Abstract
Multiple adjuvant regimens are used for HER2+ breast cancer, but experience in routine practice is not reported. We evaluated whether oncologists’ perceptions of these regimens matches clinical experience. We surveyed Wisconsin medical oncologists throughout the state regarding factors impacting selection of TCH (docetaxel, carboplatin, trastuzumab) or anthracycline-based therapy. We also reviewed 200 cases of HER2+ breast cancer treated at the University of Wisconsin and the Marshfield Clinic and collected data on patient and tumor characteristics, chemotherapy regimen, and toxicities. Two-thirds of surveyed oncologists prefer anthracycline-based therapy, particularly for node-positive cancers. However, TCH was preferred for early stage (T1a-bN0) tumors. Half of oncologists use prophylactic G-CSF with TCH. In the 200 cases reviewed at our centers, acute toxicity occurred more frequently with TCH. There were fewer dose modifications or delays for AC-TH (doxorubicin, cyclophosphamide, paclitaxel, trastuzumab) than TCH (31% vs. 47%, p=0.07), possibly due to higher use of prophylactic G-CSF with AC-TH (77% vs. 34% with TCH, p<0.001). Fifteen patients received prophylactic G-CSF during TCH; none developed neutropenic fever. In contrast, 25% developed neutropenic fever during TCH without G-CSF. There were modest declines in median left ventricular ejection fraction reaching 9% with AC-TH and 3% with TCH at 12 months, but early cessation of trastuzumab was similar for both regimens. We conclude that TCH and AC-TH are common adjuvant regimens used for HER2+ breast cancer. The preference of TCH for early-stage disease and anthracycline-based therapy for node-positive disease suggests that many oncologists perceive that TCH is safer and AC-TH more effective. Myelosuppression from TCH is greater than AC-TH, but can be mitigated with routine G-CSF.
Keywords: ErbB2, chemotherapy, trastuzumab, neutropenic fever, myelosuppression, cardiotoxicity
INTRODUCTION
Landmark clinical trials have established chemotherapy plus trastuzumab as the standard adjuvant therapy for human epidermal growth factor 2 positive (HER2+) breast cancer [1-4]. A commonly used regimen is doxorubicin with cyclophosphamide followed by paclitaxel and trastuzumab (AC-TH) [2]. Congestive heart failure is an overlapping risk of trastuzumab and doxorubicin, an anthracycline, and clinically significant heart failure occurs in 2-4% of patients treated with AC-TH [5-7]. To reduce this risk, a non-anthracycline regimen was developed combining docetaxel, carboplatin, and trastuzumab (TCH) [3,8]. Compared with a non-trastuzumab regimen, disease-free survival is improved with TCH and with AC-DH, a regimen like AC-TH with docetaxel 100mg/m2 every 3 weeks in place of paclitaxel. AC-DH is numerically superior to TCH in disease-free survival and overall survival, albeit with a 2% risk of grade 3/4 heart failure versus 0.4% with TCH [3]. Differences in dose, schedule, and drugs among anthracycline arms of the large phase III trials and shifting standards of care towards dose-dense chemotherapy have led to considerable debate about optimal adjuvant therapy, and whether anthracyclines should be used [9,10]. Moreover, the major focus on cardiotoxicity has obscured differences in other toxicities between TCH and AC-TH, such as myelosuppression. Although there may be significant regional variations in preference for TCH or AC-TH, both are used in Wisconsin.
We hypothesized that oncologists base adjuvant recommendations on factors such as toxicity, convenience and perceived effectiveness. We anticipated that toxicity profile in routine practice differs from phase III trials due to expanded populations, alterations in schedule, and adoption of new supportive medications. Both AC-TH and TCH are widely used in routine practice in Wisconsin, which allows us to capture differences in oncologist opinion and observed toxicities. Here, we determine how Wisconsin oncologists select adjuvant therapy for HER2 positive breast cancer and evaluate toxicity in patients treated in routine practice.
METHODS
In Part 1 of this study, we surveyed Wisconsin oncologists regarding how they select adjuvant therapy for HER2+ breast cancer. In Part 2, we evaluated toxicity in unselected patients seen in routine practice.
Part 1: Survey of Oncologists
Population
We identified 151 Wisconsin medical oncologists through: (1) the ASCO directory for oncologists who self-reported interest in breast cancer or general oncology, (2) the membership directory of the Wisconsin Oncology Network, and (3) updated websites of practices identified in (1) and (2).
Study design
We hypothesized that oncologists’ use of TCH or anthracycline-based therapy was based on training, experience, and perceptions about efficacy and toxicity. To test this, we invited oncologists to participate in an anonymous 12-question web survey (See Appendix). The survey was reviewed by the University of Wisconsin (UW) IRB and was considered exempt (IRB# 2010-0425). The survey was administered anonymously via the UW Carbone Cancer Center Survey Shared Service.
Part 2: Retrospective Chart Review
Population
The cancer registries at UW Hospital and Clinics and at Marshfield Clinic were used to identify women with Stage I-III breast cancer treated with adjuvant or neoadjuvant trastuzumab from 2003-2010. The UW Madison is a tertiary care facility and NCI-designated Comprehensive Cancer Center in south-central Wisconsin. The Marshfield Clinic is a multi-site multi-specialty clinic system providing care to central, western, and northern Wisconsin. To be considered, chemotherapy had to be discontinued or completed prior to collection of toxicity data (11/15/2010 at UW and 1/1/2011 at Marshfield Clinic). Charts for 200 patients met these criteria.
Study design
We performed a retrospective chart review of electronic medical records to obtain age, medical history (cardiac disease, hypertension, hyperlipidemia, diabetes, chronic kidney disease), tumor characteristics (size, number of nodes positive/collected, estrogen receptor and progesterone receptor status, cancer stage), therapy (dates of treatment, regimen, delays/modifications, use of growth factor), and toxicities (left ventricular ejection fraction [LVEF], maximum creatinine level and minimum value for hemoglobin and platelets during chemotherapy). For analysis, grade 3 or 4 anemia (hemoglobin <8 g/dL) or thrombocytopenia (platelets <50,000/uL) were documented. Acute kidney injury was defined as change in creatinine level from a normal baseline to above 1.5 mg/dL. This project was approved by the UW IRB as a minimal risk study (IRB# 2010-0346), and accepted by the Marshfield Clinic IRB as a Wisconsin IRB Consortium project.
Study Analysis
Subjects were categorized into cohorts by the first regimen received, and were analyzed regardless of completion of planned therapy. Categories were TCH, AC-TH, and ‘Other.’ The AC-TH cohort was defined as AC followed by paclitaxel with sequential or concurrent trastuzumab, regardless of dose/schedule. AC-DH was uncommon (7 patients) and was categorized as ‘other’ due to potential differences between docetaxel and paclitaxel. To evaluate cardiac function, LVEF values were grouped by time from treatment initiation and evaluated as median and quartiles for baseline LVEF, and for change from baseline at other time points. We excluded subjects for which baseline LVEF were not available, although this was available for most patients, including 96 who received AC-TH and 43 who received TCH.
Statistical Analysis (Part 1 and 2)
Comparison between TCH and AC-TH (chart review) were made using Fisher's exact test. P-values less than 0.05 were considered statistically significant. No adjustment was made for multiple comparisons.
RESULTS
Part 1: Survey of Oncologists
We first determined how oncologists select treatment for HER2+ breast cancer. One hundred fifty-one oncologists were invited to participate and 65 (42%) responded. Among respondents, the anthracycline-based and TCH regimens were perceived as equally likely to be completed (13 responded anthracycline-based, 13 TCH, 37 equal, two did not respond). For healthy women with HER2+ breast cancer, 64% of oncologists preferred anthracycline-based regimens, and 36% prefer TCH. Interestingly, there was a correlation between time in practice and regimen preference. For example, of oncologists who prefer TCH, 87% reported being in clinical practice over 10 years. In contrast, only 46% of oncologists that preferred anthracycline-based regimens were in practice for over 10 years. Prior experience with cardiotoxicity may be influential as 36% of respondents reported experience with symptomatic heart failure from anthracyclines. Thus, clinical experience and recent training correlate with treatment selection.
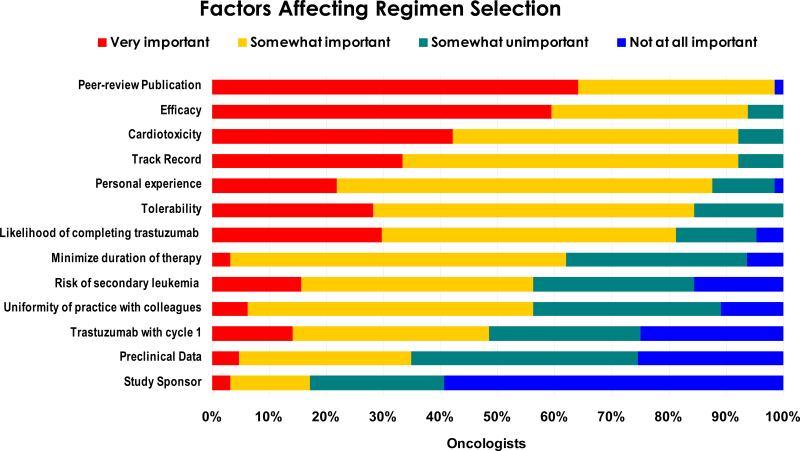
To determine influences for TCH vs. anthracycline-based preference, we asked oncologists to rate the importance of several factors (Figure 1). Several differed from our expectations: First, publication in a peer-reviewed journal was cited as the most important criterion, although BCIRG 006 had not been published as of the survey date. Second, fewer than 20% of oncologists thought early initiation and completion of trastuzumab were ‘very important.’ Finally, we anticipated that a pharmaceutical industry-sponsored trial might be viewed as potentially more biased than a study funded by the National Cancer Institute. However, study sponsor was cited by oncologists as the least important factor used to select therapy. As expected, efficacy and cardiotoxicity were cited as key criteria that oncologists use to select treatment.
Fig. 1.
Factors that affect regimen selection among surveyed Oncologists. Oncologists were asked to select the relative importance of each factor on a 4-point scale ranging from “not at all important” to “very important.” (See Appendix)
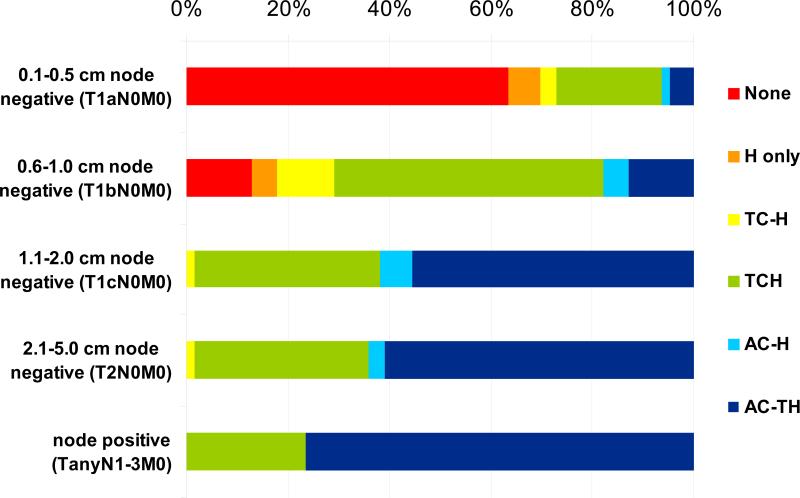
To determine how these criteria determine treatment recommendations for individual patients, we asked physicians to recommend treatment for healthy premenopausal women with HER2+ cancers of progressive stages (Figure 2). Treatment of small HER2+ tumors with trastuzumab-based regimens is controversial but is supported by the risk of recurrence of these tumors, retrospective outcome data, and eligibility for BCIRG 006 if an additional high risk feature is present [3,11-13]. As expected, adjuvant recommendations were varied. For node-negative tumors <6mm, most oncologists recommended no chemotherapy, but when treatment was recommended, anthracycline-sparing regimens such as TCH and trastuzumab alone were preferred. For these T1aN0 tumors, oncologists who prefer TCH recommend chemotherapy twice as often as physicians who favor anthracycline-based therapy (41% vs. 20%). Adjuvant chemotherapy was recommended for T1bN0 HER2+ tumors by most oncologists, with TCH as the preferred regimen (Figure 2). Nevertheless, variation in clinical recommendations reflects the uncertainty about whether adjuvant treatment improves outcome for patients with node-negative tumors measuring 1-10mm [12,13].
Fig. 2.
Regimen Selection by Stage. Surveyed oncologists were asked to select adjuvant therapy for a healthy premenopausal woman with ER- PR- HER2+ breast cancer of successive stages. Options included no therapy, trastuzumab only, TCH, AC-TH or other anthracycline+taxane based therapy, AC-H or anthracyline+trastuzumab without taxane, or TC-H (docetaxel/cyclophosphamide followed by trastuzumab)
Treatment recommendations were strikingly different for healthy women with more advanced HER2+ cancers (Figure 2). For node-positive cancers, anthracycline-based treatments were preferred by a majority of oncologists, including many who selected TCH for node-negative cancers. These data imply that oncologists are willing to accept elevated cardiotoxicity of anthracyclines when efficacy is of paramount concern.
We next sought to determine how oncologists support patients during adjuvant chemotherapy. Routine G-CSF was not used with anthracycline-based therapies for HER2+ breast cancer in large randomized trials [1,2]. However, the concept of dose density has lead to widespread adoption of G-CSF with anthracycline-based therapies based on improved efficacy without trastuzumab and safety with trastuzumab [14-16]. We found that most oncologists use G-CSF with Cycle 1 of therapy, but this is more common with AC-TH (77%) than with TCH (53%).
Part 2: Retrospective Chart Review--Patient Characteristics
To evaluate toxicities of TCH and AC-TH in routine practice, we reviewed charts of patients who received trastuzumab-based therapy. We expected that toxicity in routine practice may differ from those reported in prospective studies because of differences in patient population, regimen modifications, and adoption of new supportive care medicines. We identified 200 patients treated at the University of Wisconsin and Marshfield Clinics between 2003 and 2010 (Table 1). Over this period 47 patients received TCH, 114 received AC-TH, and the remainder received another trastuzumab-containing treatment.
Table 1.
Demographic and baseline characteristics of patients in this study.
| All Patientsa (n=200) | TCHb (n=47) | AC-TH (n=114) | ||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | No. | % |
| Age, years | ||||||
| Mean | 51.4 | 52 | 51.1 | |||
| SD (range) | 11.4 (26-97) | 13.8 (27-97c) | 11.8 (28-83) | |||
| Timing of treatment | ||||||
| Neoadjuvant | 32 | 16.0 | 5 | 10.6 | 21 | 18.4 |
| Adjuvant | 168 | 84.0 | 42 | 89.4 | 93 | 81.6 |
| Hormone Receptor status | ||||||
| ER+ | 127 | 63.5 | 27 | 57.4 | 76 | 66.7 |
| PR+ | 101 | 50.5 | 22 | 46.8 | 60 | 52.6 |
| Stage | ||||||
| I | 57 | 28.5 | 20 | 42.6 | 21 | 18.4 |
| II | 76 | 38.0 | 21 | 44.7 | 43 | 37.7 |
| III | 67 | 33.5 | 22 | 46.8 | 50 | 43.9 |
| Node positive | 104 | 52.0 | 20 | 42.6 | 69 | 60.5 |
| Comorbidities | ||||||
| Diabetes | 16 | 8.0 | 7 | 14.9 | 6 | 5.3 |
| Cardiac Disease | 10 | 5.0 | 7 | 14.9 | 2 | 1.8 |
| Chronic Kidney Disease | 1 | 0.5 | 0 | 0 | 1 | 0.9 |
| Hypertension | 56 | 28.0 | 14 | 29.8 | 27 | 23.7 |
| Hyperlipidemia | 48 | 24.0 | 13 | 27.7 | 22 | 19.3 |
Abbreviations: TCH, Taxotere+carboplatin+trastuzumab; AC-TH, adriamycin+cyclophosphamide followed by paclitaxel and trastuzumab; SD, standard deviation; ER, estrogen receptor; PR, progesterone receptor
Includes 38 patients treated with regimens other than TCH or AC-TH: AC-H (12 patients), AC-DH (7), T+H (4), TAC+H (3), FEC+H (3), EC+H (2), CMF+H (2), TAC+DH (1), sequential TH-AC-H (1), AT+T+carboplatin (1), CMF+TH(1) H only (1). A small number of patients received a non-chemotherapy investigational agent; such patients were classified by chemotherapy received.
TCH use began in 2006 and represents 35% of adjuvant therapy delivered at these centers from this date.
A 97 year old woman with T3N1M0 HER2-amplified breast cancer was treated with TCH on modified schedule with four months of trastuzumab. She experienced grade 3 anemia and grade 4 thrombocytopenia without neutropenic fever; the next oldest patient who received TCH was aged 82.
The TCH and AC-TH cohorts were balanced with respect to timing of treatment, hormone receptor status, and age (Table 1). Consistent with the oncologist survey, TCH use was common for Stage I cancers. Moreover, TCH was commonly used for patients with a history of coronary artery disease, congestive heart failure, or cardiac rhythm disturbance—patients who would have been ineligible for adjuvant HER2 trials [1-3]. Interestingly, a wide number of schedules for chemotherapy were utilized and most differed from that used in the phase III trials of trastuzumab (Table 2). The most common regimen/schedule included dose dense AC (every two weeks) with G-CSF support. Paclitaxel was commonly delivered after AC, on a weekly or every-2-week schedule. Non-anthracycline, non-TCH regimens such as trastuzumab alone or CMF plus trastuzumab were chosen for 9 patients, of whom 5 had stage I disease, and 5 were at least 70 years of age.
Table 2.
Schedule of chemotherapy with anthracycline+trastuzumab based therapy.
| no. | |
|---|---|
| AC-TH | 114 |
| AC Schedule: | |
| weekly AC | 1 |
| every 2 week AC | 93 |
| every 3 week AC | 27 |
| Paclitaxel Schedule: | |
| weekly paclitaxel | 49 |
| every 2 week paclitaxel | 54 |
| every 3 week paclitaxel | 11 |
| AC-DH | 8 |
| AC, no taxane | 12 |
| Other anthracycline-based | 10 |
Abbreviations: AC, Adriamycin and cyclophosphamide.
Toxicity
We next sought to evaluate toxicities in the AC-TH and TCH cohorts (Table 3). In the AC-TH cohort, there was a 31% rate of chemotherapy delays and dose modifications compared with 47% with TCH. Strikingly, myelosuppression was greater with TCH and represented the most common cause of hospitalizations and delays or modification in treatment. Of 10 patients hospitalized with TCH, 8 were admitted for neutropenic fever, one for bacteremia, and one with hypotension and neutropenia without fever. Additionally one patient was not hospitalized, but died during TCH treatment from a bowel obstruction thought to be unrelated to breast cancer or its treatment. There were no other deaths during treatment among the reviewed patients. There was significantly lower rate of febrile neutropenia with AC-TH (4% vs. 17% with TCH).
Table 3.
Toxicity by regimen
| Toxicity | Total (n=200) | TCH (n=47) | AC-TH (n=114) | p-valuea | |||
|---|---|---|---|---|---|---|---|
| Hospitalization | 28 | 14% | 10 | 21% | 14 | 12% | 0.152 |
| Chemotherapy delays/modifications | 68 | 34% | 22 | 47% | 35 | 31% | 0.070 |
| Febrile neutropenia | 13 | 7% | 8 | 17% | 4 | 4% | 0.006 |
| Grade 3/4 Anemia (Hgb <8 g/dl)b | 12 | 6% | 8 | 17% | 3 | 3% | 0.003 |
| Grade 3/4 Thrombocytopenia (platelets < 50K/μl)b | 23 | 12% | 8 | 17% | 5 | 4% | 0.021 |
| Acute Kidney Injury | 7 | 5 | 11% | 2 | 2% | 0.023 | |
| Use of G-CSFc | 124 | 62% | 16 | 34% | 88 | 77% | <0.001 |
Abbreviations: TCH, Taxotere+carboplatin+trastuzumab; AC-TH, adriamycin+cyclophosphamide followed by paclitaxel and trastuzumab; Hgb, hemoglobin; G-CSF, Granulocyte-colony stimulating factor
p-value for difference between TCH and AC-TH by Fisher's exact test.
Hemoglobin and platelet values were missing in 9 patients, including 4 who received AC-TH and one who received TCH.
G-CSF with first cycle; these data were available for all but one patient who received AC followed by trastuzumab.
Prophylactic G-CSF had a major impact on febrile neutropenia with TCH. When prophylactic G-CSF was not used, TCH was associated with a 26% rate of febrile neutropenia. In contrast, there was no febrile neutropenia among 16 patients who received TCH with G-CSF. This toxicity is not associated with age or comorbid conditions; half the neutropenic fevers occurred in otherwise healthy women under age 50. The lower, 4% rate of neutropenic fever with AC-TH may be attributed in part to use of G-CSF in 77% of patients treated with this regimen. However, TCH also resulted in a significantly higher rate of grade 3/4 anemia and thrombocytopenia compared with AC-TH (Table 3). Together, these data demonstrate that TCH is more myelosuppressive than AC-TH even among young patients, but febrile neutropenia can be mitigated by adopting routine G-CSF when TCH is selected.
We hypothesized that TCH may be more nephrotoxic than AC-TH, due to inclusion of carboplatin. Indeed, 11% of patients treated with TCH had acute kidney injury versus 2% patients treated with AC-TH (p = 0.023; Table 3). However, all had spontaneous recovery of elevated creatinine conservative management including intravenous and oral fluids. Additionally there were occasional but rare electrolyte disturbances associated with TCH (not shown). Overall, these data demonstrate that TCH is more likely to result in transient nephrotoxicity of unclear clinical significance.
Timing and completion of trastuzumab
In our data set, trastuzumab was given concurrently with chemotherapy in 73.5% of all patients, and in 76% of the AC-TH cohort, overlapping with paclitaxel. We found that the standard dose and schedule of trastuzumab was commonly modified in routine practice. Most patients received 6 mg/kg every 3 weeks rather than 2 mg/kg weekly based on safety, efficacy and pharmacologic analysis showing long half-life [17]. We found that the 3-week schedule of trastuzumab was also adopted during chemotherapy in 73% of patients who received TCH. In women receiving AC-TH, there was even more variation in the trastuzumab schedule, with 47% weekly, 11% every 2 weeks, and 18% every 3 weeks. Upon completion of chemotherapy, trastuzumab was most commonly given every 3 weeks. We did not find differences of toxicity by trastuzumab dose or schedule.
A total of 15% of patients in our chart review discontinued trastuzumab prior to the planned 1 year of therapy. Eleven percent of all patients stopped due to cardiac causes, including 8.7% of women receiving TCH and 8.3% who received AC-TH. Of these women who stopped early due to cardiac causes, 9 of 12 women receiving AC-TH stopped prior to 6 months of therapy in comparison to only 1 of 4 women in the TCH group. Eight women stopped trastuzumab for other reasons, including three with ACTH (after 1, 10, and 11 months of trastuzumab) and two with TCH (after 8, 10 months). Additionally, there were 25 women who had therapy held, but ultimately completed trastuzumab as planned. The most common reason for holding trastuzumab was LVEF decline. Other delays and cessation of therapy were due to a host of reasons including procedures, myelosuppression, arthralgias, rash, infections, rheumatologic conditions, poor general health, kidney or electrolyte abnormalities, and patient preference.
Cardiotoxicity
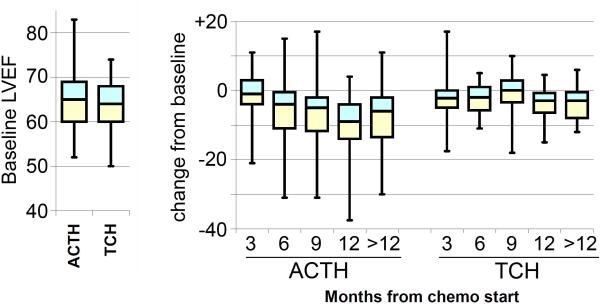
Cardiotoxicity has been extensively scrutinized for HER2+ adjuvant therapies in large randomized trials of highly selected patients [1-3,5-7]. We evaluated LVEF values collected for unselected patients in our study (Figure 3). Although there was no difference in trastuzumab discontinuation between AC-TH and TCH cohorts, we did observe differences in mean change in LVEF from baseline. Pretreatment LVEF values were similar for both regimens. However, the median LVEF decline reached 9% for AC-TH at 12 months from chemotherapy initiation, versus 3% for TCH at a similar time-point. We were not able to retrospectively determine grade 3/4 congestive heart failure. Our LVEF data concur with the differences in asymptomatic decline in LVEF previously reported for anthracycline- and non-anthracycline regimens [1-3]. The clinical significance of asymptomatic declines in LVEF is unknown [7].
Fig. 3.
LVEF values by regimen, AC-TH vs. TCH. Data are shown by box and whisker plots of median (line), quartiles (boxes), and extreme values (whiskers). Left: Baseline LVEF values. Right: Intrapatient change from baseline by month from chemotherapy initiation
DISCUSSION
Our survey provides new insight into the implementation of adjuvant therapies for HER2+ breast cancer. This survey captured a spectrum of treatment preference, ranging from oncologists who exclusively select TCH to those who use only anthracycline-based therapies. However, we also identified that a large number of oncologists take a more nuanced approach when selecting regimens, using TCH for small node-negative tumors and AC-TH for advanced-stage tumors where the additional risk of cardiotoxicity is overshadowed by risk of cancer recurrence. Moreover, we found the dose/schedule and duration of trastuzumab is frequently altered for a myriad of reasons. To our knowledge randomized studies are not planned to determine which of these treatment approaches is optimal, making analysis of patient outcomes important for optimizing current care.
As expected, cardiotoxicity is a major concern among oncologists. Cardiotoxicity of trastuzumab-based therapies has been extensively scrutinized [3,5-7,16,15,18]. Although cardiotoxicity is important, our study signals a note of caution regarding the importance of other toxicities. In particular, acute myelosuppression is more common with TCH compared with AC-TH, resulting in higher rates of febrile neutropenia, anemia, thrombocytopenia, and consequent hospitalizations. We conclude that, in addition to cardiotoxicity, myelosuppression should be considered when selecting TCH vs. AC-TH.
The perception amongst the surveyed oncologists is that TCH and anthracycline-based regimens are equally likely to be completed without delay or modification. In practice, however, we found a higher rate of dose delays and modifications with TCH than AC-TH. The major toxicity was myelosuppression and neutropenic fever with 26% risk without prophylactic G-CSF. This result seems dissonant with phase III results, where TCH is reported to have a 66% risk of grade 3/4 neutropenia, but only an 9.6% rate of febrile neutropenia[3]. It is unlikely that our population was at greater risk of myelosuppression, since half of hospitalizations were in healthy women <50 years old. However, we note that Slamon et al. reported that G-CSF use was left to the discretion of the treating oncologist, but does not specify how often this was used [3]. Moreover, these investigators report an additional 11.2% risk of grade 3/4 neutropenic infection with TCH. Using Common Terminology Criteria for Adverse Events version 2.0, neutropenic fever and neutropenic infection categories are distinguished by whether an infectious organism was identified, so total rate of neutropenic fever plus infection is 20.8% in BCIRG 006, despite use of G-CSF in some patients. Interestingly, more than half of Wisconsin oncologists who were surveyed report using prophylactic G-CSF with TCH, suggesting that oncologists recognize this toxicity through individual experience. Based on the rates of neutropenic fever/infection and current guidelines [19], we conclude that prophylactic G-CSF should be used routinely with TCH.
These findings demonstrate the importance of clear reporting for GCSF use in large phase III trials of adjuvant therapy. Previously, US Oncology 9735 reported a 5% rate of febrile neutropenia with docetaxel and cyclophosphamide, without specifying rate of G-CSF use. However, two observational studies reported rates of 33-50% with this regimen when G-CSF was not used [20,21]. Similarly, we report an unexpectedly high rate of febrile neutropenia with TCH. We conclude that phase III studies of chemotherapy regimens should carefully record and report G-CSF use among subjects, to allow oncologists to assess myelosuppression and to safely apply results to patients.
We anticipate possible increases in myelosuppression due to TCH with implementation of Isotope Dilution Mass Spectrometry (IDMS) for measurement of creatinine. IDMS has recently been adopted as a calibrated measure for creatinine, with values that are typically lower than what was determined previously by individual laboratories [22]. With TCH, carboplatin is dosed using AUC 6 [3], typically calculated Creatinine Clearance using the Calvert formula[23]. Both UW and Marshfield implemented the IDMS measurements after all patients reported here were treated. If IDMS was used, most patients would have received higher doses of carboplatin, making the likelihood of neutropenic fever and myelosuppression even higher than we report here.
When implemented in routine practice, HER2+ regimens are commonly modified from the schedules used in phase III trials. Both the NSABP trial B-31 and the NCCTG trial N9831 gave AC every 3 weeks [2], yet in our study, AC was most commonly given in a dose-dense fashion, every two weeks, followed by paclitaxel. Although this schedule differs from phase III studies, dose-dense AC is not associated with an increase risk of clinical cardiotoxicity or LVEF decline at 6 months, even when combined with HER2-targeted therapy [15,16]. We found that oncologists also routinely modify TCH schedule to use trastuzumab every 3 weeks; we did not observe unexpected toxicity associated with this modification.
Strengths of this study include a high rate of response among surveyed physicians and the inclusion of a population of patients seen in routine practice. Moreover, the variations in clinical practice in this study allowed correlation of interventions and toxicity (e.g. G-CSF and febrile neutropenia). Finally, AC-TH and TCH cohorts are well matched for factors other than tumor stage. A major limitation of the chart review is that it is retrospective with limited objective endpoints, non-randomized and limited to two regional oncology centers. Despite the limitations, retrospective analysis can provide important guidance for selecting treatment and supportive care given the lack of planned prospective studies to evaluate these questions.
In conclusion, this study demonstrates wide variation in perception and practice among oncologists who select therapy for HER2+ breast cancer. We conclude that a major focus on cardiotoxicity has obscured other differences between anthracycline- and non-anthracycline based treatments, even though all toxicities should be considered when selecting a cancer treatment regimen. In particular, we find that myelosuppression with TCH is more severe than with AC-TH and meets guidelines for routine use of G-CSF.
Supplementary Material
ACKNOWLEDGEMENTS
We thank T. Champeny (UWCCC Breast DOWG), R. Millholland (UW Tumor Registry), N. Jones, X. Zhang, and A. Trentham-Dietz (UW Shared Survey Service), A. Traynor, M.B. Wims and the Wisconsin Oncology Network physicians. This study was supported by UW Carbone Cancer Center P30 CA14520. M. Burkard is supported by CTSA 1UL1RR025011 and Flight Attendant Medical Research Foundation (FAMRI).
Abbreviations
- G-CSF
Granulocyte-colony stimulating factor, filgrastim or peg-filgrastim
- HER2
Human epidermal growth factor receptor 2
- LVEF
Left Ventricular ejection fraction
- ER
Estrogen Receptor
- PR
Progesterone Receptor
- G-CSF
Granulocyte-Colony Stimulating Factor
- TCH
Docetaxel, cyclophosphamide, trastuzumab
- AC-TH
doxorubicin, cyclophosphamide followed by paclitaxel and trastuzumab
- AC-DH
doxorubicin, cyclophosphamide followed by docetaxel and trastuzumab
Footnotes
CONFLICTS: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. doi:353/16/1659 [pii] 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 2.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. doi:353/16/1673 [pii] 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 3.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu M, Sauter G, Von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE, Jr., Martino S, Mamounas EP, Kaufman PA, Wolmark N. Four-Year Follow-Up of Trastuzumab Plus Adjuvant Chemotherapy for Operable Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Joint Analysis of Data From NCCTG N9831 and NSABP B-31. J Clin Oncol. doi: 10.1200/JCO.2011.35.0868. doi:JCO.2011.35.0868 [pii] 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT, Toi M, Coombes RC, Dodwell D, Pagani O, Madrid J, Hall M, Chen SC, Focan C, Muschol M, van Veldhuisen DJ, Piccart-Gebhart MJ. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422–3428. doi: 10.1200/JCO.2009.26.0463. doi:JCO.2009.26.0463 [pii] 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 6.Russell SD, Blackwell KL, Lawrence J, Pippen JE, Jr., Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–3421. doi: 10.1200/JCO.2009.23.6950. doi:JCO.2009.23.6950 [pii] 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 7.Morris PG, Hudis CA. Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: how worried should we be? J Clin Oncol. 2010;28(21):3407–3410. doi: 10.1200/JCO.2009.26.0125. doi:JCO.2009.26.0125 [pii] 10.1200/JCO.2009.26.0125. [DOI] [PubMed] [Google Scholar]
- 8.Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, Roche H, Martin M, Crown J, Mackey JR, Fumoleau P, Rolski J, Mrsic-Krmpotic Z, Jagiello-Gruszfeld A, Riva A, Buyse M, Taupin H, Sauter G, Press MF, Slamon DJ. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2010;29(2):149–156. doi: 10.1200/JCO.2010.28.6450. doi:JCO.2010.28.6450 [pii] 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 9.Gianni L, Norton L, Wolmark N, Suter TM, Bonadonna G, Hortobagyi GN. Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol. 2009;27(28):4798–4808. doi: 10.1200/JCO.2008.21.4791. doi:JCO.2008.21.4791 [pii] 10.1200/JCO.2008.21.4791. [DOI] [PubMed] [Google Scholar]
- 10.Press MF, Sauter G, Buyse M, Bernstein L, Guzman R, Santiago A, Villalobos IE, Eiermann W, Pienkowski T, Martin M, Robert N, Crown J, Bee V, Taupin H, Flom KJ, Tabah-Fisch I, Pauletti G, Lindsay MA, Riva A, Slamon DJ. Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol. 2011;29(7):859–867. doi: 10.1200/JCO.2009.27.5644. doi:JCO.2009.27.5644 [pii] 10.1200/JCO.2009.27.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herceptin (trastuzumab) prescribing information. Genentech; 2010. [Google Scholar]
- 12.Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO, Sahin A, Guray M, Larsimont D, Feoli F, Stranzl H, Buchholz TA, Valero V, Theriault R, Piccart-Gebhart M, Ravdin PM, Berry DA, Hortobagyi GN. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–5706. doi: 10.1200/JCO.2009.23.2025. doi:JCO.2009.23.2025 [pii] 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McArthur HL, Mahoney KM, Morris PG, Patil S, Jacks LM, Howard J, Norton L, Hudis CA. Adjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancer. Cancer. doi: 10.1002/cncr.26171. doi:10.1002/cncr.26171. [DOI] [PubMed] [Google Scholar]
- 14.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–1439. doi: 10.1200/JCO.2003.09.081. doi:10.1200/JCO.2003.09.081 JCO.2003.09.081 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Dang C, Fornier M, Sugarman S, Troso-Sandoval T, Lake D, D'Andrea G, Seidman A, Sklarin N, Dickler M, Currie V, Gilewski T, Moynahan ME, Drullinsky P, Robson M, Wasserheit-Leiblich C, Mills N, Steingart R, Panageas K, Norton L, Hudis C. The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancer. J Clin Oncol. 2008;26(8):1216–1222. doi: 10.1200/JCO.2007.12.0733. doi:26/8/1216 [pii] 10.1200/JCO.2007.12.0733. [DOI] [PubMed] [Google Scholar]
- 16.Morris PG, Dickler M, McArthur HL, Traina T, Sugarman S, Lin N, Moy B, Come S, Godfrey L, Nulsen B, Chen C, Steingart R, Rugo H, Norton L, Winer E, Hudis CA, Dang CT. Dose-dense adjuvant Doxorubicin and cyclophosphamide is not associated with frequent short-term changes in left ventricular ejection fraction. J Clin Oncol. 2009;27(36):6117–6123. doi: 10.1200/JCO.2008.20.2952. doi:JCO.2008.20.2952 [pii] 10.1200/JCO.2008.20.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baselga J, Carbonell X, Castaneda-Soto NJ, Clemens M, Green M, Harvey V, Morales S, Barton C, Ghahramani P. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23(10):2162–2171. doi: 10.1200/JCO.2005.01.014. doi:23/10/2162 [pii] 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Subar M, Lin W, Chen W, Pittman DG. Lack of Uniformity in Cardiac Assessment during Trastuzumab Therapy. Breast J. 2011;17(4):383–390. doi: 10.1111/j.1524-4741.2011.01101.x. doi:10.1111/j.1524-4741.2011.01101.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205. doi: 10.1200/JCO.2006.06.4451. doi:JCO.2006.06.4451 [pii] 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 20.Vandenberg T, Younus J, Al-Khayyat S. Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice-a retrospective analysis. Curr Oncol. 17(2):2–3. doi: 10.3747/co.v17i2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soong D, Haj R, Leung MG, Myers R, Higgins B, Myers J, Rajagopal S. High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. J Clin Oncol. 2009;27(26):e101–102. doi: 10.1200/JCO.2009.23.0508. doi:JCO.2009.23.0508 [pii] 10.1200/JCO.2009.23.0508. [DOI] [PubMed] [Google Scholar]
- 22.Ivy SP, Z J, M M. Follow-up for information letter regarding AUC-based dosing of carboplatin. Public Health Service; Bethesda, MD: 2010. [Google Scholar]
- 23.Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.