Abstract
Cryoturbated peat circles (that is, bare surface soil mixed by frost action; pH 3–4) in the Russian discontinuous permafrost tundra are nitrate-rich ‘hotspots' of nitrous oxide (N2O) emissions in arctic ecosystems, whereas adjacent unturbated peat areas are not. N2O was produced and subsequently consumed at pH 4 in unsupplemented anoxic microcosms with cryoturbated but not in those with unturbated peat soil. Nitrate, nitrite and acetylene stimulated net N2O production of both soils in anoxic microcosms, indicating denitrification as the source of N2O. Up to 500 and 10 μ nitrate stimulated denitrification in cryoturbated and unturbated peat soils, respectively. Apparent maximal reaction velocities of nitrite-dependent denitrification were 28 and 18 nmol N2O gDW−1 h−1, for cryoturbated and unturbated peat soils, respectively. Barcoded amplicon pyrosequencing of narG, nirK/nirS and nosZ (encoding nitrate, nitrite and N2O reductases, respectively) yielded ≈49 000 quality-filtered sequences with an average sequence length of 444 bp. Up to 19 species-level operational taxonomic units were detected per soil and gene, many of which were distantly related to cultured denitrifiers or environmental sequences. Denitrification-associated gene diversity in cryoturbated and in unturbated peat soils differed. Quantitative PCR (inhibition-corrected per DNA extract) revealed higher copy numbers of narG in cryoturbated than in unturbated peat soil. Copy numbers of nirS were up to 1000 × higher than those of nirK in both soils, and nirS nirK−1 copy number ratios in cryoturbated and unturbated peat soils differed. The collective data indicate that the contrasting N2O emission patterns of cryoturbated and unturbated peat soils are associated with contrasting denitrifier communities.
Keywords: permafrost-affected soil, global change, wetland, barcoded amplicon pyrosequencing, quantitative PCR
Introduction
Nitrous oxide (N2O) is a major ozone-depleting substance in the atmosphere and the third most important greenhouse gas on earth (Forster et al., 2007; Ravishankara et al., 2009). The global warming potential of N2O is 300-fold higher than that of CO2 on a 100-year basis, and the atmospheric concentration of N2O increased from 270 to 319 ppb from 1750 to 2005 (Forster et al., 2007). Agricultural and pristine tropical soils are well-recognized major sources of N2O, whereas the importance of arctic peatlands as sources of N2O is just emerging (Denman et al., 2007; Repo et al., 2009; Marushchak et al., 2011).
Areas of bare surface soil mixed by frost action in acidic tundra (pH 3–4) are termed ‘cryoturbated peat circles', and emit N2O at rates documented for tropical and agricultural soils (Maljanen et al., 2007; Werner et al., 2007; Repo et al., 2009). The estimated global N2O emission from cryoturbated peat circles is ∼0.1 Tg N2O per year, which is equivalent to 4% of the global warming potential of the arctic methane emissions and to 0.6% of the total global annual N2O emission (Christensen, 1993; Denman et al., 2007; Repo et al., 2009). Vegetation is absent from ∼12% of the area in the arctic, including cryoturbated peat circles (Walker et al., 2005). Nitrate concentrations approximate 2 m in the pore water of such unvegetated cryoturbated peat soil, and are ∼1000 × higher than in adjacent vegetated unturbated peat areas where N2O emissions are negligible (Repo et al., 2009). Repeated freezing and thawing of the cryoturbated soil leads to breakdown of soil aggregates, renders decomposable organic carbon more easily accessible to microbes and may thereby activate the microbial community including N2O producers (Mørkved et al., 2006; Sharma et al., 2006). Thus, cryoturbated peat circles represent acidic ‘hotspots' of microbial N2O emission in the tundra (Repo et al., 2009; Marushchak et al., 2011).
The main source of N2O in water-logged anoxic soils including peatlands is denitrification (Conrad, 1996; Pihlatie et al., 2004; Palmer et al., 2010). Complete denitrification is the sequential reduction of nitrate or nitrite to dinitrogen (N2) through nitric oxide (NO) and N2O; nitrite is likewise an intermediate when nitrate is used (Zumft, 1997). The oxidoreductases involved in denitrification include dissimilatory nitrate reductases encoded by narG or napA, copper- and cytochrome cd1-containing nitrite reductases (encoded by nirK and nirS, respectively), NO reductases encoded by norBC and N2O reductases encoded by nosZ (Zumft, 1997). Nitrate reductases likewise occur in dissimilatory nitrate reducers (Stolz and Basu, 2002). NirK and NirS are structurally different but functionally equivalent (Jones et al., 2008). Organisms hosting both types of nitrite reductase are unknown to date (Heylen et al., 2006). The genes coding for the above-named oxidoreductases are commonly used as structural gene markers for the analysis of nitrate reducer and denitrifier communities (Braker et al., 2000; Philippot et al., 2002; Prieme et al., 2002; Rich et al., 2003; Horn et al., 2006; Enwall et al., 2010; Jones and Hallin, 2010; Palmer et al., 2010; Bru et al., 2011). The main products of denitrification that are released into the atmosphere are N2 or N2O. Denitrifiers might lack nitrate reductases and/or N2O reductases, and occupy diverse ecological niches (Tiedje, 1988; Zumft, 1997; Shapleigh, 2006). Denitrification rates and the product ratio of N2O to N2 are regulated by the denitrifying community and in situ conditions (for example, pH, temperature, C-to-N-ratio, as well as the availability of substrates and electron acceptors; van Cleemput, 1998). Acidic pH<5 impairs denitrification and increases the product ratio of N2O to N2 (Simek and Cooper, 2002; Cuhel et al., 2010). The increased product ratio of N2O to N2 is likely caused by post-transcriptional effects of low pH on N2O reductase formation (Liu et al., 2010). However, information on denitrifier communities that thrive at pH<5 in peatlands is scarce (Palmer et al., 2010).
Denitrifier communities in permafrost-affected acidic tundra soils are unresolved to date, despite the fact that such soils are prone to react sensitively to global warming, which might accelerate cryoturbation and in turn increase N2O emissions (Bockheim, 2007; Repo et al., 2009). It is hypothesized that the observed contrasting N2O emission patterns of cryoturbated and unturbated acidic peat soil are associated with contrasting denitrifier communities. The main objectives of this study were (1) to compare ecophysiological traits (that is, capacities) of acid-tolerant denitrifier communities in cryoturbated and unturbated peat soils, (2) to develop pyrosequencing-based strategies for in-depth analysis of denitrifier communities by parallel analysis of multiple denitrification-associated genes, (3) to determine whether contrasting and new denitrifier communities occur in cryoturbated and unturbated peat soils by such pyrosequencing-based strategies and quantitative PCR and (4) thus to identify potential microbial catalysts of the exceptionally high N2O emissions from cryoturbated peat soil.
Materials and methods
Site description and soil sampling
The sampling area is located in the Russian discontinuous permafrost zone (62°57′E, 67°03′N) and was described previously (Repo et al., 2009; Supplementary Materials and methods). Cumulative N2O emissions in the field from the cryoturbated soil are 1.2±0.3 g N2O m−2, whereas those of the unturbated soil are negligible (<0.006 g N2O m−2). Topsoil was identified as the site of highest N2O production in the peat profile (data not shown), and the upper 5 cm was sampled from three cryoturbated peat circles and three adjacent, unturbated areas in September 2010. Roots were removed from unturbated soil, and soil for microcosm studies was stored at 4 °C until further processing. Soil for DNA extraction was suspended in RNAlater (Qiagen, Hilden, Germany) immediately after sampling to avoid decomposition of nucleic acids, and stored at −20 °C upon arrival at the laboratory. Experiments were conducted within 2 months after sampling. Moisture content was determined by weighing the soil before and after drying at 60 °C for 3 days and was 71% and 81% in cryoturbated and unturbated peat soils, respectively.
Assessment of denitrification in soil microcosms
Soil of the three replicate sampling sites was homogenized and pooled before microcosm experiments. Soil slurries at in situ pHH2O of ∼4 were prepared with 4–5 g of soil and 3 volumes of deionized water in 125-ml infusion flasks, and sealed using gas-tight rubber stoppers. The gasphase was 100% argon. Microcosms were incubated at 20 °C in the dark and performed in triplicate unless stated otherwise.
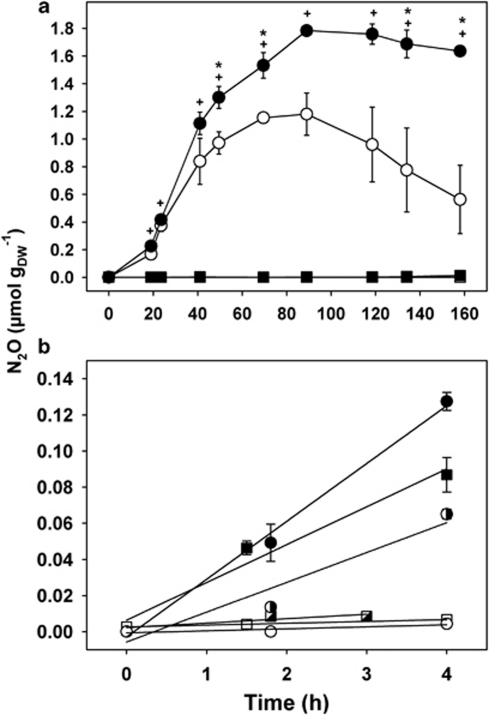
Acetylene blocks N2O reductases and thus the reduction of N2O to N2 (Yoshinari and Knowles, 1976). Parallel microcosms with and without acetylene (15% (vol/vol) in headspace) were used to differentiate between total denitrification and N2O-production potentials. Total denitrification stopped after ∼4 days (90 h) in unsupplemented microcosms with cryoturbated peat soil and acetylene, indicating that internal nitrate and nitrite were depleted (Figure 1a).
Figure 1.
(a) Denitrification and effect of acetylene on the production and consumption of N2O in anoxic microcosms with unsupplemented peat soil. Squares and circles represent unturbated and cryoturbated peat soils, respectively. Closed and open symbols represent microcosms with and without acetylene, respectively. Time points at which N2O concentrations in cryoturbated peat soil with acetylene differed significantly (P<0.05) from N2O concentrations in cryoturbated peat soil without acetylene or in unturbated peat soil with acetylene are indicated with (*) or (+), respectively. (b) Effect of 100 μ nitrite or nitrate on the production of N2O in anoxic microcosms with nitrate-depleted peat soil in the presence of acetylene. Squares and circles represent unturbated and cryoturbated peat soils, respectively. Closed symbols represent microcosms supplemented with nitrite, half-filled symbols represent microcosms supplemented with nitrate, and open symbols represent unsupplemented controls. Solid lines represent linear regression curves (R2=0.8–0.99). Mean values and s.e. of three replicate microcosms are shown in panels a and b.
For apparent Michaelis–Menten kinetics, soil was pre-incubated for 7 days under anoxic conditions to deplete internal nitrate and nitrite. Such soil was supplemented with 0–500 μ NaNO3 or NaNO2. N2O did not accumulate in anoxic microcosms containing 1 m nitrite in sterile water at pH 4 within 2 days (data not shown). Apparent Michaelis–Menten kinetics were based on the production of N2O in the presence of acetylene as described previously (Segel, 1993; Palmer et al., 2010; Supplementary Materials and methods). Soil that was pre-incubated under anoxic conditions for 9 days was used to study the effects of the electron donors acetate, ethanol, formate, propionate, butyrate and lactate on denitrification in microcosms supplemented with 1 m nitrite and 0.5 m electron donors in the presence of acetylene. After another 47 days of anoxic incubation, 1 m nitrite and 2 m electron donors (0.5 m for propionate only) were resupplied. N2O production, nitrite and electron donors were determined regularly after initial supplementation and after resupplementation. N2O production rates were calculated from 3 to 4 data points determined within 8–25 h after addition of substrates (nitrite and/or electron donors) when N2O production was linear. R2-values of the linear regressions were always >0.88.
Concentrations of electron donors were assessed by high-performance liquid chromatography, and nitrate as well as nitrite by ion chromatography (Palmer et al., 2010; Supplementary Materials and methods).
Extraction of nucleic acids, and amplification of narG, nirK, nirS and nosZ
Nucleic acids were extracted from triplicate cryoturbated and unturbated peat soil samples to account for lateral heterogeneity in microbial communities. A bead-beating protocol tailored for the efficient removal of PCR-inhibiting humic acids by aluminum sulfate precipitation before cell lysis was applied (Peršoh et al., 2008; Supplemental Materials and methods).
narG, nirK, nirS and nosZ were amplified using the primer pairs narG1960f (TAYGTSGGSCARGARAA)/narG2650r (TTYTCRTACCABGTBGC; Philippot et al., 2002), F1aCu (ATCATGGTSCTGCCGCG)/R3Cu (GCCTCGATCAGRTTGTGGTT; Throbäck et al., 2004), cd3aF (GTSAACGTSAAGGARACSGG)/R3cd (GASTTCGGRTGSGTCTTGA; Throbäck et al., 2004) and nosZF (CGCTGTTCITCGACAGYCAG)/nosZR (ATGTGCAKIGCRTGGCAGAA; Rich et al., 2003), respectively. Each primer was preceeded by a 6-bp-long barcode (AGCGTC for unturbated and ATATAC for cryoturbated soil samples) to separate sequences after pyrosequencing. In all, 8 replicate 25 μl PCR reactions per target gene were performed at 8 different annealing temperatures from 54.7 to 63.6 °C to maximize the likelihood of detecting a high diversity of target genes. All replicate PCR reactions that yielded products (that is, amplicons) of the correct size were pooled before subsequent analyses. For detailed PCR protocols, refer to Supplementary Materials and methods.
Barcoded amplicon pyrosequencing of structural genes
Previously published amplicon pyrosequencing strategies (Huber et al., 2007; Iwai et al., 2010; Will et al., 2010) were modified to maximize the likelyhood of specific amplification of denitrification-associated structural genes during amplicon generation. Pyrosequencing requires amplicons fused with sequencing adaptors. Published strategies use target gene-specific primers fused with a barcode and an ∼30-bp-long sequencing adaptor, resulting in primers with >50% of the sequence being not complementary to the target genes, and thus allowing for unspecific amplifications. In this study, amplicons were generated during PCR with target gene-specific primers fused with the barcode only (see above) rather than using primers that contain barcode and sequencing adaptors. Sequencing adaptors were ligated after PCR to gel-purified amplicons.
Amplicons of similar lengths of both soil types were combined in equal amounts (that is, narG and nosZ amplicons were pooled, as well as nirK and nirS). Amplicon mixtures were treated with PreCR Repair Mix (New England Biolabs, Frankfurt am Main, Germany) to eliminate possible PCR-blocking DNA damage that might have occurred during gel purification or storage of amplicons, and purified through isopropanol precipitation. Sequencing from 5′ (forward) and 3′ (reverse) ends of amplicons was performed after ligation of A (CGTATCGCCTCCCTCGCGCCATCAG) and B (CTATGCGCCTTGCCAGCCCGCTCAG) sequencing adaptors at the Göttingen Genomics Laboratory using the Roche GS-FLX 454 pyrosequencer and GS FLX Titanium series reagents (Roche, Mannheim, Germany) according to the manufacturer's instructions.
Sequence filtering and analysis
Sequences with ambiguities, and those with incorrect primer or barcode sequences were discarded. narG and nosZ sequences shorter than 350 bp, and nirK as well as nirS sequences shorter than 300 bp were likewise excluded from further analyses. Amplicon sequences were sorted according to their barcodes and primers, and combined subsets of sequences for each structural gene (that is, containing sequences from both cryoturbated and unturbated peat soils) were clustered (that is, assigned to operational taxonomic units (OTUs)) at species-level threshold distances of 33% (narG (Palmer et al., 2009)), 17% (nirK (PS Depkat-Jakob, HL Drake, MA Horn, personal communication)), 18% (nirS (PS Depkat-Jakob, HL Drake, MA Horn, personal communication)) or 20% (nosZ (Palmer et al., 2009)) based on DNA sequences using the JAguc2 pipeline (http://wwwagak.informatik.uni-kl.de/research/JAguc/; Nebel et al., 2011; Supplementary Figure S1). In brief, JAguc2 generates a pairwise sequence alignment before calculation of a distance matrix and clustering with the average similarity method. This approach is more reliable (that is, less sensitive to PCR and pyrosequencing noise, and thus less sensitive to an artifical inflation of diversity (Kunin et al., 2010)) than multiple alignments and/or clustering with complete linkage algorithms (Quince et al., 2009; Sun et al., 2009; Huse et al., 2010). Amplicon sequences obtained by pyrosequencing from defined template mixtures were essentially at most 10% dissimilar to template sequences due to PCR and pyrosequencing noise (Behnke et al., 2011; Quince et al., 2011). The threshold distances used to call OTUs in this study were 17–33%, which is substantially greater than the above-reported maximal PCR and pyrosequencing noise (Supplementary Figure S1). Thus, our approach was rather unaffected by PCR and pyrosequencing noise, although we did not apply flowgram-based sequence correction algorithms for pyrosequencing (as implemented in for example, AmpliconNoise; Quince et al., 2011). Clustering can be easily redone with JAguc2 at different threshold distances without the need for time-consuming re-calculation of the distance matrix to test the effect of threshold distance on the number of OTUs (Supplementary Figure S1). Rarefaction curves were generated for each sequence set using aRarefact (http://www.huntmountainsoftware.com/html/rarefaction.html) as part of a strategy to further minimize the effect of pyrosequencing noise on comparative diversity analyses (Dickie, 2010). The closest relatives of OTU representatives were determined using BLAST (Altschul et al., 1990). OTU representatives were exported from JAguc2, edited, translated in silico and aligned with reference sequences using the ClustalW algorithm implemented in MEGA 5.0 (Kumar et al., 2008). The alignments were refined manually, and phylogenetic trees were constructed with the neighbor-joining algorithm using p-distances from in silico-translated sequences with MEGA 5.0. The stability of tree topologies was assessed by calculating 10 000 bootstrap replicates (Saitou and Nei, 1987). Diversity measures with 95% confidence intervals were calculated as described previously (Sørensen, 1948; Bray and Curtis, 1957; Hill et al., 2003; Zaprasis et al., 2010). Normalized weighted UniFrac significance was calculated to evaluate differences between the communities of narG, nirK, nirS and nosZ based on phylogenetic information (Lozupone and Knight, 2005; Lozupone et al., 2007).
Quantification of narG, nirK, nirS, nosZ and 16S rRNA genes in soil
Quantitative kinetic real-time PCRs were performed as described with DNA extracts from three replicate cryoturbated and three unturbated sites in six technical replicates per DNA extract (Zaprasis et al., 2010; Supplementary Materials and methods). Thermal protocols and primers were as described previously (Supplementary Materials and methods, Table 1). Melting-curve analyses, agarose gel electrophoresis and sequencing of amplicons generated with the same primers indicated that the amplification was specific. The lower limits of quantification were ⩽101 gene copy numbers μ l−1 of DNA extract. 16S rRNA gene copy numbers were determined concomitantly for all environmental samples to quantify microorganisms that harbor genes associated with denitrification in soils relative to the total bacterial population (Muyzer et al., 1993; Zaprasis et al., 2010). Inhibition of quantitative kinetic real-time PCR was assessed per individual DNA extract according to Zaprasis et al. (2010) by spiking soil DNA with pure standard DNA. Please refer to Supplementary Materials and methods for further details.
Table 1. Thermal protocols for qPCR of narG, nirK, nirS, nosZ and 16S rRNA genes.
| Primer set |
Temperature (°C)/time (min) |
||||
|---|---|---|---|---|---|
| narG1960f/ | F1aCu/ | cd3aF/ | nosZF/ | Eub341F/ | |
| narG2650ra | R3Cub | R3cdb | nosZRc | Eub534Rd | |
| Initial denaturation | 95/10 | 95/10 | 95/10 | 95/10 | 95/10 |
| Denaturation | 95/0.75 | 95/1 | 95/0.5 | 95/0.5 | 95/0.5 |
| Annealing | 64/0.75 | 55/1 | 58.5/0.5 | 63/0.5 | 55.7/0.4 |
| Elongation | 72/1.3 | 72/1.7 | 72/0.5 | 72/0.75 | 72/0.4 |
| Recording of fluorescence | 80/0.3 | 83.5/0.3 | 80/0.3 | (72)e | (72)e |
| No. of cycles | 40 | 50 | 40 | 35 | 35 |
| Final elongation | 72/5 | 72/5 | 72/5 | 72/5 | 72/5 |
Abbreviation: qPCR, quantitative kinetic real-time PCR.
Fluorescence recorded during elongation step.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA). Mean differences between cryoturbated and unturbated peat soils and differences in slopes of linear regression curves were tested using a two-tailed t-test. The correlation between N2O to total N gases was analyzed with Spearman's rank correlation. Non-linear regressions for apparent Michaelis–Menten kinetics and the resulting vmax were compared with a sum-of-squares F test.
Nucleotide sequence accession numbers
The OTU representatives of narG, nirK, nirS and nosZ gene sequences derived from barcoded amplicon pyrosequencing were deposited in EMBL under accession numbers FR865777 to FR865864. Complete amplicon sequence libraries were deposited in the ENA Short Read Archive under submission number ERA062401.
Results
Denitrification activities in cryoturbated and unturbated peat soils
Unsupplemented cryoturbated peat soil but not unturbated peat soil produced N2O under anoxic conditions without apparent delay (Figure 1a). pH approximated 4 in microcosms with cryoturbated and unturbated peat soils. After 49.5 h of incubation, N2O concentrations were significantly higher (P<0.04) in anoxic microcosms with cryoturbated peat soil when N2O-reductase was blocked by acetylene than in those without acetylene (Figure 1a). Approximately 1.8 μmol N2O gDW−1 accumulated and remained constant after 90 h in acetylene-treated microcosms with cryoturbated peat soil, indicating that soil endogeneous nitrate and nitrite were depleted (Figure 1a). Cryoturbated peat soil contained 4.8 μmol NO3− gDW−1 before anoxic incubation. Nitrate was below the detection limit (that is, <1.5 μmol NO3− gDW−1) after 90 h of incubation, and 75% of the initially present NO3−-N was recovered in N2O. In cryoturbated peat soil microcosms without acetylene, up to 1.2 μmol N2O gDW−1 accumulated within the first 90 h (Figure 1a); N2O decreased linearly to 0.54 μmol gDW−1 within the next 70 h. Nitrate was below the detection limit in unturbated peat soil. N2O did not accumulate in anoxic unturbated peat soil microcosms without acetylene, and only minor amounts of N2O accumulated in the presence of acetylene (<0.014 μmol gDW−1 within 160 h; Figure 1a).
Effect of supplemental nitrate and nitrite on denitrification
Supplemental nitrate (10 μ) significantly stimulated the production of N2O in anoxic microcosms with unturbated (4.5 × 10−4 and 2.1 × 10−2 μmol N2O gDW−1 h−1 for unsupplemented and nitrate supplemented unturbated peat soils; P=0.001) but essentially not with cryoturbated peat soil, indicating that denitrifiers in cryoturbated peat soil were apparently saturated with soil endogenous nitrate (data not shown). However, in anoxic microcosms with nitrate-depleted peat soils, 100 μ of supplemental nitrate significantly stimulated the production of N2O by cryoturbated peat soil without apparent delay (P=0.04 when N2O production rates were compared; Figure 1b), whereas stimulation of N2O production by 100 μ of nitrate was unexpectedly not significant in microcosms with unturbated peat soil (P=0.273). The latter finding provides first evidence for substrate inhibition of unturbated peat soil denitrifiers by high nitrate concentrations (see below and Figure 2). Nitrite significantly stimulated the production of N2O without apparent delay in microcosms with cryoturbated (P=0.02) and unturbated (P=0.03) peat soils (Figure 1b). Stimulation of N2O production was higher with nitrite than with nitrate in cryoturbated (P=0.04) and unturbated peat soils (P=0.06) when N2O production rates were compared. N2O concentrations were similar after 1–2 h of incubation in anoxic cryoturbated and unturbated peat soil microcosms containing the same supplement, demonstrating similar denitrification potentials in both soils when 100 μ nitrite as electron acceptor for denitrification was supplied (Figure 1b).
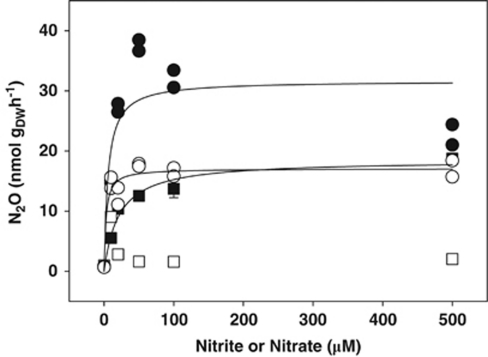
Figure 2.
Apparent Michaelis–Menten kinetics of nitrate- and nitrite-dependent denitrification in anoxic microcosms with peat soil in the presence of acetylene. Squares and circles represent unturbated and cryoturbated peat soils, respectively. Closed and open symbols represent microcosms supplemented with nitrite and nitrate, respectively. Mean values and s.e. of three replicate microcosms are shown for unturbated peat soil; individual values of duplicate microcosms are shown for cryoturbated peat soil. Solid lines indicate Michaelis–Menten curves fitted to the data.
Initial nitrite-dependent N2O production rates of microcosms with cryoturbated and unturbated peat soils and nitrate-dependent N2O production rates of microcosms with cryoturbated peat soil followed apparent Michaelis–Menten kinetics, whereas nitrate-dependent N2O production rates of microcosms with unturbated peat soil did not (Figure 2). N2O production rates were up to 4 times greater in microcosms with unturbated peat soil containing 10 μ supplemental nitrate than in those containing 20–500 μ supplemental nitrate (pairwise t-test of N2O production rates for 10 μ and rates for 0, 20, 50, 100 and 500 μ nitrate yielded P-values of <0.001, 0.001, 0.001, <0.001 and <0.001, respectively), suggesting that unturbated peat soil denitrifiers are saturated with 10 μ nitrate and subjected to substrate inhibition by higher nitrate concentrations (Figure 2). In contrast, N2O production plateaued out in microcosms with cryoturbated peat soil when supplemental nitrate concentrations were ⩾50 μ (Figure 2). Apparent maximal reaction velocities (vmax) were higher for nitrite than for nitrate in cryoturbated peat soil microcosms (P<0.001), and higher in cryoturbated than in unturbated peat soil microcosms (P=0.001; Table 2). Apparent Michaelis–Menten constants (KM) for nitrite were lower in cryoturbated than in unturbated peat soil microcosms (P=0.05; Table 2).
Table 2. Kinetic parameters of apparent Michaelis–Menten kinetics of nitrate- and nitrite-dependent denitrification in anoxic microcosms with peat soil in the presence of acetylene.
| Soil |
Nitrate amended |
Nitrite amended |
||
|---|---|---|---|---|
| vmaxa (nmol gDW−1 h−1) | KMa (μ) | vmaxa (nmol gDW−1 h−1) | KMa (μ) | |
| Unturbated | NA | NA | 18±1 | 21±5 |
| Cryoturbated | 17±1 | 3±1 | 32±3 | 6±4 |
Abbreviation: NA, not applicable.
Kinetic parameters (calculated from Figure 2)±s.e.
The ratio of N2O to total N gases (that is, N2 plus N2O) after 8 h of anoxic incubation was below 30% for 10 μ nitrate or nitrite in microcosms with cryoturbated peat soil and increased with increasing concentrations of nitrate and nitrite (Spearman's correlation coefficients of 1.0 and 0.9, respectively; Supplementary Figure S2). In unturbated peat soil microcosms, the ratio of N2O to total N gases approximated 100% for all supplied concentrations of nitrate or nitrite. Such data suggest a higher N2O consumption potential of cryoturbated relative to unturbated peat soil denitrifiers for low concentrations of electron acceptors.
Anaerobic fermentation activities and trophic links to denitrifiers
Organic acids were not detectable in cryoturbated peat soil after anoxic pre-incubation to deplete nitrate and nitrite (0 h, Supplementary Figure S3a). In unsupplemented, nitrate-depleted anoxic microcosms with cryoturbated peat soil, only trace amounts of formate were transiently produced, and up to 0.1 m of lactate accumulated within 58 days of incubation (that is, 9 days of preincubation plus 49 days of treatment; Supplementary Figure S3a). In contrast, ∼0.7 m of acetate and trace amounts of formate, propionate, butyrate and lactate were produced in unsupplemented anoxic microcosms with unturbated peat soil during the 9 days of pre-incubation (0 h, Supplementary Figure S3a). Up to 1.7 m of acetate, and 0.1 to 0.2 m of propionate, butyrate and lactate accumulated within 58 days of incubation (that is, 9 days of preincubation plus 49 days of treatment; Supplementary Figure S3a). Formate was below the detection limit after 58 days of incubation, indicating formate consumption. Such data suggest that fermentation potentials are lower in cryoturbated than in unturbated peat soils.
Six low-molecular-weight organic electron donors were tested for their potential to stimulate denitrification in anoxic peat soil microcosms supplied with nitrite and acetylene to identify putative substrates of acid-tolerant permafrost denitrifiers (Table 3; Supplementary Results). Nitrite was consumed in all treatments (data not shown). Supplemental acetate tended to stimulate N2O production after the first pulse, and when resupplied after 47 days of anoxic incubation (second pulse) in microcosms with cryoturbated peat soil (Table 3). Supplemental acetate, formate and propionate tended to stimulate initial N2O production after the first pulse (that is, when electron donors and nitrite were supplemented to the microcosms for the first time; Table 3). Such data suggest that acid-tolerant denitrifiers in cryoturbated and unturbated peat soils are capable of acetate consumption.
Table 3. Effect of supplemented electron donors on N2O production in nitrite-amended anoxic peat soil microcosms.
| Treatment |
First pulsea |
Second pulseb |
||
|---|---|---|---|---|
| Unturbated | Cryoturbated | Unturbated | Cryoturbated | |
| Controlc | 100 (98–102)d,e | 100f (79–121) | 100 (96–104)g | 100 (77–123)h |
| Ethanol | 103 (84–122) | 70 (57–82) | 93 (89–97) | 123 (115–132) |
| Acetate | 118 (111–124) | 111 (103–120) | 100 (88–113) | 151 (143–159) |
| Formate | 121 (114–128) | 92 (83–100) | 85 (82–87) | 72 (72) |
| Propionate | 118 (108–127) | 86 (76–97) | 78 (75–81) | 95 (75–115) |
| Butyrate | 89 (81–97) | 82 (71–93) | 70 (60–79) | 51 (47–55) |
| Lactate | 70 (68–74) | 81 (78–84) | 82 (80–84) | 145 (127–163) |
Percentage of N2O production in each treatment as compared with microcosms supplemented with nitrite only (that is, control).
Nitrite and electron donors added after 9 days of pre-incubation.
Nitrite and electron donors resupplied 47 days after the first pulse.
Microcosms supplemented with nitrite only.
N2O production = 23.1 nmol gDW−1 h−1.
Ratios of the duplicates and the mean of the control microcosms (%).
N2O production=54.9 nmol gDW−1 h−1.
N2O production=16.7 nmol gDW−1 h−1.
N2O production=22.6 nmol gDW−1 h−1.
Phylogenetic analysis of denitrifiers by structural gene targeted amplicon pyrosequencing
In total, 48 917 quality-filtered sequences of the structural gene markers narG, nirK, nirS and nosZ were analyzed. On average, 6115±1447 sequences per gene marker and soil with an average sequence length of 444±19 bp were obtained (Supplementary Figure S4). As forward and reverse reads of nirK and nirS amplicons overlapped almost completely (∼470 and 410 bp average read length, respectively), forward and reverse reads of nirK, and forward and reverse reads of nirS were analyzed together. Overlaps of forward and reverse reads were not sufficient for narG and nosZ amplicons (∼670 and 700 bp, respectively). Thus, forward and reverse reads of both narG and nosZ were analyzed separately (Table 4). Only few non-target sequences occurred and were excluded from further analyses; 95±7% of sequences generated from amplicons obtained with a certain structural gene-specific (that is, narG, nirK, nirS or nosZ) primer set were related to publicly available target genes of that primer set. Such amplification specificity is above or in the range of values obtained for other structural gene marker analyses (for example, average of 87±11% for narG, dsrAB, [FeFe]-hydrogenase and dioxygenase genes; Philippot et al., 2002; Loy et al., 2004; Iwai et al., 2010; Schmidt et al., 2010).
Table 4. Analyses of in silico-translated amino-acid sequences of narG, nirK, nirS and nosZ derived from peat soil.
| Gene marker |
Unturbated |
Cryoturbated |
β-Diversity |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of sequences | Library coverage (%)a | No. of OTUs observed | No. of OTUs estimatedb | Hc | Ed | No. of sequences | Library coverage (%)a | No. of OTUs observed | No. of OTUs estimatedb | Hc | Ed | SSe | BCSf | |
| narG forward | 1 825 | 99.8 | 16 | 17 (16–27) | 1.28 (1.23–1.34) | 0.46 (0.44–0.48) | 2526 | 99.8 | 14 | 25 (17–78) | 1.36 (1.33–1.40) | 0.50 (0.49–0.52) | 0.84 | 0.67 |
| narG reverse | 2 047 | 99.9 | 9 | 10 (9–23) | 0.89 (0.85–0.94) | 0.41 (0.39–0.43) | 3806 | 99.9 | 8 | 9 (8–22) | 0.81 (0.77–0.84) | 0.39 (0.37–0.40) | 0.93 | 0.70 |
| nirK | 12 187 | 100 | 19 | 20 (19–24) | 1.17 (1.15–1.19) | 0.40 (0.39–0.40) | 10 219 | 100 | 10 | 13 (10–33) | 0.13 (0.12–0.15) | 0.06 (0.05–0.06) | 0.48 | 0.03 |
| nirS | 2 942 | 99.9 | 14 | 16 (14–30) | 1.78 (1.75–1.80) | 0.67 (0.66–0.68) | 285 | 98.6 | 6 | 9 (6–30) | 0.69 (0.59–0.79) | 0.39 (0.33–0.44) | 0.30 | 0.12 |
| nosZ forward | 2 097 | 100 | 7 | 7 (7) | 0.23 (0.19–0.27) | 0.12 (0.10–0.14) | 3709 | 100 | 9 | 9 (9) | 0.77 (0.73–0.80) | 0.35 (0.33–0.36) | 0.59 | 0.72 |
| nosZ reverse | 1 919 | 99.9 | 6 | 6 (6) | 0.23 (0.19–0.27) | 0.13 (0.11–0.15) | 3664 | 99.9 | 11 | 14 (12–22) | 0.75 (0.72–0.79) | 0.31 (0.30–0.33) | 0.67 | 0.68 |
Abbreviation: OTU, operational taxonomic unit.
Percentage library coverage C=(1−ns nt−1) × 100 (ns=OTUs that occur only once, nt=total number of sequences).
Chao1 richness estimate with upper and lower 95% confidence intervals given in parentheses.
Shannon–Weaver diversity index with upper and lower 95% confidence intervals given in parentheses.
Species evenness with upper and lower 95% confidence intervals given in parentheses.
Sørensen similarity index.
Bray–Curtis similarity index.
Sequences were assigned to OTUs on the basis of threshold distances. The number of OTUs decreased rapidly with increasing threshold distance from 0 to 10%, which might be attributed to inaccuracies during PCR and pyrosequencing (Supplementary Figure S1; Behnke et al., 2011; Quince et al., 2011). However, the number of OTUs stabilized from 10 to 30% of threshold distance, indicating that the threshold distances of 17–33% used for diversity analyses in this study rendered the analysis insensitive to such potential inaccuracies (Supplementary Figure S1; see the ‘Materials and methods' section for details). Coverages were always >98%, the number of taxa as estimated by Chao1 was essentially identical to those observed in the amplicon libraries (Table 4), and rarefaction curves essentially plateaued out for most genes analyzed (Supplementary Figure S5), indicating that the number of obtained sequences sufficed for the structural gene-based diversity analysis of denitrifiers.
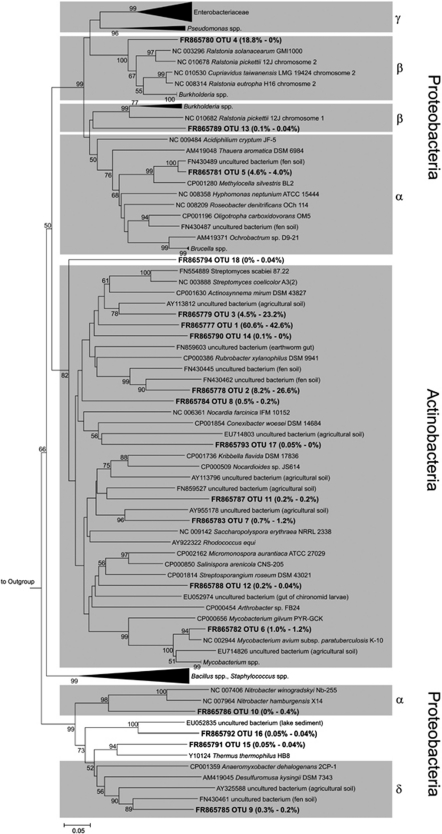
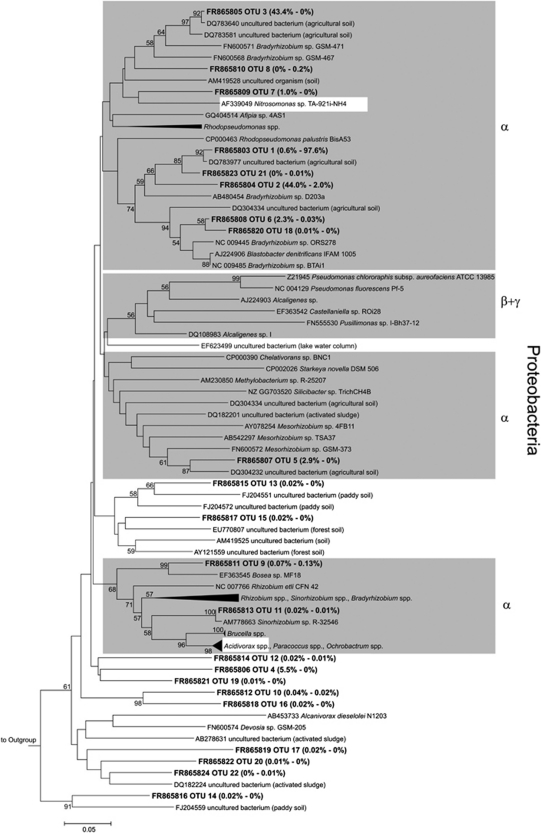
Forward reads of narG amplicons yielded more OTUs than did reverse reads, although a similar number of sequences was obtained, indicating that the utility of forward reads of narG amplicons is higher for diversity analyses than reverse reads (Table 4, Supplementary Figure S5). Results obtained from reverse reads show similar overall trends to those from forward reads (Figure 3, Supplementary Figures S5 and 6). Thus, the information presented below refers to forward reads only. In total, narG sequences were assigned to 18 species-level OTUs (Figure 3). OTU 1 dominated narG in both soils. In all, 16 of the 18 OTUs including OTU 1 were only distantly related to narG of cultured organisms or environmental sequences (that is, sequence dissimilarities of OTU representatives were 20–35%), indicating phylogenetic new narG in cryoturbated and unturbated peat soils (Figure 3). Overall, 95% and 76% of narG from cryoturbated and unturbated peat soils, respectively, affiliated with Actinobacterial narG. OTUs 2 and 3 were more abundant in cryoturbated than in unturbated peat soil amplicon libraries (Figure 4a). OTU 4 was exclusively detected in unturbated peat soil amplicon libraries and accounted for 19% of narG (Figure 3). Confidence intervals of Shannon–Weaver diversity indices and species evenness values of narG from cryoturbated and unturbated peat soils overlapped. Sørensen and Bray–Curtis indices for the β-diversity of narG were high (that is, 0.84 and 0.67, respectively; Table 4) indicating a high proportion of shared OTUs among both soils. UniFrac analysis of narG phylogenetic trees likewise indicated that the narG communities were similar (P≈1).
Figure 3.
Phylogenetic tree of representative narG sequences (forward reads) retrieved from unturbated and cryoturbated peat soils. The tree is based on in silico-translated amino-acid sequences. One representative sequence per OTU is shown. Codes preceeding sequence names represent sequence accession numbers in public databases. Values in parentheses represent relative abundances of sequences from unturbated (left) and cryoturbated (right) peat soils. In total, 1825 and 2526 sequences from forward reads were obtained from unturbated and cryoturbated peat soils, respectively. Gray boxes indicate branches where the majority of sequences are derived from reference strains of a certain phylogenetic class. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (10 000 replicates) are shown next to the branches. Bootstrap values below 50% are not shown. The outgroup was narG of Haloarcula marismortui ATCC 43049 (NC 006396).
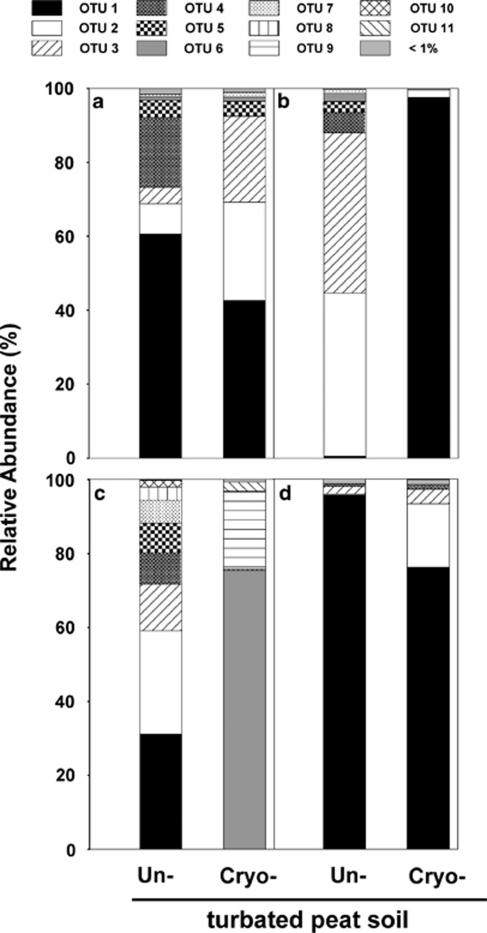
Figure 4.
Relative abundances of (a) narG- (forward reads), (b) nirK-, (c) nirS- and (d) nosZ- (forward reads) derived OTUs retrieved from unturbated and cryoturbated peat soils. Sequences were assigned to OTUs using sequence similarity thresholds of 67% (narG), 83% (nirK), 82% (nirS) and 80% (nosZ). All OTUs that had relative abundances below 1% in both soils were grouped.
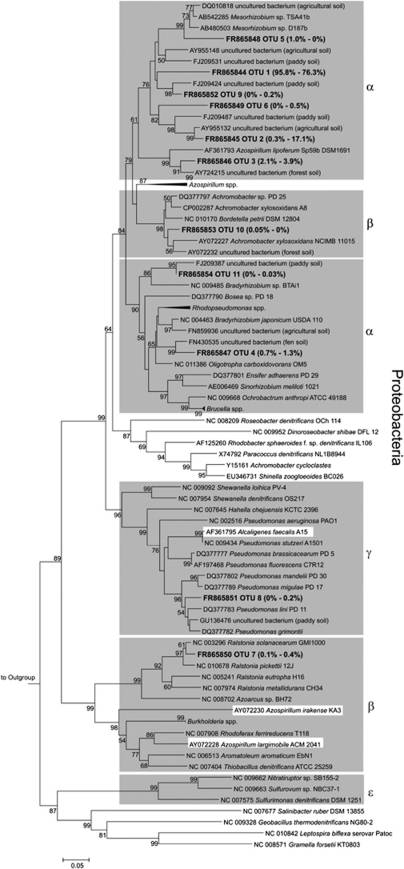
In total, nirK was assigned to 22 species-level OTUs (Figure 5). Less OTUs were detected in cryoturbated than in unturbated peat soil (Table 4). nirK of cryoturbated peat soil was dominated by OTU 1 with a relative abundance of 97% (Figures 4 and 5). OTUs 2 and 3 both had a relative abundance of ∼44% and dominated nirK in unturbated soil amplicon libraries (Figures 4 and 5). Overall, 99% and 94% of nirK from cryoturbated and unturbated peat soils, respectively, affiliated with Alphaproteobacterial nirK (Figures 4 and 5). Major OTUs of both soils were related to environmental nirK from upland soil. In all, 10 of the 22 nirK OTUs were only distantly related (that is, sequence dissimilarities of OTU representatives were 15–23%) to nirK of cultured organisms or environmental sequences, indicating phylogenetic new nirK.
Figure 5.
Phylogenetic tree of representative nirK sequences retrieved from unturbated and cryoturbated peat soils. The tree is based on in silico-translated amino-acid sequences. One representative sequence per OTU is shown. Values in parentheses represent relative abundances of sequences from unturbated (left) and cryoturbated (right) peat soils. Codes preceeding sequence names represent sequence accession numbers in public databases. In total, 12 187 and 10 219 sequences were obtained from unturbated and cryoturbated peat soils, respectively. Gray boxes indicate branches where the majority of sequences are derived from reference strains of a certain phylogenetic class; white boxes indicate minority sequences from genera not affiliated with the indicated class. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (10 000 replicates) are shown next to the branches. Bootstrap values below 50% are not shown. The outgroup was nirK of Nitrosomonas sp. C-56 (AF339044).
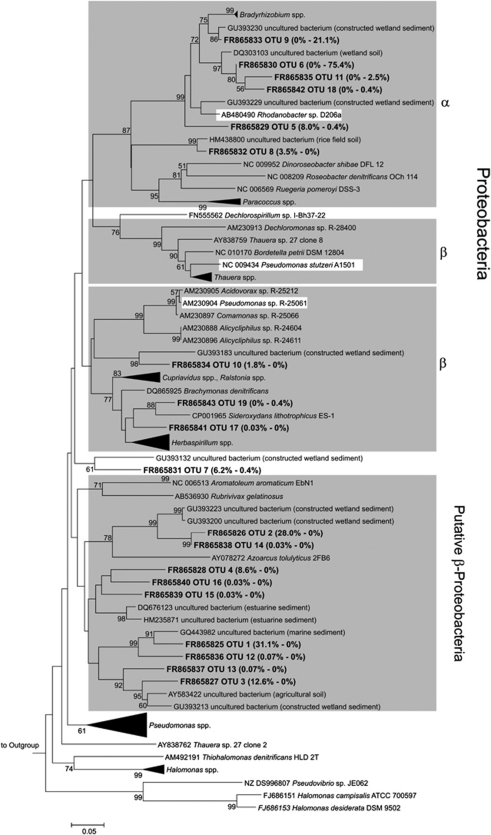
In total, 19 species-level OTUs of nirS occurred (Figure 6). Less OTUs occurred in cryoturbated than in unturbated peat soil (Figure 6, Table 4). nirS of cryoturbated peat was dominated by OTU 6, whereas OTUs 1 and 2 dominated nirS in unturbated soil (Figures 4 and 6). Overall, 99% and 12% of nirS from cryoturbated and unturbated peat soils, respectively, affiliated with Alphaproteobacterial nirS. Overall, 1% and 82% of nirS from cryoturbated and unturbated peat soils, respectively, affiliated with putative Betaproteobacterial nirS. Many OTUs from both soils were related to environmental nirS from wetlands or marine sediments, and distantly related to pure cultures (Figure 6). In all, 8 of the 19 nirS OTUs were only distantly related (that is, sequence dissimilarities of OTU representatives were 15–25%) to nirS of cultured organisms or environmental sequences, indicating phylogenetically new nirS.
Figure 6.
Phylogenetic tree of representative nirS sequences retrieved from unturbated and cryoturbated peat soils. The tree is based on in silico-translated amino-acid sequences. One representative sequence per OTU is shown. Values in parentheses represent relative abundances of sequences from unturbated (left) and cryoturbated (right) peat soils. Codes preceeding sequence names represent sequence accession numbers in public databases. In total, 2942 and 285 sequences were obtained from unturbated and cryoturbated peat soils, respectively. Gray boxes indicate branches where the majority of sequences are derived from reference strains of a certain phylogenetic class; white boxes indicate minority sequences from genera not affiliated with the indicated class. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (10 000 replicates) are shown next to the branches. Bootstrap values below 50% are not shown. The outgroup was nirS of Rhodothermus marinus DSM 4252 (CP001807).
Diversity measures of nirK and nirS were consistently lower in cryoturbated than in unturbated peat soil (Table 4, Supplementary Figure S5). The 95% confidence intervals of Shannon–Weaver indices and evenness values did not overlap indicating that the detected diversity of nirK- and nirS-type denitrifier communities was lower in cryoturbated than in unturbated peat soil (Table 4). A similar trend was observed in the rarefaction curves generated from nirK and nirS obtained from both soils, even though 95% confidence intervals overlapped in the case of nirK (Supplementary Figure S5). The Sørensen and Bray–Curtis indices for β-diversity of nirK and nirS were low (0.48 and 0.03 for nirK and 0.30 and 0.12 for nirS, respectively), suggesting differences in the community composition in cryoturbated and unturbated peat soils. UniFrac analysis confirmed significant differences in nirK and nirS community compositions of cryoturbated and unturbated peat soils (P<0.002 and P<0.002, respectively).
In total, nosZ forward reads were assigned to 11 species-level OTUs (Figure 7). OTU 1 dominated nosZ of cryoturbated and unturbated peat soils (Figures 4 and 7). Most of the nosZ from both soils were affiliated with Alphaproteobacterial nosZ. In all, 7 of the 11 nosZ OTUs from cryoturbated and unturbated peat soils were only distantly related (that is, sequence dissimilarities of OTU representatives were 16–22%) to nosZ of cultured organisms, and 2 were likewise distantly related to nosZ from environmental sequences, indicating hitherto uncultured acid-tolerant denitrifiers capable of N2O reduction in both soils. nosZ sequences clustered with nosZ of wetland and upland soils (Figure 7). nosZ reverse reads yielded similar results (Table 4, Supplementary Figure S7). Diversity measures of nosZ were higher in cryoturbated than in unturbated peat soil (Table 4, Supplementary Figure S5). Confidence intervals of Shannon–Weaver diversity indices and species evenness values did not overlap. Similar trends were indicated by the rarefaction curves of nosZ forward and reverse sequences, even though 95% confidence intervals overlapped (Supplementary Figure S5). β-Diversity as indicated by Sørensen and Bray–Curtis diversity indices of nosZ tended to be lower than those of narG but higher than for nirK and nirS (Table 4). However, UniFrac analysis of nosZ phylogenetic trees did not reveal significant differences in nosZ communities (P≈1). Such analyses might suggest a marginally higher detected diversity of putative denitrifiers capable of N2O reduction in cryoturbated than in unturbated peat soil.
Figure 7.
Phylogenetic tree of representative nosZ sequences retrieved from unturbated and cryoturbated peat soils. The tree is based on in silico-translated amino-acid sequences. One representative sequence per OTU is shown. Values in parentheses represent relative abundances of sequences from unturbated (left) and cryoturbated (right) peat soils. Codes preceeding sequence names represent sequence accession numbers in public databases. In total, 2097 and 3709 sequences from forward reads were obtained from unturbated and cryoturbated peat soils, respectively. Gray boxes indicate branches where the majority of sequences are derived from reference strains of a certain phylogenetic class; white boxes indicate minority sequences from genera not affiliated with the indicated class. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (10 000 replicates) are shown next to the branches. Bootstrap values below 50% are not shown. The outgroup was nosZ of Haloarcula marismortui ATCC 43049 (NC 006396).
Quantification of narG, nirK, nirS and nosZ relative to 16S rRNA genes
Copy numbers of all genes determined in this study were corrected by inhibition factors that were experimentally determined for every DNA extract and gene analyzed to overcome the effect of PCR-interfering substances that contaminate most environmental DNA (see the ‘Materials and methods' section). Copy numbers of narG approximated 7 × 104 per ng DNA in cryoturbated peat soil, and accounted for 8% of 16S rRNA gene copy numbers, indicating that a substantial portion of bacteria in cryoturbated peat soil was capable of dissimilatory nitrate reduction (Table 5). narG copy numbers in unturbated peat soil were significantly lower (∼100 times, P=0.02) than those in cryoturbated peat soil (Table 5). The data are in agreement with the high and low capacities of cryoturbated and unturbated peat soils, respectively, to sustain nitrate-dependent denitirification (Figure 2).
Table 5. Abundance of denitrification-associated genes in peat soil.
| Gene marker |
Unturbated |
Cryoturbated |
||
|---|---|---|---|---|
| Copy no. per | Copy no. per | Copy no. per | Copy no. per | |
| 16S rRNA gene (%)a,b | ng DNAa | 16S rRNA gene (%)a,c | ng DNAa | |
| narG | (3.8±1.3) × 10−2 | (6.5±2.5) × 102 | (7.6±2.8) × 100 | (6.5±2.0) × 104 |
| nirK | (7.7±1.6) × 10−3 | (3.5±1.1) × 101 | (5.2±1.6) × 10−4 | (5.1±2.1) × 100 |
| nirS | (8.8±1.3) × 10−1 | (7.2±0.9) × 103 | (3.4±0.8) × 10−1 | (4.6±1.0) × 103 |
| nosZ | (1.0±0.6) × 10−4 | (2.7±1.2) × 100 | (1.7±0.4) × 10−3 | (1.2±0.2) × 101 |
Mean of 3 (sites) × 6 (technical) replicates±s.e. (see the ‘Materials and methods' section).
16S rRNA gene copy numbers were (1.9±0.2) × 106 per ng DNA.
16S rRNA gene copy numbers were (8.0±1.7) × 105 per ng DNA.
Copy numbers of nirK were 5 and 35 per ng DNA in cryoturbated and unturbated peat soils, suggesting a minor role of nirK-type denitrifiers and marginally significant differences in the abundance of nirK-type denitrifiers (P=0.066; Table 5). The same tendency was reflected in nirK/16S rRNA gene copy number ratios. Copy numbers of nirS were in the same range (that is, 5–7 × 103 per ng DNA, P=0.650) for both soils and accounted for up to 1% of 16S rRNA gene copy numbers (Table 5). nirS nirK−1 copy number ratios approximated 1000 and 100 for cryoturbated and unturbated peat, respectively, and differed significantly (P=0.02). nirS narG−1 copy number ratios approximated 0.05 and 15 for cryoturbated and unturbated peat, respectively, and differed significantly (P=0.05).
Copy numbers of nosZ approximated 101 per ng DNA in cryoturbated peat soil, and accounted for 0.002% of 16S rRNA and 0.6% of nirS gene copy numbers (Table 5). Detected nosZ copy numbers per ng DNA detected in cryoturbated peat soil were five times higher than in unturbated peat soil, although such differences were not significant (P=0.247; Table 5). In unturbated peat soil, nosZ 16S rRNA−1 and nosZ nirS−1 gene copy number ratios were 0.0001% and 0.02%, respectively. nosZ narG−1 copy number ratios approximated 0.0002 and 0.003 for cryoturbated and unturbated peat, respectively. Such diferrences were only marginally significant (P=0.08).
Discussion
Denitrification as major source of N2O in cryoturbated peat
Cryoturbated acidic peat circles are ‘hotspots' of N2O emission in the arctic permafrost region, which was previously regarded as an insignificant source of N2O (Denman et al., 2007; Repo et al., 2009; Marushchak et al., 2011). Denitrification, dissimilatory nitrate reduction to ammonium, nitrification and chemodenitrification are potential sources of N2O in soils (Smith, 1983; Conrad, 1996; Bremner, 1997; van Cleemput, 1998; Kresovic et al., 2009). Although chemodenitrification of nitrite might occur under anoxic conditions at low pH, major products are NO and NO2 rather than N2O, and biotic denitrification is much quicker (van Cleemput, 1998; Kappelmeyer et al., 2003; Kresovic et al., 2009). Nitrification is suggested to be the main source of N2O in well-aerated soils with a water-filled pore space of <60% (Conrad, 1996; Pihlatie et al., 2004). However, a water-filled pore space of 70–80% in cryoturbated peat circles, nitrate concentrations in the m range, correlation of high water contents with high N2O emission and a C-to-N-ratio of ∼25 suggest denitrification rather than nitrification as the primary source of N2O (Pihlatie et al., 2004; Repo et al., 2009; Marushchak et al., 2011). Indeed, anoxic microcosms at in situ pH with cryoturbated peat soil showed an immediate production of N2O from endogenous nitrate, and 75% of the initial nitrate-N was recovered in N2O, indicating denitrification rather than dissimilatory nitrate reduction of non-denitrifiers (DNR) (Figure 1a). Organisms catalyzing DNR produce N2O by an unspecific reaction of nitrate reductase with accumulated nitrite (Smith 1983; Tiedje, 1988). Nitrite is virtually absent in cryoturbated peat (Repo et al., 2009), indicating that DNR is negligible as a direct source of N2O. Nitrate, nitrite and acetylene stimulated net N2O production under anoxic conditions (Figures 1b and 2). Extrapolation of N2O production from soil endogenous nitrate in microcosms to the field level largely exceed N2O emissions measured in situ. Thus, current findings demonstrate that cryoturbated peat soil denitrifiers (1) are prone to react to anoxia, (2) are active under acidic conditions and (3) have the potential to account for the in situ N2O emissions of cryoturbated peat circles (Repo et al., 2009; Marushchak et al., 2011).
Contrasting denitrifiers in cryoturbated and unturbated peat soils
Denitrification potentials, affinities for electron acceptors as indicated by KM, vmax, nitrate tolerance and the potential to consume N2O were higher in cryoturbated than in unturbated peat soil (Figures 1 and 2, Supplementary Figure S2, Table 2). Such data provided ecophysiological evidence that denitrifier communitities of cryoturbated and unturbated peat soils were dissimilar.
Pyrosequencing and quantitative kinetic real-time PCR of denitrification-associated genes substantiated the previous conclusion (Figure 4, Tables 4 and 5). narG copy numbers of cryoturbated peat soil were higher than or in the same range as in agricultural soils or glacier forelands (Deiglmayr et al., 2006; Kandeler et al., 2006; Bru et al., 2007), and significantly higher than in unturbated peat soil (Table 5). Such findings are in agreement with the high in situ concentrations of nitrate in cryoturbated peat soil (Repo et al., 2009), and the inability of unturbated peat soil to cope with high nitrate concentrations (Figure 2).
nirS and nirK diversity, nirS nirK−1 copy number ratios and dominant OTUs in cryoturbated differed from those in unturbated peat soil (Tables 4 and 5, Figures 4, 5 and 6). nirS diversity is higher than nirK diversity in some aquifers, marsh and costal sediments, suggesting that (semi-)aquatic systems sustain diverse nirS-type denitrifiers (Braker et al., 2000; Prieme et al., 2002; Santoro et al., 2006). Such findings are in agreement with the high detected nirS diversity in acidic peat soils (Table 4). Copy numbers of nirS outnumbered nirK by 2 to 3 orders of magnitude in both acidic peat soils (Table 5), indicating that nirS- rather than nirK-type denitrifiers were associated with denitrification in acidic peat soils. Indeed, nirS abundance in spruce forest soil was positively correlated with decreasing pH from 6.1 to 3.7, whereas nirK abundance was negatively correlated (Barta et al., 2010). Such data suggest that low pH and high moisture contents might favor nirS-type rather than nirK-type denitrifiers in acidic peat soils and highlight differences in detected nitrite reductase gene containing denitrifier communities of cryoturbated and unturbated peat soils.
In both soils, the proportion of detected denitrifiers that possess a N2O reductase was rather low, as suggested by the low nosZ nirS−1 ratios (Table 5). The relative abundance of N2O reductases in the bacterial community is reflected in the ratio of N2O to total N gases (Philippot et al., 2009), and an increased percentage of denitrifiers lacking N2O reductase can increase the relative amount of emitted N2O (Philippot et al., 2011). Indeed, N2O to total N-gas ratios approximated 100% for both soils, when 500 μ of nitrate or nitrite was supplied (Supplementary Figure S2). Diversity measures of detected nosZ consistently suggested that denitrifiers capable of N2O reduction (that is, harboring the nosZ gene) were more diverse in cryoturbated than in unturbated peat soil (Table 4 and 5, Supplementary Figure S5). Condsidering the contrasting response of cryoturbated and unturbated peat soil denitrifiers to various concentrations of nitrate and nitrite in terms of their N2O to total N gas production (Supplementary Figure S2), and the consistent (although sometimes marginal) differences in diversity measures of nosZ, the data indicate that denitrifers capable of N2O reduction likewise differed between both soils.
Regulation of net N2O production by peat denitrifiers
Stimulation of denitrifiers in cryoturbated and unturbated peat soils by nitrite was more pronounced than by nitrate (Figures 1 and 2), suggesting that denitrifiers lacking nitrate reductases might contribute to N2O production and/or nitrate reduction is rate limiting (Vangnai and Klein, 1974; Mahne and Tiedje, 1995; Zumft, 1997).
Denitrifiers in cryoturbated peat thrive at a low pH of 4 (Figures 1 and 2, Supplementary Figure S2). Denitrification occurs at acidic soil pH in other systems as well, although denitrification capacities of neutral soils are often higher (Parkin et al., 1985). However, denitrification capacities of cryoturbated peat soil were much higher than those of many more neutral habitats, indicating an acid-tolerant denitrifier community in cryoturbated peat that can cope remarkably well with low pH (Cuhel et al., 2010). Apparent KM values for both nitrate and nitrite at pH 4 were <10 μ for cryoturbated peat soil denitrifiers (Table 2), indicating a high affinity of the denitrifiers for both substrates. Apparent KM values for both nitrate and nitrite were in the same range or lower than in other more neutral soil types or pure cultures (Betlach and Tiedje, 1981; Strong and Fillery, 2002; Palmer et al., 2010), supporting the conclusion that peat denitrifiers cope well with low pH.
Unturbated peat has the same acidic pH as cryoturbated peat soil, but a dissimilar denitrifier community, and does not emit N2O in situ (Repo et al., 2009, Marushchak et al., 2011). Although soil pH has a significant impact on denitrifiers in temperate soils (Bru et al., 2011), data suggest that the low nitrate content of the vegetated unturbated peat soil and the dissimilar denitrifier communities rather than soil pH might account for the contrasting N2O emission patterns of cryoturbated and unturbated peat soils (Figure 1; Repo et al, 2009, Marushchak et al., 2011). The contrasting denitrifier communites of such soils reacted differently to nitrate and nitrite supplementations (Figures 1b and 2, and Supplementary Figure S2), lending further support to the hypothesis that denitrifier community composition impacts regulation and thus prediction of N2O fluxes (Holtan-Hartwig et al., 2000; Philippot et al., 2009, 2011; Ma et al., 2011).
Cryoturbated peat soil consumed N2O that was initially produced from internal-N sources (Figure 1a), indicating the capability of peat soil denitrifiers for complete denitrification to N2 under acidic conditions, which is in agreement with capabilities of a previously analyzed acidic fen denitrifier community and the genetic potential for complete denitrification detected in acidic Antarctic permafrost-affected wetland soils (Yergeau et al., 2007; Yergeau and Kowalchuk, 2008; Palmer et al., 2010). Ratios of N2O to total N gases were below 40% at low nitrate and nitrite concentrations and ∼100% at 500 μ (Supplementary Figure S2). Increasing concentrations of nitrate and nitrite were correlated with an increase in the ratio of N2O to total N gases, a phenomenon that has been observed in various soils (Blackmer and Bremner, 1978; Gaskell et al., 1981; Palmer et al., 2010). Low pH and low electron donor availability favor increased ratios of N2O to total N gases when nitrate is not limiting (Blackmer and Bremner, 1978; Schalk-Otte et al., 2000; Simek and Cooper, 2002; van den Heuvel et al., 2010). Indeed, denitrifiers of cryoturbated peat were saturated with less than half of the nitrate concentrations occurring in situ, suggesting that electron donor availabiltity might limit denitrification (Figure 2). In situ nitrate concentrations exceed 1 m and might be explained by constant replenishment of carbon and nitrogen due to mixing in the cryoturbated soil and by the absence of plants as competitors for nitrate (Bockheim, 2007; Repo et al., 2009; Kuhry et al., 2010). Thus, cryoturbation favors denitrifiers and N2O as the main end product of denitrification in cryoturbated peat soil.
New acid-tolerant peat denitrifers
Most of the narG OTUs retrieved from acidic peat soils contained hitherto unknown sequences, and the major ones clustered with Actinobacterial narG (for example, OTUs 1 and 3; Figures 3 and 4). Interestingly, detected agricultural soil narG communities are likewise dominated by Actinobacteria-related narG, indicating a wide distribution of Actinobacterial nitrate reducers (Philippot et al., 2002).
Many OTUs of nirK and nirS contained new sequences indicative of new and uncultured denitrifiers (Figures 5 and 6). Major OTUs affiliated with Alphaproteobacterial sequences and were substantially more abundant in cryoturbated peat nirS amplicon libraries than in those from unturbated peat (Figures 4 and 6). nirS-based phylogenies are more congruent with the 16S rRNA-based phylogenies of their hosts than nirK-based phylogenies; thus, the data suggest that uncultured acid-tolerant denitrifiers of the Alphaproteobacteria occur in cryoturbated peat soil (Heylen et al., 2006). Certain nirK harboring acid-tolerant Rhodanobacter strains of the Gammaproteobacteria that are known to be capable of complete denitrification to N2 at pH 4 were not detected (van den Heuvel et al., 2010). However, the primers used for the amplification of nirK from the peat soils do not target nirK of Rhodanobacter sp. (Green et al., 2010). Thus, it is still unclear whether Rhodanobacter-like denitrifiers occur in acidic peat soils. nosZ OTUs were also indicative of new and uncultured denitrifiers capable of N2O reduction (Figure 7). Thus, the collective analysis of denitrification gene-associated data suggests that the permafrost-affected, acidic tundra peat soil harbors diverse, new and acid-tolerant, uncultured denitrifiers.
Conclusions and limitations
Microbial communities including denitrifiers in permafrost-affected habitats are rather stable under repeated freeze-thaw cycles and rapidly resume activity upon the onset of soil thawing (Yergeau and Kowalchuk, 2008; Männistö et al., 2009; Sawicka et al., 2010). Increasing thaw-depth and frequencies of freeze-thaw cycles may increase the availability of organic carbon and nitrogen stored in permafrost-affected soils, finally fueling denitrification-associated N2O emissions (Mørkved et al., 2006; Sharma et al., 2006; Kuhry et al., 2010). This highlights the potential susceptibility of such systems to global change. Ecophysiological and molecular data collected in this study indicate pronounced differences and a high diversity of denitrifier communities in cryoturbated and unturbated peat soils. However, the molecular data are largely dependent on the choice of primers. Although the primer systems used in this study are well evaluated and widely applied for estimating denitrifier diversity, and four denitrification-associated genes were analyzed in parallel to maximize the detectability of denitrifiers, it is known that not all denitrifers are detectable by the primer systems used (Throbäck et al., 2004; Enwall et al., 2010; Green et al., 2010; Palmer et al., 2010). Considering the rather high threshold distances used in this study for calling OTUs, the diversity analyses of cryoturbated and unturbated peat soil denitrifiers might be regarded as a minimal estimate of the ‘real' denitrifier diversity.
Within these limitations, this study nonetheless provides evidence that (1) the exceptionally high N2O emissions from cryoturbated peat circles are associated with a specific diverse, and acid-tolerant denitrifier community, (2) contrasting denitrifier community compositions are associated with high and low N2O emission patterns in acidic permafrost-affected peat soil and (3) such soils represent a hitherto overlooked reservoir of new microbial diversity associated with N2O production. Such new and uncultured diversity might coincide with new ecophysiological traits, necessitating future in-depth studies addressing denitrifiers in permafrost-affected peat soils with respect to global warming.
Acknowledgments
Support for this work was provided by the Suomen Akatemia, the Deutsche Forschungsgemeinschaft (DFG HO 4020/2-2) and the University of Bayreuth. We are grateful to Christian Hofmann for help with gas measurements, Chistine Stöcker for analysis of nitrate and nitrite, Rolf Daniel and Andrea Thürmer for pyrosequencing, Steffen Kolb, Markus Nebel and Sebastian Wild for support with JAguc2, as well as Mirjam Selzer and Peter Dörsch for helpful discussions.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Barta J, Melichova T, Vanek D, Picek T, Santruckova H. Effect of pH and dissolved organic matter on the abundance of nirK and nirS denitrifiers in spruce forest soil. Biogeochem. 2010;101:123–132. [Google Scholar]
- Behnke A, Engel M, Christen R, Nebel M, Klein RR, Stoeck T. Depicting more accurate pictures of protistan community complexity using pyrosequencing of hypervariable SSU rRNA gene regions. Environ Microbiol. 2011;13:340–349. doi: 10.1111/j.1462-2920.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- Betlach MR, Tiedje JM. Kinetic explanation for accumulation of nitrite, nitric-oxide, and nitrous-oxide during bacterial denitrification. Appl Environ Microbiol. 1981;42:1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmer AM, Bremner JM. Inhibitory effect of nitrate on reduction of N2O to N2 by soil-microorganisms. Soil Biol Biochem. 1978;10:187–191. [Google Scholar]
- Bockheim JG. Importance of cryoturbation in redistributing organic carbon in permafrost-affected soils. Soil Sci Soc Am J. 2007;71:1335–1342. [Google Scholar]
- Braker G, Zhou JZ, Wu LY, Devol AH, Tiedje JM. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific northwest marine sediment communities. Appl Environ Microbiol. 2000;66:2096–2104. doi: 10.1128/aem.66.5.2096-2104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monographs. 1957;27:326–349. [Google Scholar]
- Bremner JM. Sources of nitrous oxide in soils. Nutr Cyc Agroecosys. 1997;49:7–16. [Google Scholar]
- Bru D, Ramette A, Saby NPA, Dequiet S, Ranjard L, Jolivet C, et al. Determinants of the distribution of nitrogen-cycling microbial communities at the landscape scale. ISME J. 2011;5:532–542. doi: 10.1038/ismej.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru D, Sarr A, Philippot L. Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl Environ Microbiol. 2007;73:5971–5974. doi: 10.1128/AEM.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen TR. Methane emission from arctic tundra. Biogeochemistry. 1993;21:117–139. [Google Scholar]
- van Cleemput O. Subsoils: chemo- and biological denitrification, N2O and N2 emissions. Nutr Cyc Agroecosys. 1998;52:187–194. [Google Scholar]
- Conrad R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO) Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuhel J, Simek M, Laughlin RJ, Bru D, Cheneby D, Watson CJ, et al. Insights into the effect of soil pH on N2O and N2-emissions and denitrifier community size and activity. Appl Environ Microbiol. 2010;76:1870–1878. doi: 10.1128/AEM.02484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiglmayr K, Philippot L, Tscherko D, Kandeler E. Microbial succession of nitrate-reducing bacteria in the rhizosphere of Poa alpina across a glacier foreland in the Central Alps. Environ Microbiol. 2006;8:1600–1612. doi: 10.1111/j.1462-2920.2006.01051.x. [DOI] [PubMed] [Google Scholar]
- Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, et al. 2007Couplings between changes in the climate system and biogeochemistryIn: Solomon, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds).Climate Change 2007; the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press: Cambridge, UK; New York, NY; 499–587. [Google Scholar]
- Dickie IA. Insidious effects of sequencing errors on perceived diversity in molecular surveys. New Phytol. 2010;188:916–918. doi: 10.1111/j.1469-8137.2010.03473.x. [DOI] [PubMed] [Google Scholar]
- Enwall K, Throbäck IN, Stenberg M, Soderstrom M, Hallin S. Soil resources influence spatial patterns of denitrifying communities at scales compatible with land management. Appl Environ Microbiol. 2010;76:2243–2250. doi: 10.1128/AEM.02197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, et al. 2007Changes in atmospheric constituents and in radiative forcingIn: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds).Climate Change 2007; the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press: Cambridge, UK; New York, NY; 129–234. [Google Scholar]
- Gaskell JF, Blackmer AM, Bremner JM. Comparison of effects of nitrate, nitrite, and nitric-oxide on reduction of nitrous-oxide to dinitrogen by soil-microorganisms. Soil Sci Soc Am J. 1981;45:1124–1127. [Google Scholar]
- Green SJ, Prakash O, Gihring TM, Akob DM, Jasrotia P, Jardine PM, et al. Denitrifying bacteria isolated from terrestrial subsurface sediments exposed to mixed-waste contamination. Appl Environ Microbiol. 2010;76:3244–3254. doi: 10.1128/AEM.03069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel RN, van der Biezen E, Jetten MSM, Hefting MM, Kartal B. Denitrification at pH 4 by a soil-derived Rhodanobacter-dominated community. Environ Microbiol. 2010;12:3264–3271. doi: 10.1111/j.1462-2920.2010.02301.x. [DOI] [PubMed] [Google Scholar]
- Heylen K, Gevers D, Vanparys B, Wittebolle L, Geets J, Boon N, et al. The incidence of nirS and nirK and their genetic heterogeneity in cultivated denitrifiers. Environ Microbiol. 2006;8:2012–2021. doi: 10.1111/j.1462-2920.2006.01081.x. [DOI] [PubMed] [Google Scholar]
- Hill TCJ, Walsh KA, Harris JA, Moffett BF. Using ecological diversity measures with bacterial communities. FEMS Microbiol Ecol. 2003;43:1–11. doi: 10.1111/j.1574-6941.2003.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Holtan-Hartwig L, Dörsch P, Bakken LR. Comparison of denitrifying communities in organic soils: kinetics of NO3− and N2O reduction. Soil Biol Biochem. 2000;32:833–843. [Google Scholar]
- Horn MA, Drake HL, Schramm A. Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Appl Environ Microbiol. 2006;72:1019–1026. doi: 10.1128/AEM.72.2.1019-1026.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JA, Mark Welch DB, Morrison HG, Huse SM, Neal PR, Butterfield DA, et al. Microbial population structures in the deep marine biosphere. Science. 2007;318:97–100. doi: 10.1126/science.1146689. [DOI] [PubMed] [Google Scholar]
- Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol. 2010;12:1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai S, Chai BL, Sul WJ, Cole JR, Hashsham SA, Tiedje JM. Gene-targeted-metagenomics reveals extensive diversity of aromatic dioxygenase genes in the environment. ISME J. 2010;4:279–285. doi: 10.1038/ismej.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Hallin S. Ecological and evolutionary factors underlying global and local assembly of denitrifier communities. ISME J. 2010;4:633–641. doi: 10.1038/ismej.2009.152. [DOI] [PubMed] [Google Scholar]
- Jones CM, Stres B, Rosenquist M, Hallin S. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol Biol Evol. 2008;25:1955–1966. doi: 10.1093/molbev/msn146. [DOI] [PubMed] [Google Scholar]
- Kandeler E, Deiglmayr K, Tscherko D, Bru D, Philippot L. Abundance of narG, nirK, nirS, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl Environ Microbiol. 2006;72:5957–5962. doi: 10.1128/AEM.00439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmeyer U, Kuschk P, Stottmeister U. Model experiments on the influence of artificial humic compounds on chemodenitrification. Water Air Soil Poll. 2003;147:317–330. [Google Scholar]
- Kresovic M, Jakovljevic M, Blagojevic S, Maksimovic S. Specific transformations of mineral forms of nitrogen in acid soils. J Serb Chem Soc. 2009;74:93–102. [Google Scholar]
- Kuhry P, Dorrepaal E, Hugelius G, Schuur EAG, Tarnocai C. Potential remobilization of belowground permafrost carbon under future global warming. Permafrost Periglac. 2010;21:208–214. [Google Scholar]
- Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin V, Engelbrektson A, Ochman H, Hugenholtz P. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol. 2010;12:118–123. doi: 10.1111/j.1462-2920.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- Liu BB, Mørkved PT, Frostegård Å, Bakken LR. Denitrification gene pools, transcription and kinetics of NO N2O, and N2 production as affected by soil pH. FEMS Microbiol Ecol. 2010;72:407–417. doi: 10.1111/j.1574-6941.2010.00856.x. [DOI] [PubMed] [Google Scholar]
- Loy A, Küsel K, Lehner A, Drake HL, Wagner M. Microarray and functional gene analyses of sulfate-reducing prokaryotes in low-sulfate, acidic fens reveal co-occurrence of recognized genera and novel lineages. Appl Environ Microbiol. 2004;70:6998–7009. doi: 10.1128/AEM.70.12.6998-7009.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73:1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WK, Farrell RE, Siciliano SD. Nitrous oxide emissions from ephemeral wetland soils are correlated with microbial community composition. Front Microbiol. 2011;2:110. doi: 10.3389/fmicb.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahne I, Tiedje JM. Criteria and methodology for identifying respiratory denitrifiers. Appl Environ Microbiol. 1995;61:1110–1115. doi: 10.1128/aem.61.3.1110-1115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljanen M, Hytönen J, Mäkiranta P, Alm J, Minkkinen K, Laine J, et al. Greenhouse gas emissions from cultivated and abandoned organic croplands in Finland. Boreal Environ Res. 2007;12:133–140. [Google Scholar]
- Männistö MK, Tiirola M, Häggblom MM. Effect of freeze-thaw cycles on bacterial communities of arctic tundra soil. Microb Ecol. 2009;58:621–631. doi: 10.1007/s00248-009-9516-x. [DOI] [PubMed] [Google Scholar]
- Marushchak ME, Pitkämäki A, Koponen H, Biasi C, Seppälä M, Martikainen PJ. Hot spots for nitrous oxide emissions found in different types of permafrost peatlands. Glob Change Biol. 2011;17:2601–2614. [Google Scholar]
- Mørkved PT, Dörsch P, Henriksen TM, Bakken LR. N2O emissions and product ratios of nitrification and denitrification as affected by freezing and thawing. Soil Biol Biochem. 2006;38:3411–3420. [Google Scholar]
- Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel ME, Wild S, Holzhauser M, Hüttenberger L, Reitzig R, Sperber M, et al. Jaguc-a software package for environmental diversity analyses. J Bioinform Comput Biol. 2011;9:749–773. doi: 10.1142/s0219720011005781. [DOI] [PubMed] [Google Scholar]
- Palmer K, Drake HL, Horn MA. Association of novel and highly diverse acid-tolerant denitrifiers with N2O fluxes of an acidic fen. Appl Environ Microbiol. 2010;76:1125–1134. doi: 10.1128/AEM.02256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K, Drake HL, Horn MA. Genome-derived criteria for assigning environmental narG and nosZ sequences to operational taxonomic units of nitrate reducers. Appl Environ Microbiol. 2009;75:5170–5174. doi: 10.1128/AEM.00254-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin TB, Sexstone AJ, Tiedje JM. Adaptation of denitrifying populations to low soil-pH. Appl Environ Microbiol. 1985;49:1053–1056. doi: 10.1128/aem.49.5.1053-1056.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peršoh D, Theuerl S, Buscot F, Rambold G. Towards a universally adaptable method for quantitative extraction of high-purity nucleic acids from soil. J Microbiol Meth. 2008;75:19–24. doi: 10.1016/j.mimet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Philippot L, Andert J, Jones CM, Bru D, Hallin S. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob Change Biol. 2011;17:1497–1504. [Google Scholar]
- Philippot L, Cuhel J, Saby NPA, Cheneby D, Chronakova A, Bru D, et al. Mapping field-scale spatial patterns of size and activity of the denitrifier community. Environ Microbiol. 2009;11:1518–1526. doi: 10.1111/j.1462-2920.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- Philippot L, Piutti S, Martin-Laurent F, Hallet S, Germon JC. Molecular analysis of the nitrate-reducing community from unplanted and maize-planted soils. Appl Environ Microbiol. 2002;68:6121–6128. doi: 10.1128/AEM.68.12.6121-6128.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlatie M, Syväsalo E, Simojoki A, Esala M, Regina K. Contribution of nitrification and denitrification to N2O production in peat, clay and loamy sand soils under different soil moisture conditions. Nutr Cyc Agroecosys. 2004;70:135–141. [Google Scholar]
- Prieme A, Braker G, Tiedje JM. Diversity of nitrite reductase (nirK and nirS) gene fragments in forested upland and wetland soils. Appl Environ Microbiol. 2002;68:1893–1900. doi: 10.1128/AEM.68.4.1893-1900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quince C, Lanzen A, Curtis TP, Davenport RJ, Hall N, Head IM, et al. Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Methods. 2009;6:639–641. doi: 10.1038/nmeth.1361. [DOI] [PubMed] [Google Scholar]
- Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ. Removing noise from pyrosequenced amplicons. BMC Bioinformatics. 2011;12:38. doi: 10.1186/1471-2105-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- Repo ME, Susiluoto S, Lind SE, Jokinen S, Elsakov V, Biasi C, et al. Large N2O emissions from cryoturbated peat soil in tundra. Nat Geosci. 2009;2:189–192. [Google Scholar]
- Rich JJ, Heichen RS, Bottomley PJ, Cromack K, Myrold DD. Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Appl Environ Microbiol. 2003;69:5974–5982. doi: 10.1128/AEM.69.10.5974-5982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method–A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Santoro AE, Boehm AB, Francis CA. Denitrifier community composition along a nitrate and salinity gradient in a coastal aquifer. Appl Environ Microbiol. 2006;72:2102–2109. doi: 10.1128/AEM.72.3.2102-2109.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka JE, Robador A, Hubert C, Jørgensen BB, Brüchert V. Effects of freeze-thaw cycles on anaerobic microbial processes in an Arctic intertidal mud flat. ISME J. 2010;4:585–594. doi: 10.1038/ismej.2009.140. [DOI] [PubMed] [Google Scholar]
- Schalk-Otte S, Seviour RJ, Kuenen JG, Jetten MSM. Nitrous oxide (N2O) production by Alcaligenes faecalis during feast and famine regimes. Water Res. 2000;34:2080–2088. [Google Scholar]
- Schmidt O, Drake HL, Horn MA. Hitherto unknown [Fe-Fe]-hydrogenase gene diversity in anaerobes and anoxic enrichments from a moderately acidic fen. Appl Environ Microbiol. 2010;76:2027–2031. doi: 10.1128/AEM.02895-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. John Wiley & Sons: New York; 1993. [Google Scholar]
- Shapleigh J.2006The denitrifying prokaryotesIn: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds). The Prokaryotes Springer: New York; 769–792. [Google Scholar]
- Sharma S, Szele Z, Schilling R, Munch JC, Schloter M. Influence of freeze-thaw stress on the structure and function of microbial communities and denitrifying populations in soil. Appl Environ Microbiol. 2006;72:2148–2154. doi: 10.1128/AEM.72.3.2148-2154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek M, Cooper JE. The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. Eur J Soil Sci. 2002;53:345–354. [Google Scholar]
- Smith SM. Nitrous oxide production by Escherichia coli is correlated with nitrate reductase activity. Appl Environ Microbiol. 1983;45:1545–1547. doi: 10.1128/aem.45.5.1545-1547.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen T. A method of establishing groups of equal amplitude in plant sociology based on similatity of species and its application to analyses of the vegetation on Danish commons. Biol Skr. 1948;5:1–34. [Google Scholar]
- Stolz JF, Basu P. Evolution of nitrate reductase: molecular and structural variations on a common function. Eur J Chem Biol. 2002;3:198–206. doi: 10.1002/1439-7633(20020301)3:2/3<198::AID-CBIC198>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Strong DT, Fillery IRP. Denitrification response to nitrate concentrations in sandy soils. Soil Biol Biochem. 2002;34:945–954. [Google Scholar]
- Sun YJ, Cai YP, Liu L, Yu FH, Farrell ML, McKendree W, et al. ESPRIT: estimating species richness using large collections of 16S rRNA pyrosequences. Nucleic Acids Res. 2009;37:e76. doi: 10.1093/nar/gkp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throbäck IN, Enwall K, Jarvis A, Hallin S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol. 2004;49:401–417. doi: 10.1016/j.femsec.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Tiedje JM.1988Ecology of denitrification and dissimilatory nitrate reduction to ammoniumIn: Zehnder JB (ed.). Biology of Anaerobic Microorganisms John Wiley & Sons: New York; 179–243. [Google Scholar]
- Vangnai S, Klein DA. Study of nitrite-dependent dissimilatory microorganisms isolated from Oregon soils. Soil Biol Biochem. 1974;6:335–339. [Google Scholar]
- Walker DA, Raynolds MK, Daniels FJA, Einarsson E, Elvebakk A, Gould WA, et al. The circumpolar arctic vegetation map. J Veg Sci. 2005;16:267–282. [Google Scholar]
- Werner C, Butterbach-Bahl K, Haas E, Hickler T, Kiese R. A global inventory of N2O emissions from tropical rainforest soils using a detailed biogeochemical model. Global Biogeochem Cy. 2007;21:GB3010. [Google Scholar]
- Will C, Thürmer A, Wollherr A, Nacke H, Herold N, Schrumpf M, et al. Horizon-specific bacterial community composition of German grassland soils, as revealed by pyrosequencing-based analysis of 16S rRNA genes. Appl Environ Microbiol. 2010;76:6751–6759. doi: 10.1128/AEM.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yergeau E, Kang S, He Z, Zhou J, Kowalchuk GA. Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. ISME J. 2007;1:163–179. doi: 10.1038/ismej.2007.24. [DOI] [PubMed] [Google Scholar]
- Yergeau E, Kowalchuk GA. Responses of Antarctic soil microbial communities and associated functions to temperature and freeze-thaw cycle frequency. Environ Microbiol. 2008;10:2223–2235. doi: 10.1111/j.1462-2920.2008.01644.x. [DOI] [PubMed] [Google Scholar]
- Yoshinari T, Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Co. 1976;69:705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]
- Zaprasis A, Liu YJ, Liu SJ, Drake HL, Horn MA. Abundance of novel and diverse tfdA-like genes, encoding putative phenoxyalkanoic acid herbicide-degrading dioxygenases, in soil. Appl Environ Microbiol. 2010;76:119–128. doi: 10.1128/AEM.01727-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft WG. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–615. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.