Abstract
Background:
Clausine B, a carbazole alkaloid isolated from the stem bark of Clausena excavata, was investigated for its antiproliferative activities against five human cancer cell lines: HepG2 (hepatic cancer), MCF-7 (hormone-dependent breast cancer), MDA-MB-231 (non-hormone-dependent breast cancer), HeLa (cervical cancer), and CAOV3 (ovarian cancer).
Methods:
Chang liver (normal cells) was used as a control. The effect of clausine-B was measured using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.
Results:
Clausine-B was found to be active (IC50<30 μg/mL) against four of the cancer cell lines tested. The IC50 values for these four lines were: 21.50 μg/mL (MDA-MB-231), 22.90 g/ml (HeLa), 27.00 μg/mL (CAOV3) and 28.94 μg/mL (HepG2). Clausine-B inhibited the MCF-7 cancer cell line at 52.90 μg/mL, and no IC50 value was obtained against Chang liver.
Conclusion:
It is possible that the phenolic group in clausine-B responsible for the antiproliferative activities found in this study.
Keywords: Clausine-B, Clausena excavata, ethnopharmacology, cell survival, medical sciences
Introduction
Natural sources of foods that are believed to have a high antiproliferative activity on tumour cells have become an important element of cancer prevention and treatment strategies. For this reason, the potential of naturally-occurring dietary substances to prevent disease has become an important area of scientific interest. Several years ago, researchers began to consider whether a drug-resistant cell line could be used to screen samples for such agents and, in particular, to evaluate whether non-toxic extracts and compounds could be found that would possess this selective activity. Furthermore, with a number of compounds available, it was also regarded as an opportunity to evaluate a broad range of natural product structure types (1). Plants have many phytochemicals that possess various bioactivities, including antioxidant and anticancer properties. For example, some studies have reported that extracts from natural products, such as fruits, vegetables and medicinal herbs, have positive effects against cancer that are comparable to those of chemotherapy or recent hormonal treatments (2–3). Many plants have therefore been examined in an attempt to identify new and effective antioxidant and anticancer compounds (4–6).
Clausena excavata is commonly known as Chemama among Malays. This species is also known as Kemantu hitam, Pokok cherek (diarrhoea tree), Cherek hitam and Secherek in Malaysia; Tikusan and Bagal tikus (in Java); Bajetah and Ki bechetah (in Sundanese); Sicherek (in Sumatra); and as Fia fan (in Thailand) (7). It is a strong-smelling shrub, found from the Himalayas and China to (and throughout) Malaysia, especially in the Peninsula. The leaflets have a characteristic curry-like smell when crushed. The decoction of the roots can be used for bowel-complaints, chiefly colic. The pounded root and leaves are used as a poultice for sores, including ulceration of the nose. It has been recorded that pounded leaves have been applied to the head for headaches. Ulceration of the nose may be treated by fumigation from burning the leaves and bark (8). The flowers and leaves may be boiled and the decoction taken for colic; and a decoction of leaves has been given after childbirth. The juice of the plant was used in Java for coughs and as a vermifuge and cold treatment (9). The leaves of this plant are used as a traditional medicine to cure colds, abdominal pain, malaria and dysentery (10). The principal active constituents of this plant are carbazole alkaloids and coumarins (11–17). Some coumarins that have been isolated from the leaves have been found to inhibit tumour promotion (17).
Limited data is available on the antiproliferative properties of clausine-B, one of the carbazole alkaloids that have been isolated from the stem bark of Clausena excavata. Therefore, we developed this study to determine whether clausine-B has antiproliferative activity against several human cancer cell lines, including breast and liver.
Materials and Methods
Human cancer cell lines and reagents
HepG2 (hepatic cancer), MCF-7 (hormone-dependent breast cancer), MDA-MB-231 (non-hormone-dependent breast cancer), HeLa (cervical cancer), CAOV3 (ovarian cancer) and Chang (normal liver) cell lines were obtained from the American Type Culture Collection (ATCC), USA. The normal cell line, Chang, was used for comparison. The growth media: RPMI-Dulbecco’s Modified Eagle Medium (DMEM) with 1640, Minimum Essential Medium (MEM) and high glucose and low glucose concentrations, and the phosphate buffer solution (PBS) tablets were obtained from Sigma Chemical Co., St Louis, USA.
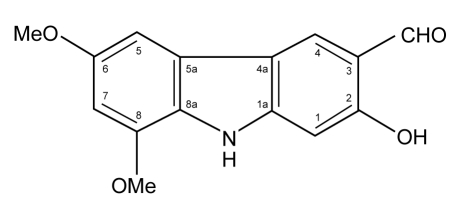
Foetal calf serum, penicillin-streptomycin and trypsin were from PAA Laboratories, GmbH, Austria. The MTT kit, which consists of the MTT labelling reagent and the solubilisation solution, was supplied by Roche Diagnostics GmbH, USA. Clausine-B (Figure 1), a carbazole alkaloid isolated from the stem bark of C. excavata, was obtained from Professor Taufiq Yap Yun Hin, from the Department of Chemistry, Universiti Putra Malaysia. It was dissolved (1 mg/mL) in dimethyl sulphoxide (DMSO) (Sigma Chemical Co).
Figure 1:
The chemical structure of clausine-B
Cell culture
HepG2, MCF-7 and HeLa were grown in RPMI-1640. MDA-MB-231 was grown in DMEM with low glucose, and CAOV3 was grown in DMEM with high glucose. MEM was used to grow Chang. The cells were cultured in the appropriate medium, supplemented with 5–10% foetal calf serum and 1% penicillin-streptomycin, using 25 cm2 flasks in a 37°C incubator with 5% CO2.
Cell subculture
To subculture the cells, the cells were divided and the culture medium was replaced with fresh medium as follows: First, the old medium was removed, and then the cells were rinsed briefly with PBS to wash the cells. 1–2 mL of trypsin was then added, and the flask was incubated at 37°C and 5% CO2 for 5 minutes. After the cells had detached from the lower part of the flask, 20 mL of medium was added to the flask and the culture was divided in two parts. One part was then transferred to a new flask.
Cellular behaviour
The cells were grown until they were confluent. Then, the cells were trypsinised and the number of viable cells was counted with a hemocytometer to prepare a cell suspension. One hundred microlitres of suspension containing 1 x 105 cells was seeded in each well of a 96-well microtiter plate (Nunc, Denmark). The plate was then incubated overnight at 37°C with 5% CO2. The following day, the medium was replaced (18).
Cell proliferation assay
Each cancer cell line was grown in a 96-well microtiter plate (Nunc, Denmark) in a final volume of 100 μL culture medium per well. The cells were then treated with clausine-B at a dose of 10, 20, 30, 40, 60, 80 or 100 μg/mL and maintained at 37°C with CO2 for 24–72 hours. After the incubation period, 10 μL of MTT labelling reagent (Roche Diagnostics, USA) was added to each well. The microtiter plate was then incubated again for 4 hours at 37°C with 5% CO2. Then, 100 μL of the solubilisation solution (Roche Diagnostics, USA) was added into each well. The plate was allowed to stand overnight in the incubator at 37°C and 5% CO2. The cell viability was measured using an ELISA reader (ELx 800) at 550 nm. The experiment was carried out in six replicates.
Measurement of the growth inhibitory effect
Cells were grown in 6-well plates (Nunc, Denmark) in a final volume of 1 mL of culture medium per well. Each well contained 1 x 105 cell/mL and was incubated for 24 hours in a 5% CO2 incubator at 37°C. The method used for cell culture was similar to that described above. The HeLa cell line was used as it has the lowest IC50 value (the concentration of sample that can inhibit 50% of the cancer cells from proliferating) for clausine-B, which had been screened using the MTT assay. The cells were treated with the sample at a concentration that was similar to the IC50 value. The plate was then incubated again in the 5% CO2 incubator at 37°C for 24, 48 and 72 hours. The untreated cells (control) were also incubated for 24, 48 and 72 hours. The growth of the cells was photographed using a phase contrast microscope (Olympus, USA).
Results
Cell proliferation assay
Table 1 presents the IC50 values of clausine-B. Clausine-B was found to inhibit 50% of the proliferation of HeLa cancer cells at 22.90 μg/mL, MDA-MB-231 at 21.50 μg/mL, CAOV3 at 27.00 μg/mL and HepG2 at 28.94 μg/mL. Clausine-B inhibited the MCF-7 cancer cell line at 52.90 μg/mL. There was no IC50 value obtained from clausine-B against the Chang liver cell line.
Table 1:
The IC50 values of clausine-B
| Cancer cell lines | IC50(μg/mL)a |
|---|---|
| HeLa | 22.90 ± 0.45 |
| MCF-7 | 52.90 ± 8.49 |
| HepG-2 | 28.94 ± 0.00 |
| MDA-MB-231 | 21.50 ± 0.04 |
| CAOV3 | 27.00 ± 0.29 |
| Chang | None |
Values are expressed as the mean ± standard deviation of six replicate measurements. The IC50 value is defined as the concentration of sample necessary to inhibit 50% of the cancer cells from proliferating.
Growth inhibitory effect
The ability of clausine-B to inhibit proliferation of the HeLa cell line at 22.90 μg/mL was estimated by analysing its effect on the growth of the cells (Figure 2). The growth of the untreated (control) and treated HeLa cell line after incubation for 24, 48 and 72 hours was photographed using a phase contrast microscope. Figure 2 (A, C and E) shows the untreated cells after 24 to 72 hours incubation. It can be seen that the cells are growing normally and that they are attached to each other. The observation of HeLa cells showed that after 24 hours of treatment, most of the cells were attached in the culture containing 22.90 μg/mL of clausine-B (Figure 2 B). They were viable and adhered to the lower surface of the well after 48 hours of treatment, although a large proportion of the cells were poorly attached (Figure 2 D). However, all of the cells were shrunken and detached after 72 hours of incubation (Figure 2 F).
Figure 2:
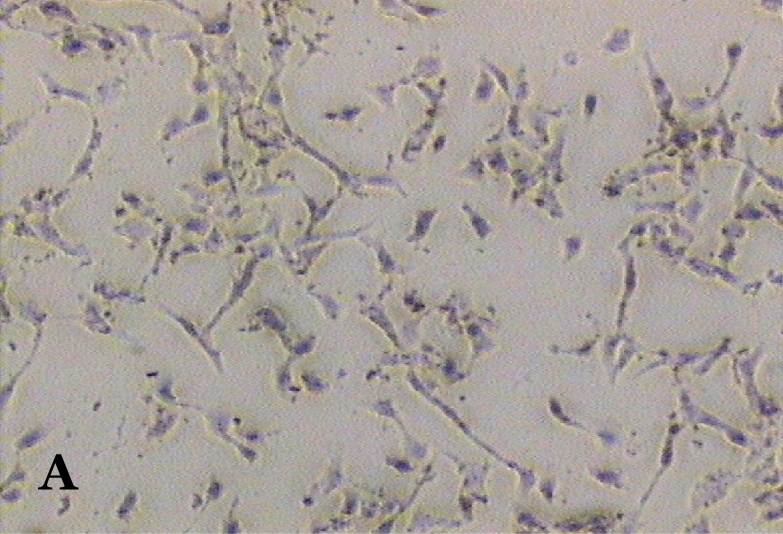
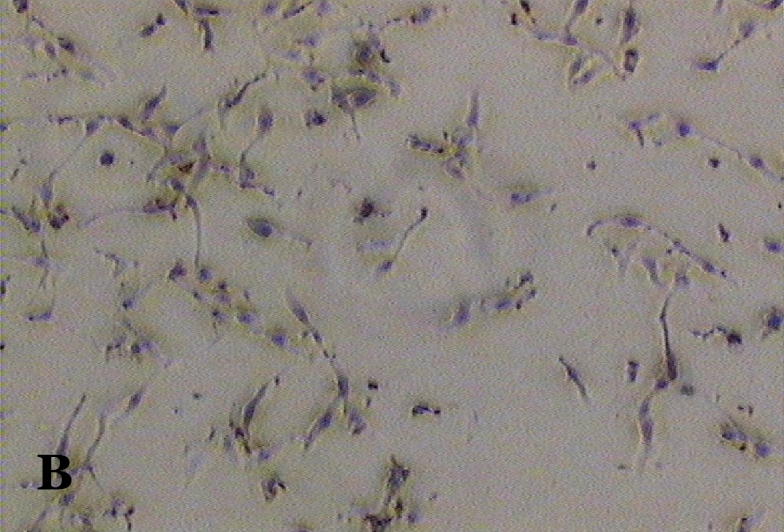
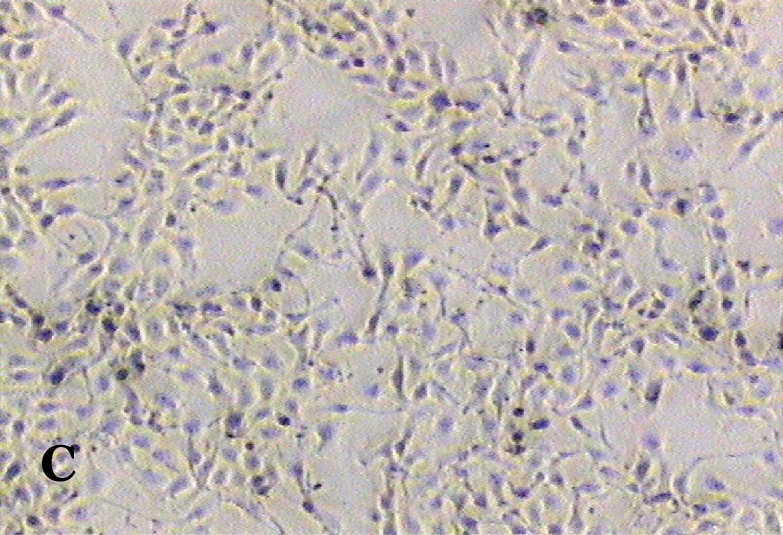
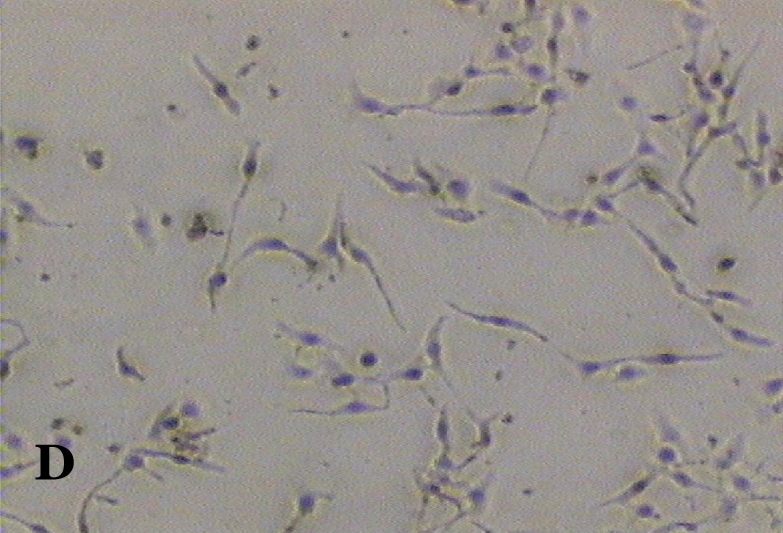
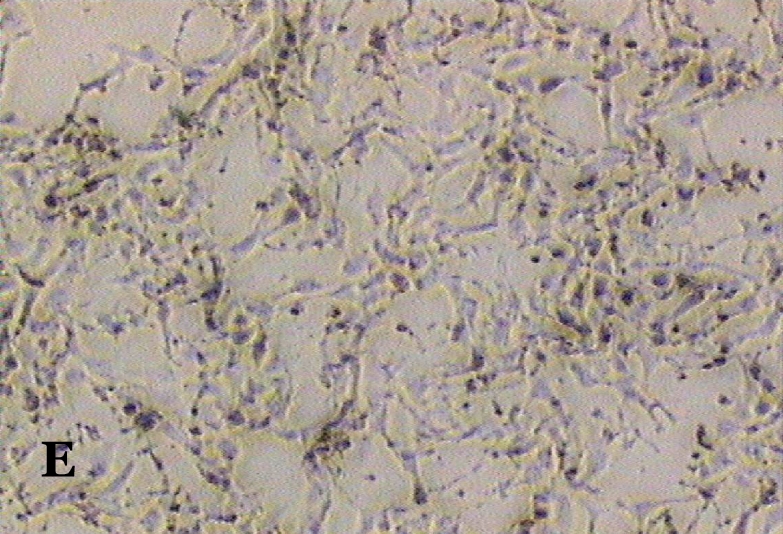
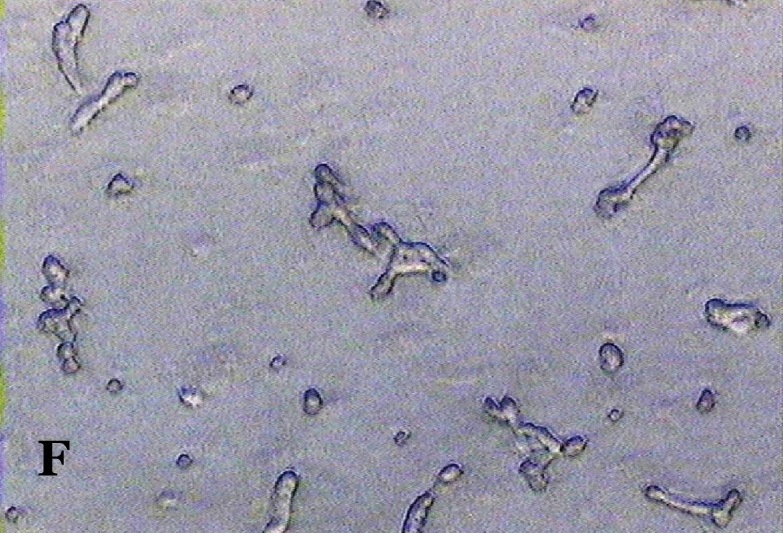
Phase contrast micrographs (x4 magnification) of the antiproliferative activity of clausine-B on the HeLa cell line after 24, 48 and 72 hours of incubation (IC50 = 22.90 μg/ml). A. The untreated HeLa cell line (control) after 24 hours incubation. B. The treated HeLa cell line after 24 hours incubation. C. The untreated HeLa cell line (control) after 48 hours incubation. D. The treated HeLa cell line after 48 hours incubation. E. The untreated HeLa cell line (control) after 72 hours incubation. F. The treated HeLa cell line after 72 hours incubation.
Discussion
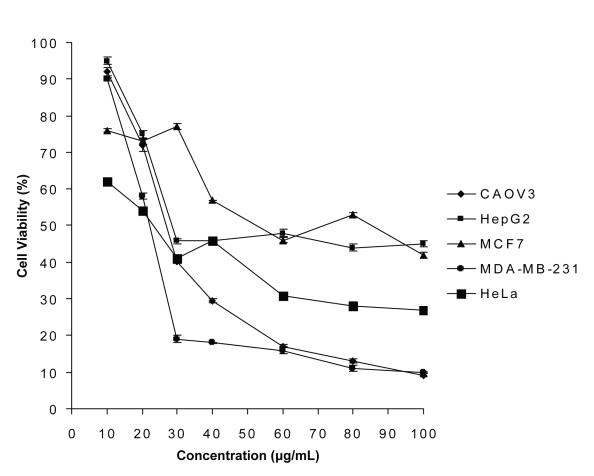
The Micro-culture Tetrazolium Salt (MTT) assay was used in this study to measure the amount of cell viability. The effect of clausine-B on the proliferation of human cancer cells was determined. The percentage of cell viability was measured by comparing the optical density (OD) against the control. The antiproliferative activity of clausine-B is presented as a percentage of the cell viability versus concentration (Figure 3). Cells grown in a 96-well tissue culture plate were incubated with the yellow MTT solution for approximately 4 hours. After this incubation period, purple formazan salt crystals were formed. These salt crystals are insoluble in aqueous solution, but may be solubilised by adding the solubilisation solution and incubating the plates overnight in a humidified atmosphere (37°C, 5% CO2). The solubilised formazan product was quantified spectrophotometrically using an ELISA reader. An increase in the number of living cells resulted in an increase in the total metabolic activity in the clausine-B sample. This increase directly correlated with the amount of the purple formazan crystals formed, as monitored by the absorbance.
Figure 3:
Antiproliferative activities of clausine-B on cancer cell lines. Values are expressed as the mean ± standard deviation of six replicate measurements.
Clausine-B, the pure compound isolated from C. excavata, causes 50% cell death in all human cancer cell lines tested (MCF-7, MDA-MB-231, HepG-2, HeLa and CAOV3). The best IC50 values obtained were 21.50 and 22.90 μg/mL, causing 50% of the MDA-MB-231 and HeLa cancer cell lines to die, respectively. In this study, clausine-B has been proven to inhibit the proliferation of four cancer cell lines out of five that were tested at a strong IC50 value (<30 μg/mL). Clausine-B was not active (IC50>30 μg/ml) against MCF-7. It was shown that clausine-B exhibited no effect on Chang, the normal cell line that was used as a comparison.
Phenolic compounds such as furanocoumarins, flavonoids and carbazole alkaloids are the main constituents of C. excavata (3,17). These phenolic compounds can stimulate or suppress the immune system due to the presence of hydroxyl groups (19). Therefore, the presence of the phenolic group may be assumed to be responsible for the antiproliferative activities of clausine-B that were found in this study. Several studies demonstrating the cytotoxic effect of carbazole alkaloids isolated from C. excavata have been reported previously (13,14,20). Clausine-B has also been found to show significant cytotoxicity against the CEM-SS cell line (21). However, limited data was obtained on the cytotoxic effects of clausine-B. Perhaps data from this research and previous studies will yield better insights into the cytotoxic properties of clausine-B.
The morphology of HeLa cancer cells that were treated with clausine-B at 22.90 μg/mL was studied to prove the effect of clausine-B on the cell growth. Our observations showed that the growth of the cells was inhibited. However, further study is needed to determine whether the inhibitory effect of clausine-B on the growth of the cells is due to the inhibition of the proliferation of the cells or the induction of cell death. Clausine-B proved to possess antiproliferative properties against all the cancer cell lines tested. Therefore, it may have potential as an anticancer agent. However, the mechanism behind the antiproliferative effects of clausine-B need to be studied to determine if the effect is due to an increase in apoptosis. Further investigations will yield clearer answers.
It has also been suggested that an in vivo study should be carried out in conjunction with the in vitro study, so that the results can be compared and more information regarding the antiproliferative properties of clausine-B will become clear. This is important, because there may be many factors that affect the antiproliferative activity in the in vivo study. Furthermore, in vitro studies may not produce the same results as in vivo experiments.
Acknowledgments
This project was financially supported by Universiti Putra Malaysia, Fundamental Research Grant – 55166.
Footnotes
Author’s contributions
Data collection, analysis and interpretation: WNIWMZ All authors have contributed equally to the conception and design, critical revision of the article for important intellectual content and final approval of the article.
References
- 1.Cordell GA, Beecher CW, Pezzuto JM. Can ethnopharmacology contribute to the development of new anticancer drugs? J Ethnopharmacol. 1998;32:117–133. doi: 10.1016/0378-8741(91)90110-y. [DOI] [PubMed] [Google Scholar]
- 2.Pezzuto JM. Plant-derived anticancer agents. Biochem Pharmacol. 1997;53:121–133. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Wu Y, Yang BB. Anticancer activity of Hemsleya amabilis extract. Life Sci. 2002;71:2161–2170. doi: 10.1016/s0024-3205(02)02013-1. [DOI] [PubMed] [Google Scholar]
- 4.Pietta P, Siimonetti P, Mauri P. Antioxidant activity of selected medicinal plants. J of Agri Food Chem. 1998;46:4487–4490. [Google Scholar]
- 5.Kim JH, Park MK, Lee JY, Okuda H, Kim S, Hwang WI. Antioxidant and antitumor effects of Manda. Biochem Arch. 1998;14:211–219. [Google Scholar]
- 6.Swamy SMK, Tan BKH. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol. 2000;70:1–7. doi: 10.1016/s0378-8741(98)00241-4. [DOI] [PubMed] [Google Scholar]
- 7.Soepadmo E. Conservation status of medicinal plants in Peninsular Malaysia; Proceedings of the Medicinal Products from Tropical Rain Forest Conference; 1991 May 13–15; FRIM, Kuala Lumpur. [Google Scholar]
- 8.Ridley HN. The Flora of the Malay Peninsula. Volume I. London: Reeve and Co. Ltd; 1967. [Google Scholar]
- 9.Heyne K. Tumbuhan berguna Indonesia III. Badan Lsitbang Kehutanan Jakarta. Jakarta: Yayasan Sarana Wana Jaya; 1927. p. 1754. [Google Scholar]
- 10.Wu TS, Shiow CH, Jeng SL, Che MT, Feng NK, Chang SK. Chemical and antiplatelet aggregative investigation of the leaves of Clausena excavata. Phytochemistry. 1992;32:449–451. [Google Scholar]
- 11.Wu TS, Furukawa H. Biological and phytochemical investigation of Clausena excavata. J Nat Prod. 1982;45:718–720. doi: 10.1021/np50024a013. [DOI] [PubMed] [Google Scholar]
- 12.Chihiro I, Hideki O, Hugh TW, Hiroshi F. Constituents of Clausena excavata: isolation and structural elucidation of seven new carbazole alkaloids and new coumarin. Chem Pharm Bull. 1996;44:2231–2235. [Google Scholar]
- 13.Wu TS, Huang SC, Wu PL, Teng CM. Carbazole alkaloids from Clausena excavata and their biological activity. Phytochemistry. 1996a;43:133–140. doi: 10.1016/0031-9422(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 14.Wu TS, Huang SC, Wu PL. Carbazole alkaloids from stem bark of Clausena excavata. Phytochemistry. 1996b;43:1427–1429. doi: 10.1016/0031-9422(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 15.Wu TS, Huang SC, Wu PL. Carbazole – pyranocoumarin dimmer and binary carbazole alkaloid from Clausena excavata. Tetrahedron Lett. 1996c;37:7819–7822. [Google Scholar]
- 16.Huang SC, Wu PL, Wu TS. Two coumarins from the root bark of Clausena excavata. Phytochemistry. 1997;44:179–181. [Google Scholar]
- 17.Chihiro I, Masataka I, Shinya K, Mitsuo O, Harukuni T, Hoyoku N, et al. Chemical constituents of Clausena excavata: isolation and structure elucidation of novel furanone-coumarins with inhibitory effects for tumorpromotion. J Nat Prod. 2000;63:1218–1224. doi: 10.1021/np990619i. [DOI] [PubMed] [Google Scholar]
- 18.Freshney RI. Culture of Animal Cells: A Manual of Basic Technique. 4th ed. New York: Wiley Liss; 2000. [Google Scholar]
- 19.Manosroi A, Saraphanchotiwitthaya A, Manosroi J. Immunomodulatory activities of Clausena excavata Burm. F. wood extracts. J Ethnopharmacol. 2003;89:155–160. doi: 10.1016/s0378-8741(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 20.Peh TH. Chemical constituents and biological activities of Clausena excavata and some citrus species (Rutaceae) Serdang, Selangor: Universiti Putra Malaysia; 2001. [Master’s Thesis] [Google Scholar]
- 21.Taufiq-Yap YH, Peh TH, Ee GCL, Rahmani M, Sukari MA, Ali AM, et al. A new cytotoxic carbazole alkaloid from Clausena excavata. Nat Prod Res. 2007;21:810–813. doi: 10.1080/14786410701258875. [DOI] [PubMed] [Google Scholar]