Abstract
Congenic mapping is a powerful strategy to identify genomic loci regulating quantitative traits. Generating congenic lines is an iterative process of refinement that is both time and resource intensive. Here we report an alternative to traditional microsatellite marker analysis or costly high-density oligonucleotide Single Nucleotide Polymorphism (SNP) arrays for congenic genotyping. The identification of inherited SNP variability in congenic lines using High Resolution Melting Analysis (HRMA) represents a novel application of the method. The Blocked Probe HRMA approach offers a scalable, low cost, closed-tube system that benefits from rapid turnaround times, and unequivocal interpretation. The markedly higher prevalence of SNPs relative to microsatellites in the genome allows much greater flexibility for the identification of new genotyping landmarks as congenic intervals are refined. We have adopted this approach in our development of B6.C3-Bbaa2 congenic lines for the identification of loci regulating murine Lyme arthritis severity. As a result, we have been able to fully genotype individuals prior to weaning age, and expand our number of breeding cages without increasing our colony budget. Thus far, 26 SNP markers have been successfully mapped to the Bbaa2 locus. This has facilitated the identification of 20 novel B6.C3-Bbaa2 congenic lines spanning the original interval.
Keywords: High Resolution Melting Analysis, Congenic Mice, Genotyping, Lyme Arthritis, Backcrossing, Fine Mapping
The Central Dogma of Molecular Biology is that heritable differences in DNA lead to changes in mRNA transcripts, proteins, and ultimately to observable variations in phenotypes such as disease susceptibility (1). Inbred mouse strains provide an invaluable resource of fixed genomic variability to scientifically investigate this genetic component of disease susceptibility. The ability to generate intercross populations between susceptible and resistant inbred mouse strains, combined with powerful statistical analysis, allows regulatory loci of quantitative traits to be mapped to regions of individual chromosomes. Once identified, the breeding of congenic mouse lines allows individual regulatory loci to be isolated in the context of a controlled genetic background, and then to be narrowed to finer resolution in an unbiased manner through additional backcrossing to parental lines. The genetic resolution that is possible to achieve through such breeding strategies is limited by the number and position of experimentally discernable genetic landmarks across the region of interest. Microsatellite markers have long been a key resource for genetic mapping due to their prevalence and ease of analysis by PCR.
The Bbaa2 murine Lyme arthritis susceptibility locus was identified in multiple independent intercrosses between resistant and susceptible inbred strains (2–3). Roper et al. used Composite Interval Mapping (CIM) on reciprocal backcross populations between C3H/HeN (C3H) and either C57BL/6 (B6) or Balb/c to identify complex regulation by up to four putative QTL within Bbaa2Bbaa3, with a maximum LOD score of 10.2. Reciprocal interval specific congenic lines (ISCLs) for the Bbaa2Bbaa3 region on Chromosome 5 developed on both the B6 and C3H backgrounds each retained a significant portion of the disease susceptibility phenotype conferred by the parental line (4). However, the full B6.C3-Bbaa2Bbaa3 congenic interval spanned 72.29 – 141.16 Mbp and included more than 870 genes and ESTs, making candidate gene selection impractical.
Efforts have been made to further refine regulatory loci within Bbaa2 to a resolution of 1 Mb or narrower through additional ISCL backcrossing. However, given the putative complexity of the Bbaa2 locus, the availability of microsatellite markers across the interval became a limiting factor, prompting the evaluation of alternative genotyping strategies. At present, the dbSNP database contains more than 8,000 Single Nucleotide Polymorphisms (SNPs) which differ between B6 and C3H inbred lines across the Bbaa2 interval (5). Although the C3H/HeN mouse strain used to create our congenic lines is not one of the eighteen currently included in the Sanger SNP re-sequencing project (6), data for the closely related C3H/HeJ strain is available. The Sanger database contains over 19,000 high quality SNPs which differ between C3H/HeJ and C57BL/6NJ SNPs in the Bbaa2 interval. A SNP genotyping strategy thus offered the potential to dramatically improve genetic resolution by increasing the number of discernable landmarks available. Several previously described SNP genotyping methodologies were evaluated: Amplification Refractory Mutation System (ARMS)-PCR (7), Small Amplicon High Resolution Melting Analysis (HRMA) (8), and Blocked Probe HRMA (9). We found that both methods of HRMA were useful for the genotyping of inherited intervals in congenic animals, with Blocked Probe HRMA being especially practical. Recent studies have emphasized the application of HRMA analysis for clinical diagnostics, but its application toward precise characterization of congenic mice has not previously been described.
Materials and methods
All mice used in this study were maintained in a pathogen free facility, and cared for in accordance with protocols approved by the University of Utah Institutional Animal Care and Use Committee (IACUC).
Genomic DNA was prepared from 2–3 mm tail clips from 14 to 17 day old mice by incubating in 600 μL of 50mM NaOH at 95C for one hour, following by neutralization with 50 μL of 1M Tris-HCl pH 8. Samples were then centrifuged at 6000 g for 6 min in a tabletop centrifuge and transferred to a clean tube. Two μL of 1:10 diluted DNA was used in each PCR reaction.
Amplification Refractory Mutation System (ARMS)-PCR is a tetra-primer SNP genotyping strategy that combines two inner SNP-specific and two outer primers in a single reaction volume (7). Reactions are designed to produce up to three amplicons of diagnostic sizes which are then evaluated by agarose gel electrophoresis, as shown in Supplemental Figure S-1A. ARMS-PCR primer sets for ten SNPs across the Bbaa2 region were developed using the recommended design program available online. (http://cedar.genetics.soton.ac.uk/public_html/primer1.html). ARMS-PCR primer sequences are available in Supplemental Table S-1. PCR was performed in 96-well plates on a PTC-200 Thermal Cycler using Platinum Taq (Invitrogen, Carlsbad, CA, USA) following the manufacturer's recommendations. PCR products were subjected to 1% agarose gel electrophoresis in 1X TBE buffer (89 mM Tris Base, 89 mM Boric Acid, 2 mM EDTA), and bands were visualized by ethidium bromide staining on a Gel Doc XR+ platform (Bio-Rad Laboratories, Hercules, CA, USA).
Small Amplicon HRMA employs two primers closely surrounding the SNP of interest (8). Amplicon size is kept at a minimum, between 50–70 bp. The hydrogen bonding characteristics of the nucleotide at the SNP position alters the melting temperature of the small amplicon, which can be detected by HRMA. The melting temperature differentials of homozygous Small Amplicons are reported to vary from 0.8 – 1.2 C, while heteroduplex amplicons are reported to melt at 2.0-3.0 C lower temperatures, as shown in Supplemental Figure S-1B.
Blocked (Unlabeled) Probe HRMA employs a 3′phosporylated oligonucleotide probe that overlaps the SNP of interest but cannot be extended in the PCR reaction, and two outer primers that produce a 65 – 150 bp amplicon (9). By including a limiting amount of primer on the same strand as the probe, asymmetric PCR produces an excess of opposite strand product to serve as a probe binding partner during HRMA. Probes form either perfect match or mismatch duplexes, with reported melting temperature differentials of 5.0 – 8.0 C, as shown in Supplemental Figure S-1C.
Small Amplicon primer sets for eleven SNPs, and Blocked Probe primers and probes for 33 SNPs were developed using online Primer3 design software (http://frodo.wi.mit.edu/primer3/). Small Amplicon and Blocked Probe primer sets are available in Supplemental Tables S-2 and S-3, respectively. PCR and HRMA were performed in 96-well plates using Platinum Taq and 1x LCGreen Plus+ DNA intercalating dye (Idaho Technologies, Salt Lake City, UT, USA) on a LightCycler 480 platform (Roche Applied Science, Indianapolis, IN, USA). HRMA results were evaluated using Light-Cycler 480 Software Version 1.5.
LC480 Cycling Parameters were as follows: Initial Denaturation: 95C (8 min); Amplification: 60 cycles of 95C denature (4 s) 65C annealing (6 s) 72C extension (12 s); Melt: 95C denature (15 s) 50C anneal (60 s), 50–95C continuous ramp at 0.06C/second, 10 measurements per degree. Cool: 50C (10 s).
Each primer set was evaluated with B6, C3H, and 1:1 mixed B6+C3H (obligate heterozygous) genomic DNA, as well as a H2O negative control.
Due to different assay design requirements, individual SNPs were not generally compatible with all three methodologies. SNPs were selected from the Perlegen2 database (10). The Sanger Mouse Genomes Project database provides a useful additional resource to select or verify candidate SNPs for genotyping (6).
Results and discussion
The generation of congenic mouse strains is an iterative process. The ability to detect unique meiotic recombination events producing a novel isolated genomic interval in individual mice in a timely and cost effective manner is essential for efficient progress. By definition, an average of 25 out of 100 pups are expected to contain a unique crossover event within a 25 cM locus. Therefore, as targeted intervals decrease in size, the number of expected new recombinants also decreases, leading to diminishing returns and increasing colony management costs. Theoretical calculations indicate that to narrow a single 25 cM locus to 5 cM by breeding of Interval Specific Congenic Lines requires approximately 300 individuals, while further reducing each locus from 5 cM to 1 cM requires approximately 380 additional individuals per regulatory gene (11). For the complex Bbaa2 locus, with multiple putative regulators within a 20+ cM interval, this predicts that approximately 1820 individuals, the vast majority of which will be non-informative, will be required to achieve our goal of 1 cM resolution for each putative locus.
Congenic breeders and their young pups can be maintained in a single cage, but are generally separated into 3 or more cages at weaning age. Since so many pups are non-informative as congenic intervals become narrow, rapid genotyping that allows efficient culling prior to weaning age may therefore provide a multiplier effect on cage cost savings, depending on the unique characteristics of individual colonies and institutional protocols. This process greatly benefits from the flexibility afforded by a high-throughput genotyping assay that can be rapidly and inexpensively performed on either small or large numbers of samples and can be targeted specifically to regions of interest within a sub-interval.
We previously relied on a panel of 11 microsatellite markers, some of which were poorly spaced or tightly clustered together, to genotype Bbaa2 recombinants. To improve upon this, several methods of SNP genotyping were evaluated.
To ensure backward compatibility with previous microsatellite-based congenic genotyping data, SNP genotyping assays were first designed to approximate the positions of microsatellite markers in the Bbaa2 interval. Additional assays were then designed to further improve the distribution of landmarks across the interval.
All three tested SNP genotyping methodologies produced interpretable assays. However, ARMS-PCR (7) was not adopted for routine genotyping due to concerns about assay design and optimization, throughput, and the added risk of amplicon contamination during the obligatory handling and gel electrophoresis of PCR products. Another commonly used PCR-based SNP genotyping method, Restriction Fragment Length Polymorphism (RFLP), relies on incubation of the PCR amplicon with restriction endonucleases, followed by gel electrophoresis. These steps require additional reagents and equipment, and significantly increase assay turnaround time, which dramatically reduces throughput. Only a limited sub-set of SNPs are amenable to RFLP, and if RFLP-SNP mining is limited to commonly used, cost-effective restriction endonucleases that number is reduced even further. For the 22 Mb Bbaa2 interval, only 538 such RFLP amenable SNPs were identified by SNP2RFLP (12) from over 19,000 high quality SNPs. Methodologically reducing the available SNPs in this way by more than 35-fold may severely impair fine mapping of single genes or SNPs within specific congenic lines, especially in low-SNP areas of the genome. Like ARMS-PCR, these manipulations also require an open-tube assay that may increase the risk of amplicon contamination of laboratory space or equipment if samples are not handled appropriately.
Small Amplicon HRMA (8) was found to be a reliable and efficient methodology. Nine out of eleven Small Amplicon SNP assays (81%) produced interpretable results using standard reaction conditions. Heterozygous genotypes were readily apparent, due to the presence of a definitive heteroduplex melting peak. However, when scaled up to evaluate many unknown samples in parallel, inter-sample melting peak variability was observed, making it difficult to objectively assign a genotype to some individual homozygous samples. In all such cases, an objective genotype assignment could be made by repeating the HRMA analysis after spiking the unknown sample with known B6 or C3H control DNA and looking for the presence of a heteroduplex melting peak.
Blocked Probe HRMA (9) produced interpretable results under standard conditions in twelve out of sixteen assays (75%) as originally designed. Even when evaluating many unknown samples in parallel, interpretation of working blocked probe sets was objective and unequivocal. Eleven out of 17 additional blocked probe assays designed across the region also produced interpretable assays, including three out of four designed as nearby replacements for non-working assays from the first round.
Both Small Amplicon and Blocked Probe HRMA were very rapid and efficient assays, generating SNP genotyping data in a 96-well format within 120 min. For very large scale applications, is unclear whether the observed difference in assay design success rates (81% vs 75%) would strongly influence assay selection, or if other concerns would take precedence. Blocked Probe HRMA offers the additional benefit over Small Amplicon HRMA of theoretically greater specificity due to the addition of a third sequence-specific oligonucleotide as a probe, as well as dramatically better separation between diagnostic peaks so that spiking and HRMA repetition was not necessary. For these reasons we have adopted Blocked Probe HRMA as our preferred methodology for high-throughput SNP genotyping.
The estimated time, reagent cost, and utility of the ARMS-PCR, Small Amplicon, Blocked Probe, and RFLP methods based on uniform manufacturer recommended 50 μL PCR cycling volumes are compared in Table 1.
Table 1.
PCR-Based SNP Genotyping Assay Comparison
| Estimated Reagent Cost and Throughput (per 96-well plate) | |||||
|---|---|---|---|---|---|
| ARMS-PCR | Small Amplicon | Blocked Probe | RFLP | ||
| Reagents ($) | |||||
| Taq / dNTP | 100 | 100 | 100 | 100 | |
| Primers / Probe | 2 | 1 | 2 | 1 | |
| Analysis | 2 | 10 | 10 | 22 | |
| Total Cost | 104 | 111 | 112 | 123 | |
| Time (Minutes) | |||||
| PCR Setup | 30 | 30 | 30 | 30 | |
| Cycling | 90 | 75 | 75 | 90 | |
| Handling | 30 | 0 | 0 | 45 | |
| Endonuclease | 0 | 0 | 0 | 60 | |
| Electrophoresis | 60 | 0 | 0 | 60 | |
| Analysis | 15 | 15 | 15 | 15 | |
| Total Time | 225 | 120 | 120 | 300 | |
| Bbaa2 Genotyping (26 positions, 96-well plates, run back-to-back) | |||||
| ARMS-PCR | Small Amplicon | Blocked Probe | RFLP | ||
| 9 Unknowns + 3 Control Samples | |||||
| Total Cost ($) | 340 | 360 | 365 | 400 | |
| Total Time (Hrs) | 15 | 8 | 8 | 20 | |
| 21 Unknowns + 3 Control Samples | |||||
| Total Cost ($) | 675 | 720 | 730 | 800 | |
| Total Time (Hrs) | 27 | 14 | 14 | 35 | |
| Assay Design / Utility | |||||
| ARMS-PCR | Small Amplicon | Blocked Probe | RFLP | ||
| Success Rate | 10% (1/10) | 81% (9/11) | 75% (12/16) | Not Tested | |
| Interpretation | Unequivocal | Subjective* | Unequivocal | Unequivocal | |
An objective genotype assignment can be made by spiking unclear samples with control DNA and repeating the HRMA
All Bbaa2 HRMA genotyping assays have been successfully scaled down to 10 μL reaction volumes in 96-well plates while retaining diagnostic integrity, further reducing reagent costs. Rapid turnaround times have enabled complete genotyping of the Bbaa2 interval or appropriate sub-interval in all congenic offspring prior to weaning age, also reducing cage costs. This has allowed resources to be better focused on expanding breeding cages to accelerate the generation of new recombinants and on maintenance of novel sub-interval congenic lines.
The use of SNP genotyping by HRMA is becoming increasingly accessible as equipment capable of performing HRMA becomes more common in research institutions around the world. A search of the PubMed database in October 2011 identified over 240 publications using High Resolution Melting in the past year alone, primarily relating to human diagnostics. For this purpose, the SNPs of interest are pre-defined due to their established linkage to or causation of human disease. While we have found that a large majority of SNPs can be genotyped in this way, not every such SNP is amenable to HRMA genotyping, depending upon the unique characteristics and complexity of surrounding DNA sequence. However, for the purposes of mapping inbred congenic mouse strains, the genomic location of a SNP rather than its potential biological function is the primary consideration, allowing flexibility for several different SNPs to be evaluated in a given area. Based on the Perlegen high-density resequencing project, SNPs differing between any two pairwise combinations of 12 classic inbred strains occur with an average frequency ranging from 1 in 440 bp to 1 in 21,000 bp in high-SNP or low-SNP regions of the mouse genome, respectively (13). Even in low frequency regions, this represents a potential resolution for congenic mapping on the order of 0.01 – 0.10 cM.
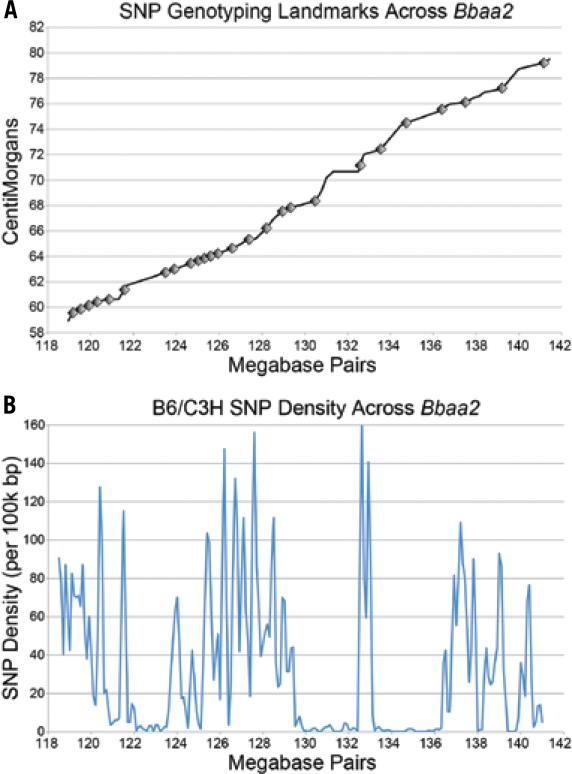
The Bbaa2 region of mouse chromosome 5 is complex. It exhibits a nonlinear relationship between basepair position and centiMorgan distance (Figure 1A), suggesting that meiotic recombination does not occur with uniform frequency across the interval. It also exhibits a highly variable SNP density, with some areas highly conserved between our parental B6 and C3H inbred strains of interest (Figure 1B). These characteristics impact the capacity to generate congenic animals and then to identify where recombination has occurred. Despite this complexity, diagnostic SNP genotyping assays were successfully designed at regular intervals. A total of 26 SNP genotyping assays across the Bbaa2 region are now used for routine genotyping of backcross progeny in our congenic colony. By adopting SNP genotyping methods, all previously used microsatellite markers have been replaced with approximately equivalent high-throughput SNP assays, and 15 additional landmarks have been added. As shown in Table 2, the average and median resolutions between genotyping landmarks across the Bbaa2 interval have improved from 2.20 Mbp and 1.56 Mbp to 0.88 Mbp and 0.75 Mbp, respectively. The ability to readily design new genotyping assays targeted to intervals containing novel breakpoints in individual mice contributes to a harmonious and efficient refinement process. In many cases, the prevalence of discernable SNPs will provide great enough resolution to exclude single genes, or even single SNPs within genes, from a congenic interval. This is especially important when attempting to precisely define the boundaries of similar or overlapping recombinant congenic lines. The use of HRMA based SNP genotyping may also be of special interest to anyone working with Recombinant Inbred (RI) lines such as those produced by the Collaborative Cross (14), or performing iterative backcrossing, such as during the transfer of a targeted gene knockout to a specific genetic background. A basic SNP genotyping panel for a broad interval surrounding the gene of interest may help speed up the backcrossing process by quickly identifying which individuals within litters inherited the narrowest surrounding interval, and would therefore best serve as breeders for successive backcrosses.
Figure 1. Distribution of SNP Genotyping Assays and SNP Density Across Bbaa2.
(A) Microsatellites from the MGD database (http://www.informatics.jax.org/) with assigned cM values were plotted against their basepair position to obtain a linear plot of cM versus genomic location (Black Line). Markers indicate the positions of SNP Genotyping Assays developed across the interval. (B) SNP Density across Bbaa2 was calculated from all dissimilar B6/C3H SNPs present in the MGD database.
Table 2.
Comparison of Discernable Genetic Landmarks in Bbaa2
| Bbaa2 Microsatellite Markers | Bbaa2 SNP Genotyping Assays | ||
|---|---|---|---|
| bp | Δ bp | bp | Δ bp |
| 119,175,776 | N.A. | 119,172,092 | N.A. |
| 119,539,274 | 367,182 | ||
| 119,914,546 | 375,272 | ||
| 120,256,981 | 1,081,205 | 120,298,004 | 383,458 |
| 120,858,582 | 560,578 | ||
| 121,575,713 | 717,131 | ||
| 123,489,225 | 1,913,512 | ||
| 123,905,461 | 416,236 | ||
| 124,668,171 | 762,710 | ||
| 125,034,265 | 366,094 | ||
| 125,309,605 | 5,052,624 | 125,316,596 | 282,331 |
| 125,581,535 | 271,930 | 125,602,080 | 285,484 |
| 125,931,691 | 350,156 | 125,940,137 | 338,057 |
| 126,607,294 | 667,157 | ||
| 127,404,260 | 1,472,569 | 127,391,403 | 784,109 |
| 128,208,712 | 817,309 | ||
| 128,954,717 | 746,005 | ||
| 129,042,314 | 1,638,054 | 129,338,863 | 384,146 |
| 130,476,763 | 1,137,900 | ||
| 132,603,109 | 3,560,795 | 132,593,675 | 2,116,912 |
| 133,538,654 | 944,979 | ||
| 134,737,636 | 1,198,982 | ||
| 136,395,980 | 3,792,871 | 136,401,340 | 1,663,704 |
| 137,534,245 | 1,138,265 | 137,497,474 | 1,096,134 |
| 139,207,488 | 1,710,014 | ||
| 141,162,992 | 3,628,747 | 141,158,294 | 1,950,806 |
| Average | 2,198,722 | Average | 879,448 |
| Median | 1,555,312 | Median | 746,005 |
| StDev | 1,664,367 | StDev | 576,607 |
| Max | 5,052,624 | Max | 2,116,912 |
| Min | 271,930 | Min | 282,331 |
Distribution of previously used microsatellite markers and newly mapped SNP genotyping landmarks on mouse chromosome 5.
Our adoption of HRMA-based SNP genotyping has facilitated the generation, identification, and more precise discernment of 20 novel ISCLs carrying unique subintervals of the Bbaa2 locus, as shown in Table 3. Many of these newly defined advanced congenics coincide with regions previously predicted to encode loci with positive and negative effects on Lyme arthritis severity, thereby establishing tools for the formal analysis of regulatory regions on chromosome 5. Current congenic lines allow the pairwise interrogation of intervals as small as approximately 0.6 Mbp, which will greatly facilitate efforts to identify causal genes in the region, and may be of general interest for the investigation of any phenotypic differences assigned to this region of mouse chromosome 5.
Table 3.
Development of Advanced Bbaa2 Sub-Interval Congenic Lines
| Chr5 Position (Mb) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 119.9 | 120.3 | 120.9 | 121.6 | 123.5 | 123.9 | 124.7 | 125.0 | 125.3 | 125.6 | 125.9 | 126.6 | 127.4 | 128.2 | 129.0 | 129.3 | 130.5 | 132.6 | 133.5 | 134.7 | 136.4 | 137.5 | 139.2 | 141.2 |
| B | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C |
| B | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | B | B | B |
| B | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | B | B | B | B |
| B | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | B | B | B | B | B |
| B | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | B | B | B | B | B | B | B |
| B | C | C | C | C | C | C | C | C | C | C | C | C | C | B | B | B | B | B | B | B | B | B | B |
| B | C | C | C | C | C | C | C | C | C | C | C | B | B | B | B | B | B | B | B | B | B | B | B |
| B | C | C | C | C | C | C | C | C | C | B | B | B | B | B | B | B | B | B | B | B | B | B | B |
| B | C | C | C | C | C | C | C | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B |
| B | C | C | C | C | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B |
| B | C | C | C | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B |
| B | B | C | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B |
| B | B | B | B | B | B | B | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C |
| B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | C | C | C | C | C | C | C |
| B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | C | C | C | C | C |
| B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | C | C | C | C |
| B | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | B | B | B | B | B | B | B |
| B | B | B | B | B | B | B | B | C | C | C | C | C | C | B | B | B | B | B | B | B | B | B | B |
| B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | C | C | B | B | B | B | B |
| B | B | B | B | B | B | B | B | B | B | B | B | B | B | C | C | C | B | B | B | B | B | B | B |
| B6C3F1 × C3H/HeN BC1 | |||||||||||||||||||||||
| B6C3F1 × C57BL/6 BC1 | |||||||||||||||||||||||
| B6C3F2 Intercross | |||||||||||||||||||||||
Rows - Individual B6.C3-Bbaa2 congenic lines. Columns - SNP assay positions. Genotypes designated: C – C3H Homozygote, B – B6 Homozygote.
The bottom three rows indicate the positions of regulatory loci previously predicted for each B6C3F1 backcross and for the B6C3F2 intercross. (3)
Supplementary Material
Acknowledgments
We would like to acknowledge the helpful advice and expertise provided by Dr. Carl Wittwer and Luming Zhou during our implementation of High Resolution Melting Analysis assays. Heydon Kaddas provided helpful assistance with the design and testing of Bbaa2 SNP genotyping primer sets. The project described was supported by Award Number T32AI055434 (KCB), AI-24158 & AI-088451(JHW), AI32223 (JJW) from the National Institute Of Allergy And Infectious Diseases and AR-43521 (CT & JJW) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The DNA/Peptide Core is supported in part by the Cancer Center Support Grant P30 CA04201. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This paper is subject to the NIH Public Access Policy.
Footnotes
Supplementary material for this article is available at www.BioTechniques.com/article/[last 6 digits of DOI no.].
Competing interests
The authors declare no competing interests.
References
- 1.Crick F. Central dogma of molecular biology. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 2.Weis JJ, McCracken BA, Ma Y, Fairbairn D, Roper RJ, Morrison TB, Weis JH, Zachary JF, et al. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 1999;162:948–956. [PubMed] [Google Scholar]
- 3.Roper RJ, Weis JJ, McCracken BA, Green CB, Ma Y, Weber KS, Fairbairn D, Butterfield RJ, et al. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2001;2:388–397. doi: 10.1038/sj.gene.6363801. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, Miller JC, Crandall H, Larsen ET, Dunn DM, Weiss RB, Subramanian M, Weis JH, et al. Interval-specific congenic lines reveal quantitative trait Loci with penetrant lyme arthritis phenotypes on chromosomes 5, 11, and 12. Infect. Immun. 2009;77:3302–3311. doi: 10.1128/IAI.00396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smigielski EM, Sirotkin K, Ward M, Sherry ST. dbSNP: a database of single nucleotide polymorphisms. Nucleic Acids Res. 2000;28:352–355. doi: 10.1093/nar/28.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29:E88–8. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew M, Pryor R, Palais R, Meadows C, Erali M, Lyon E, Wittwer C. Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clin. Chem. 2004;50:1156–1164. doi: 10.1373/clinchem.2004.032136. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Myers AN, Vandersteen JG, Wang L, Wittwer CT. Closed-tube genotyping with unlabeled oligonucleotide probes and a saturating DNA dye. Clin. Chem. 2004;50:1328–1335. doi: 10.1373/clinchem.2004.034322. [DOI] [PubMed] [Google Scholar]
- 10.Cox D, Frazer KA. SNP data, 8.2+ million locations for 16 inbred strains of mice. MPD:Perlegen2. The Jackson Laboratory; Bar Harbor, Maine USA: 2011. Mouse Phenome Database web site http://phenome.jax.org. [Google Scholar]
- 11.Darvasi A. Experimental strategies for the genetic dissection of complex traits in animal models. Nat. Genet. 1998;18:19–24. doi: 10.1038/ng0198-19. [DOI] [PubMed] [Google Scholar]
- 12.Beckstead WA, Bjork BC, Stottmann RW, Sunyaev S, Beier DR. SNP2RFLP: a computational tool to facilitate genetic mapping using benchtop analysis of SNPs. Mamm. Genome. 2008;19:687–690. doi: 10.1007/s00335-008-9149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- 14.Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J, Beavis WD, Belknap JK, et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 2004;36:1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.