Abstract
Introduction
Many physicians are inadequately familiar with the clinical features of achalasia. Often, it is not diagnosed until years after the symptoms arise. This is unfortunate, because a delay in diagnosis worsens the prognosis.
Methods
Selective review of the literature.
Results
Achalasia has a lifetime prevalence of 1:10 000. It is a neurodegenerative disorder in which the neurons of the myenteric plexus are lost, leading to dysfunction of the lower esophageal sphincter and to a derangement of esophageal peristalsis. In the final stage of achalasia, esophageal motility is irreversibly impaired, and complications ensue because of the retention of food that is no longer transported into the stomach. Aspiration causes pulmonary disturbances in up to half of all patients with achalasia. There may also be inflammation of the esophageal mucosa (retention esophagitis); this, in turn, is a risk factor for esophageal cancer, which arises in 4% to 6% of patients. The cause of achalasia is not fully known, but autoimmune processes appear to be involved in patients with a genetic susceptibility to the disease.
Conclusion
Achalasia should be diagnosed as early as possible, so that complications can be prevented. In addition, guidelines should be established for cancer prevention in achalasia patients. Currently ongoing studies of the molecular causes of achalasia will probably help us understand its pathophysiology.
Primary, or idiopathic, achalasia mainly affects the lower esophageal sphincter (LES). Physiologically, the LES is contracted and prevents reflux of stomach acid into the esophagus. The LES relaxes and opens only on swallowing, so that food can enter the stomach. This is a muscle reflex that is coordinated with the peristalsis of the muscles of the esophagus.
In achalasia, relaxation of the LES gradually becomes more and more restricted. Due to persistent contraction, the contents of the esophagus cannot be transported into the stomach. They enter the stomach only when the hydrostatic pressure on the accumulated food exceeds the contraction pressure of the LES. In the final stage of achalasia, LES contraction can be so severe that very little food enters the stomach.
The persistent contraction of the LES is caused by degeneration of the neurons of the myenteric plexus (1), which controls the motility of the digestive tract. Achalasia is therefore considered to be a neurodegenerative disease. Degeneration mainly affects neurons that have an inhibitory effect on LES muscles and enable it to relax. However, loss of inhibitory innervation also affects the muscles of the adjacent distal esophagus, leading in turn to a derangement of esophageal peristalsis (1).
Although there have been various indications of the causes of neurodegeneration since achalasia was first described in 1674 by the English doctor Sir Thomas Willis, the etiology of achalasia remains poorly understood (2).
Epidemiologically, achalasia is a rare disease, with an incidence of 1:100 000 per year and a lifetime prevalence of 1:10 000 (3). However, achalasia is less well known by practicing physicians than other rare diseases of the gastrointestinal tract, because in early stages its clinical symptoms are mostly nonspecific. As a result, achalasia is not diagnosed until an average of five years after initial complaints begin (4, 5). This leads to an unnecessary loss of quality of life and to complications, such as megaesophagus and esophageal carcinoma.
This article therefore aims to increase awareness of achalasia. It describes the clinical features, diagnosis, and current conclusions of research on the etiology of the disease. It also provides a critical evaluation of the various treatment options.
Methods
The Medline database was used for a selective search of the literature. Various combinations of relevant keywords were used.
Clinical picture, stages of achalasia
The main symptom of achalasia is dysphagia, which develops slowly over several weeks to years and increases as the disease progresses (6). As a rule, dysphagia manifests initially with solid food and later also with liquid food (7). As the disease progresses, the increasing contraction of the LES on swallowing leads to regurgitation of food that has not entered the stomach and to retrosternal pain, particularly when there is extreme dilatation of the esophagus. Restricted transportation of food and avoidance of food lead to weight loss. This can occur swiftly (8) but typically takes place over a longer period lasting several months or years and rarely leads to a loss of more than 10% of bodyweight (9). Complications may be caused by food that remains in the esophagus and is not transported any further. Food entering the trachea causes bronchopulmonary infections and even aspiration pneumonia in 7% to 8% of patients (10). These are typically caused by nighttime regurgitations while lying down (6). Meanwhile, up to 50% of patients suffer from restricted pulmonary function resulting from chronic microaspirations (11). Chronic retention of food also leads to esophagitis as a result of mechanical and chemical irritation of the mucosa, with a risk of metaplasia and squamous-cell carcinoma (12– 14). This affects approximately 4% to 6% of all achalasia patients, leading to a risk of esophageal carcinoma approximately 30 times higher than in the general population (13, 15, 16). There is also an increased frequency of adenocarcinoma (14), although its pathophysiological relationship to achalasia is less well understood. However, to date there are no guidelines on cancer screening.
Different forms of achalasia
When achalasia is not a consequence of any other disease, it is called primary or idiopathic achalasia. Primary achalasia can occur either alone or together with other abnormalities. In the latter case, it is referred to as syndromic achalasia. Isolated forms represent the overwhelming majority of all cases of primary achalasia. The typical age of onset is between 25 and 60 years, although any age group can be affected (7). Achalasia in children and young adults is mostly isolated; however, syndromic achalasia typically occurs in this age group.
The most common achalasia syndrome is triple-A syndrome. This is an autosomal recessive disease characterized by the trio of achalasia, alacrimia, and Addison’s disease. In children, neurological and dermatological disorders are also observed, in addition to facial dysmorphias (17, 18). The gene responsible, AAAS, is located on chromosome 12q13 (19). Achalasia is also more frequent in patients with trisomy 21, or Down syndrome. Approximately 75% of all children with trisomy 21 present gastrointestinal disorders (20) and around 2% develop achalasia. This means that achalasia is approximately 200 times more frequent in patients with Down syndrome than in the overall population (21). This higher frequency may be caused by a gene dosage effect, for example, but it is unclear which or how many genes on chromosome 21 are involved. Achalasia is also seen in patients with other rare genetic diseases—such as familial visceral neuropathy and achalasia microcephaly syndrome (2)—but its molecular genetic cause is not yet clear.
Achalasia symptoms also occur in diseases that do not affect the myenteric plexus. These cases of secondary achalasia are usually caused by a narrowing at the gastroesophageal junction. They are frequently also described as “pseudoachalasia,” because they can often be clinically distinguished from primary achalasia. The most common cause of pseudoachalasia is esophageal and cardiac carcinoma (22, 23). This is distinguished by more acute dysphagia, faster weight loss, and other differences.
Diagnosis and differential diagnosis
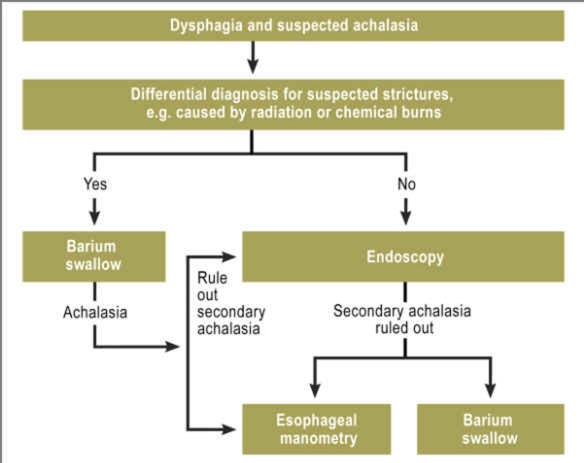
In cases of suspected achalasia, the diagnostic procedure is aimed mostly at the main symptom, dysphagia, and is shown in Figure 1.
Figure.
As a rule, endoscopy is the first step in diagnosing the cause of dysphagia. It can also be used to rule out secondary achalasia, e.g. inflammation, stenosis, or tumors, and to obtain tissue biopsies. However, if there are grounds in the patient’s medical history for suspecting a stricture, e.g. caused by radiation or chemical burns, a barium swallow and X-ray of the esophagus are recommended first, as endoscopy poses a higher risk of perforation. In such cases, endoscopy is performed only to rule out secondary achalasia. Once secondary achalasia has been ruled out endoscopically, an esophageal manometry and/or barium swallow and X-ray of the esophagus are performed
Esophagogastroscopy (if possible, in combination with endoscopic ultrasonography), together with barium swallow and manometry, is part of standard diagnostics for suspected achalasia. However, diagnosis can only be established endoscopically in approximately a third of all achalasia patients, and this depends on the stage the disease has reached (8). In these cases, resistance is typically encountered at the gastroesophageal junction but can still be overcome relatively easily by the endoscope. In advanced stages of achalasia, the esophagus is dilated and contains food (6). Endoscopic examination is particularly important in ruling out other possible causes of a patient’s complaints. These include esophageal and stomach tumors, strictures (caused by scar tissue or inflammation), and narrowing caused by aberrant blood vessel position (dysphagia lusoria), for example.
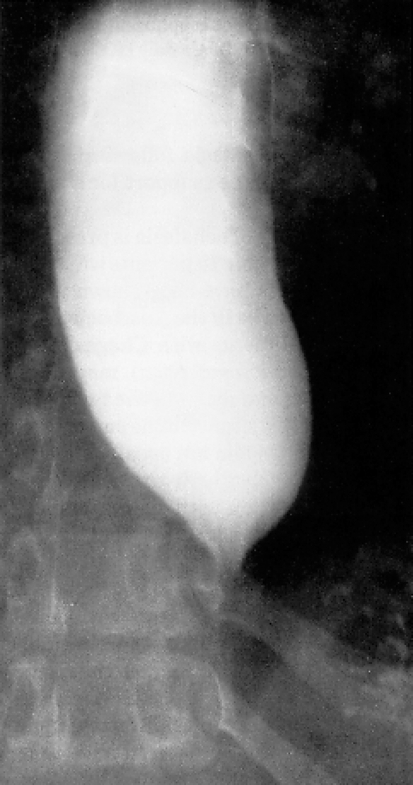
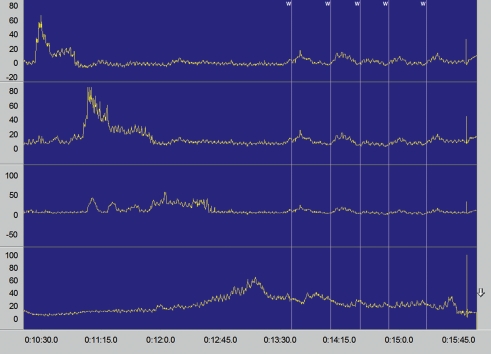
Depending on the stage of the disease, the esophagus appears dilated in a barium swallow, shows a loss of peristalsis in the distal portion, and shows a tapered, thread-like area of contrast substance absorption in the cardiac region, called a “bird’s beak” because of its shape (Figure 2). Although a barium swallow reveals abnormalities in almost every patient, they are often nonspecific, so achalasia is successfully diagnosed in only approximately two-thirds of cases (8). Although there are no studies that have directly compared the diagnostic predictive power of a barium swallow with that of esophageal manometry, the latter is the gold standard in achalasia diagnosis. It can successfully diagnose significantly over 90% of all cases (8). Depending on the stage of achalasia, patients present a derangement of esophageal peristalsis and incomplete relaxation of the LES on swallowing (Figure 3). Typically there is increased resting tone of the LES with simultaneous and therefore inefficient contractions of the tubular esophagus.
Figure 2.
Characteristic X-ray of achalasia patient following barium swallow, with bird’s beak. At an advanced stage of achalasia, esophageal dilatation can be seen even on a chest X-ray with no contrast
Figure 3.
Manometry results in achalasia: increased resting tone of LES with absence of relaxation on swallowing and simultaneous contractions of the tubular esophagus; “W” indicates a wet swallow. The manometry probe can measure the amplitude of esophageal contractions at three different locations in the tubular esophagus (top three sections); the bottom section provides a direct representation of the region of the lower esophageal sphincter (absence of relaxation). The y-axis shows the pressure in mm Hg, and the x-axis the time in second
Etiology and pathophysiology
Esophageal peristalsis with relaxation of the LES is controlled by the enteric nervous system (ENS). This is a complex network of nerves that surrounds the entire gastrointestinal tract and can be modulated by the CNS via the vagus nerve. The neurons of the ENS that control motility and peristalsis are located in the myenteric plexus, which lies between the circular and longitudinal layers of the smooth muscles. Two types of neurons can be distinguished: inhibitory neurons use mainly nitrous oxide (NO) and vasoactive intestinal peptide (VIP) as neurotransmitters and lead to muscle relaxation. Excitatory neurons, meanwhile, use acetylcholine (Ach) as a neurotransmitter and enable muscles to contract.
Histopathological and functional investigation indicates that achalasia is mainly caused by the degeneration of NO- and VIP-releasing inhibitory neurons (1, 24). This seems to affect the neurons of the myenteric plexus that innervate the LES and the distal esophagus selectively. Although this provides the first insight into the pathophysiology, the underlying causes of this “selective” neurodegeneration are poorly understood. It is thought that autoimmune processes in persons with genetic susceptibility are significant (2). Large clinical institutions and human genetics centers have therefore merged so that the underlying risk genes can be identified in the largest possible achalasia populations. This, for example, was the aim of the founding of the Achalasia Risk Consortium, arc (www.achalasie-konsortium.de [in German]), which includes Belgian and Italian institutions in addition to German institutions.
Treatment and prognosis
The aim of treatment is always to improve achalasia symptoms and esophageal clearance, and to prevent megaesophagus. Both endoscopic procedures (pneumatic balloon dilatation, Botox injection into the LES, stenting, peroral endoscopic myotomy [POEM]) and surgery (Heller laparoscopic myotomy) can be used for these purposes.
The endoscopic treatment that can best be assessed is pneumatic balloon dilatation. Complications include perforation (0% to 10%) and hemorrhaging (0.4%); balloon size, diverticulum, and torquing of the esophagus are risk factors for these (25). The long-term success rate (no complaints for more than 5 years) of a single pneumatic balloon dilatation is reported as 40% to 85% and patients under the age of 40 years have less favorable outcomes than patients over 40 years of age (e1, e2). Other unfavorable predictive factors for treatment success are high resting tone of the LES following the intervention, a need for multiple dilatations, and male sex (e3). However, it must be taken into account that all the reported studies were retrospective. In a prospective study by Eckardt et al. (e1), only 59% of patients were free of complaints following a single pneumatic balloon dilatation after one year, and only 26% after five years. According to other studies, multiple pneumatic balloon dilatations with increasing balloon diameter (graded dilatation) is more effective than single pneumatic balloon dilatation. In a prospective study by Vela et al. (e4), improvement in symptoms (dysphagia less than three times per week) following graded pneumatic balloon dilatation was observed in 90%, 82%, and 44% of cases after six months, two years, and six years respectively. In contrast, single pneumatic balloon dilatation yielded success rates of only 62%, 50%, and 28% after the same lengths of time (e4).
In contrast to endoscopic approaches, laparoscopic cardiomyotomy has a success rate of 90%, according to a meta-analysis by Campos et al. (e5) that included more than 100 publications. As cardiomyotomy also has a low complication rate (e5), it is often viewed as first-line therapy. A recent prospective, multicenter study comparing pneumatic balloon dilatation to Heller myotomy showed no significant differences between the two procedures (e6). Nevertheless, the results in favor of pneumatic balloon dilatation seem to be distorted, as some patients who underwent dilatation were excluded from the study due to esophageal perforations. In addition, the follow-up time of one or two years was relatively short.
A further endoscopic option to expand the LES is Botox injection, which inhibits the release of acetylcholine in the myenteric plexus. Although many prospective studies report swift improvement in symptoms in 65% to 90% of patients (e7, e8), complaints recurred after 3 to 12 months. Because its effect is so short-lived, Botox treatment is recommended only for patients with high comorbidity (e9). One promising new endoscopic procedure is peroral endoscopic myotomy (POEM), for which the short-term results available to date of a prospective study have shown improvement in symptoms (no complaints after three months) and a significant reduction in resting tone of the LES (e10). However, this is a new treatment, which means that as yet there are no prospective studies comparing it with other treatment methods. To date there are few data available on implantation of a metal stent, another endoscopic treatment option. Results that show this procedure to be superior to pneumatic balloon dilatation, however, could often not be confirmed by other studies (e11). In addition, stent migration is a complication in up to 27% of cases (e10).
The results presented here indicate that various factors should be taken into account when choosing a treatment option. The hospitals in which the authors practice and many other institutions therefore favor the following treatment guideline: For younger patients with a low surgical risk (e.g. less than 40 to 45 years), primary laparoscopic myotomy is recommended. Pneumatic balloon dilatation is recommended for patients who refuse surgery or whose surgical risk is high. However, patients are made aware that in approximately 50% of cases repeat dilatation will be needed in the next five years. Patients who show no improvement in symptoms even after dilatation should undergo surgical myotomy.
There are no guidelines or prospective studies for cancer screening in achalasia patients, even though their risk of esophageal carcinoma is approximately 30 times higher than that of the general population (13, 15, 16). Patients receiving treatment at the institutions at which the authors practice currently undergo repeat endoscopy every two years. Patients diagnosed with achalasia more than 10 years ago undergo endoscopy as frequently as every year.
Conclusion and outlook
Primary achalasia is a neurodegenerative disease involving the neurons of the myenteric plexus. It leads to loss of LES function, with corresponding clinical consequences. Many physicians are inadequately familiar with its clinical picture. It is characterized by dysphagia, regurgitation, chest pain, and weight loss. Diagnosis often takes years, despite the presence of symptoms. Prognosis in these cases is usually worse. Complications such as megaesophagus or esophageal carcinoma occur more frequently. There are no binding guidelines on cancer screening even when achalasia has been diagnosed. Many patients therefore receive inadequate care.
Pathophysiologically, autoimmune factors seem to trigger achalasia in patients with genetic susceptibility. In addition, genetic risk factors that are not involved in autoimmune processes also seem to play a role. This is also indicated by syndromic forms of achalasia. For example, in triple-A syndrome the AAAS gene encodes for a protein that is involved in signal transduction and RNA processing and is less involved in immune processes (2). However, the highly accurate methods currently available for the analysis of diseases with a genetic component provide hope that the first risk genes for isolated and frequent forms of primary achalasia will be identified in the next few years.
Key Messages.
Many physicians are inadequately familiar with the clinical picture of achalasia. Diagnosis often takes years, despite the presence of symptoms.
Typical symptoms of achalasia are dysphagia, regurgitation, chest pain, and weight loss.
Although retention esophagitis, which is a complication of achalasia and leads to dysplasia of the esophageal mucosa, should be considered precancerous, there are no guidelines on cancer screening.
The endoscopic procedure pneumatic balloon dilatation offers good long-term results, as does Heller laparoscopic myotomy, a surgical procedure. The long-term success of new endoscopic procedures such as peroral endoscopic myotomy (POEM) and esophageal stenting, meanwhile, has yet to be verified.
Achalasia seems to be caused by autoimmune processes in persons with genetic susceptibility.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
Footnotes
Conflict of interest statement
Dr. Müller holds shares in the Rhön Group (Rhön-Konzern).
Prof. Gockel and Dr. Schumacher declare that no conflict of interest exists.
References
- 1.Park W, Vaezi MF. Etiology and pathogenesis of achalasia: the current understanding. Am J Gastroenterol. 2005;100:1404–1414. doi: 10.1111/j.1572-0241.2005.41775.x. [DOI] [PubMed] [Google Scholar]
- 2.Gockel HR, Schumacher J, Gockel I, Lang H, Haaf T, Nothen MM. Achalasia: will genetic studies provide insights? Hum Genet. 2010;128:353–364. doi: 10.1007/s00439-010-0874-8. [DOI] [PubMed] [Google Scholar]
- 3.Vela MF, Vaezi MF. Cost-assessment of alternative management strategies for achalasia. Expert Opin Pharmacother. 2003;4:2019–2025. doi: 10.1517/14656566.4.11.2019. [DOI] [PubMed] [Google Scholar]
- 4.Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am. 2001;11:281–292. [PubMed] [Google Scholar]
- 5.Eckardt VF, Kohne U, Junginger T, Westermeier T. Risk factors for diagnostic delay in achalasia. Dig Dis Sci. 1997;42:580–585. doi: 10.1023/a:1018855327960. [DOI] [PubMed] [Google Scholar]
- 6.Boeckxstaens GE. Achalasia. Best Pract Res Clin Gastroenterol. 2007;21:595–608. doi: 10.1016/j.bpg.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27:21–35. doi: 10.1097/00004836-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Howard PJ, Maher L, Pryde A, Cameron EW, Heading RC. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut. 1992;33:1011–1015. doi: 10.1136/gut.33.8.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis. 2007;2 doi: 10.1186/1750-1172-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junginger T, Eckardt VF, Hecker A. Die Achalasie. Dtsch Arztebl. 1996;93:A 610–A 614. [Google Scholar]
- 11.Makharia GK, Seith A, Sharma SK, et al. Structural and functional abnormalities in lungs in patients with achalasia. Neurogastroenterol Motil. 2009;21:603–608. doi: 10.1111/j.1365-2982.2009.01268.x. [DOI] [PubMed] [Google Scholar]
- 12.Porschen R, Molsberger G, Kuhn A, Sarbia M, Borchard F. Achalasia-associated squamous cell carcinoma of the esophagus: flow-cytometric and histological evaluation. Gastroenterology. 1995;108:545–549. doi: 10.1016/0016-5085(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 13.Streitz JM, Ellis FH, Gibb SP, Heatley GM. Achalasia and squamous cell carcinoma of the esophagus: analysis of 241 patients. Ann Thorac Surg. 1995;59:1604–1609. doi: 10.1016/0003-4975(94)00997-l. [DOI] [PubMed] [Google Scholar]
- 14.West RL, Hirsch DP, Bartelsman JF, et al. Long term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol. 2002;97:1346–1351. doi: 10.1111/j.1572-0241.2002.05771.x. [DOI] [PubMed] [Google Scholar]
- 15.Just-Viera JO, Haight C. Achalasia and carcinoma of the esophagus. Surg Gynecol Obstet. 1969;128:1081–1095. [PubMed] [Google Scholar]
- 16.Meijssen MA, Tilanus HW, van Blankenstein M, Hop WC, Ong GL. Achalasia complicated by oesophageal squamous cell carcinoma: a prospective study in 195 patients. Gut. 1992;33:155–158. doi: 10.1136/gut.33.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allgrove J, Clayden GS, Grant DB, Macaulay JC. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet. 1978;1(8077):1284–1286. doi: 10.1016/s0140-6736(78)91268-0. [DOI] [PubMed] [Google Scholar]
- 18.Prpic I, Huebner A, Persic M, Handschug K, Pavletic M. Triple A syndrome: genotype-phenotype assessment. Clin Genet. 2003;63:415–417. doi: 10.1034/j.1399-0004.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 19.Tullio-Pelet A, Salomon R, Hadj-Rabia S, et al. Mutant WD-repeat protein in triple-A syndrome. Nat Genet. 2000;26:332–335. doi: 10.1038/81642. [DOI] [PubMed] [Google Scholar]
- 20.Moore SW. Down syndrome and the enteric nervous system. Pediatr Surg Int. 2008;24:873–883. doi: 10.1007/s00383-008-2188-7. [DOI] [PubMed] [Google Scholar]
- 21.Zarate N, Mearin F, Gil-Vernet JM, Camarasa F, Malagelada JR. Achalasia and Down’s syndrome: coincidental association or something else? Am J Gastroenterol. 1999;94:1674–1677. doi: 10.1111/j.1572-0241.1999.01161.x. [DOI] [PubMed] [Google Scholar]
- 22.Carter M, Deckmann RC, Smith RC, Burrell MI, Traube M. Differentiation of achalasia from pseudoachalasia by computed tomography. Am J Gastroenterol. 1997;92:624–628. [PubMed] [Google Scholar]
- 23.Gockel I, Eckardt VF, Schmitt T, Junginger T. Pseudoachalasia: A case series and review of the literature. Scand J Gastroenterol. 2005;40:378–385. doi: 10.1080/00365520510012118. [DOI] [PubMed] [Google Scholar]
- 24.Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest. 1993;23:724–728. doi: 10.1111/j.1365-2362.1993.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 25.Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66:605–611. doi: 10.1016/j.gie.2007.03.1028. [DOI] [PubMed] [Google Scholar]
- e1.Eckardt VF, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut. 2004;53:629–633. doi: 10.1136/gut.2003.029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Katz PO, Gilbert J, Castell DO. Pneumatic dilatation is effective long-term treatment for achalasia. Dig Dis Sci. 1998;43:1973–1977. doi: 10.1023/a:1018886626144. [DOI] [PubMed] [Google Scholar]
- e3.Dagli U, Kuran S, Savas N, et al. Factors predicting outcome of balloon dilatation in achalasia. Dig Dis Sci. 2009;54:1237–1242. doi: 10.1007/s10620-008-0493-6. [DOI] [PubMed] [Google Scholar]
- e4.Vela MF, Richter JE, Khandwala F, et al. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol. 2006;4:580–587. doi: 10.1016/s1542-3565(05)00986-9. [DOI] [PubMed] [Google Scholar]
- e5.Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for Achalasia. A systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- e6.Boeckxstaens GE, Annese V, Bruley des Varannes S, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- e7.Pasricha PJ, Ravich WJ, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774–778. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- e8.Annese V, Bassotti G, Coccia G, Dinelli M, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. Gut. 2000;46:597–600. doi: 10.1136/gut.46.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–374. doi: 10.1053/j.gastro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- e10.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- e11.Li YD, Tang GY, Cheng YS, Chen NW, Chen WX, Zhao JG. 13-year follow-up of a prospective comparison of the long-term clinical efficacy of temporary self-expanding metallic stents and pneumatic dilation for the treatment of achalasia in 120 patients. AJR Am J Roentgenol. 2010;195:1429–1437. doi: 10.2214/AJR.10.4407. [DOI] [PubMed] [Google Scholar]