, Blanco-Abad V, Varela-Pet J, Garcia-Martin O, Martinez-Urtaza J. Prevalence and genetic diversity of pathogenic populations of Vibrio parahaemolyticus in coastal waters of Galicia, Spain. Environ Microbiol Rep. 2010;2:58–66. doi: 10.1111/j.1758-2229.2009.00064.x. [DOI] [PubMed] [Google Scholar]
- Ruppert D, Wand MP, Carroll RJ. Semiparametric Regression. Cambridge University Press: Cambridge; 2003. [Google Scholar]
- Tada J, Ohashi T, Nishimura N, Shirasaki Y, Ozaki H, Fukushima S, et al. Detection of the thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Mol Cell Probes. 1992;6:477–487. doi: 10.1016/0890-8508(92)90044-x. [DOI] [PubMed] [Google Scholar]
- Terzi G, Büyüktanỳr Ö, Yurdusev N. Detection of the tdh and trh genes in Vibrio parahaemolyticus isolates in fish and mussels from Middle Black Sea Coast of Turkey. Lett Appl Microbiol. 2009;49:757–763. doi: 10.1111/j.1472-765X.2009.02736.x. [DOI] [PubMed] [Google Scholar]
- Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF. Diversity and dynamics of a North Atlantic Coastal Vibrio community. Appl Environ Microbiol. 2004;70:4103–4110. doi: 10.1128/AEM.70.7.4103-4110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JW, Good B, Cole D, Lipp EK. Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J. 2009;3:1082–1092. doi: 10.1038/ismej.2009.50. [DOI] [PubMed] [Google Scholar]
- Vos M, Didelot X. A comparison of homologous recombination rates in bacteria and archaea. ISME J. 2009;3:199–208. doi: 10.1038/ismej.2008.93. [DOI] [PubMed] [Google Scholar]
- Wood SN. Thin plate regression splines. J R Stat Soc B. 2003;65:95–114. [Google Scholar]
- Wood SN. Generalized Additive Models: an Introduction with R. Texts in Statistical Science. Chapman and Hall/CRC Press: Boca Raton, Florida; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.