Abstract
Background
Because of improved survival rates and recent advances in reproductive medicine, fertility preservation methods in women of reproductive age with malignant or autoimmune diseases have risen in importance.
Methods
Selective literature review based on the authors’ clinical and scientific experience.
Results
Fertility-preserving techniques are recommended for all girls and women up to age 40 who are at high risk of ovarian failure. As these techniques are complex, special expertise in counseling and treatment is needed; in the German-speaking countries, such expertise is available in centers belonging to the FertiPROTEKT network (www.fertiprotekt.eu). Most of these techniques carry a very low risk and can be performed in two weeks or less. Success rates depend on the patient’s age, the experience of the center, and the particular technique used. The highest attainable likelihood of pregnancy after the use of a combination of cryopreservation techniques is estimated at 40% to 50%. Fertility preservation is generally not covered by health insurance; its cost ranges from several hundred to several thousand euros.
Conclusion
Girls and women up to age 40 who are about to undergo gonadotoxic treatment should be counseled about the availability of fertility-preserving techniques and, if appropriate, should be treated with such techniques in a specialized center.
Nowadays, achieving pregnancy after cancer treatment is possible. Due to new developments in reproductive medicine, new fertility-preserving techniques are available and some of these are well evaluated.
Indications for fertility-preserving treatment in children, young adults and women up to the age of 40 include
infra-diaphragmal radiotherapy to the pelvis
gonadotoxic chemotherapy which is often used for the treatment of Hodgkin’s lymphoma, breast cancer, autoimmune disease, and bone marrow transplants
diseases which carry a high risk of ovarian destruction (e.g. ovarian borderline tumors).
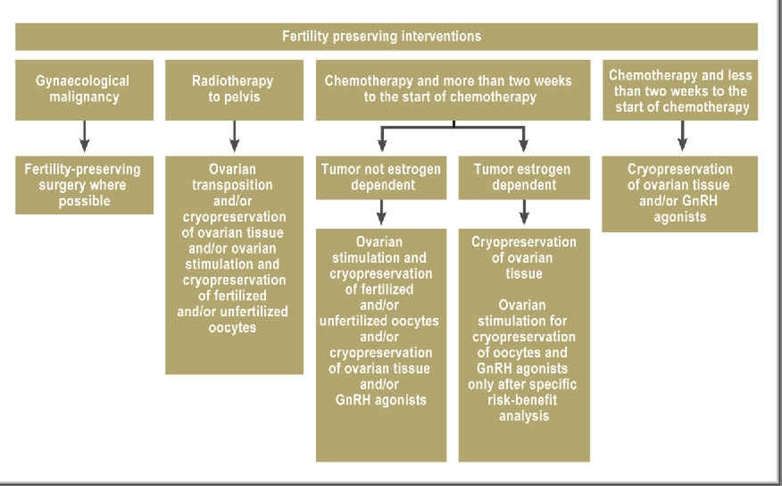
The measures for the preservation of fertility are divided into
preventative measures that aim to conserve natural fertility (e.g. transposition of the ovaries before pelvic irradiation, treatment with GnRH analogues) and
measures where oocytes or ovarian tissue are removed and conserved for later use (Figure 1).
Figure 1.
Fertility-preserving techniques for postmenarcheal women below the age of 35–40 in relation to oncological therapies and time available before the start of chemotherapy (modified according to [4])
The choice of technique depends on the diagnosis, the patient’s age, and the time available. As the techniques are complicated, specialist advice and treatment expertise is needed. This should be offered by a team of physicians, biologists and oncologists working in reproductive medicine who have special knowledge of fertility-preserving techniques.
In the following article the indications, the techniques, their risks and success rates are described.
Methods/results
This article is based on a selective literature search and the clinical and research experience of the authors.
Effects of chemotherapy and radiotherapy on fertility
The effects of radiotherapy on the ovaries depend on the dose given and the patient’s age. Radiation toxicity varies depending on the time of the cycle. Oocytes in the small primary follicles are more sensitive to radiation than oocytes in larger follicles. Younger women are particularly likely to develop transient amenorrhea which is reversible after 6 to 18 months. Wallace et al. (1) estimated the sensitivity of oocytes to radiation. According to this calculation, a dose of <2 Gy would already lead to the destruction of 50% of the oocytes. Based on this estimate the following age-dependent doses that would lead to immediate and irreversible ovarian failure in 97.5% of patients were calculated (2):
20.3 Gy at birth
18.5 Gy at age 10
16.5 Gy at age 20
14.3 Gy at age 30.
Whereas with radiotherapy the risk of ovarian damage can be estimated quite reliably, risk calculation for chemotherapy is very difficult as different therapies are used. A rough estimate can be made if the following are known: the kind of chemotherapy that will be used, the ovarian reserve that was estimated by ultrasound scan, and the level of anti-Müllerian hormone. Generally it can be noted that for example the ABVD chemotherapy regimen used for Hodgkin’s lymphoma is only minimally ovarian-toxic (3) and therefore no ovarian protective measures are needed. On the other hand, chemotherapy with alkylating agents and bone marrow transplants carry a high risk of complete ovarian A. failure even in young women. For more information see the recommendations of the network FertiPROTEKT (4).
Preventative measures
To prevent loss of fertility with chemotherapy, various therapeutic options are available.
Transposition of the ovaries
If pelvic radiotherapy is planned, the ovaries can be moved which means one or both ovaries are rotated out of the area of planned radiation (5). With transposition of the ovaries the exposure to radiation can be significantly reduced. It is still possible that stray radiation induces premature ovarian failure at a later stage without causing immediate amenorrhea.
According to the published literature nearly 90% of patients under the age of 40 who have this treatment will still have regular cycles with ovulation following radiotherapy (5). The evidence for the actual pregnancy rate after transposition is limited. Internationally only a few case reports of pregnancies have been published (6).
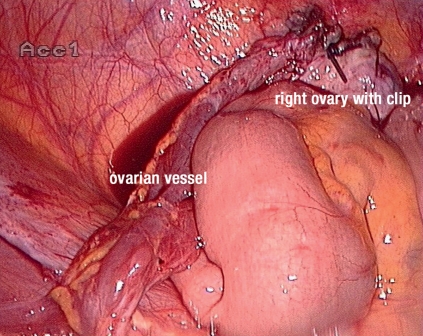
A disadvantage of transposition of the ovaries which can be done laparoscopically is the development of ovarian cysts which occurs in about 25% of patients (7). The risk of ovarian ischemia which leads to amenorrhea is about 4% (independent of radiotherapy) and therefore not significant considering the benefit (7). It is important to consider that moving the ovaries further, for example below the diaphragm, requires ligation of the fallopian tubes. This means in future in vitro fertilization is required. Returning the ovaries to their original positions and reconnecting the fallopian tubes should only be considered in exceptional cases and if this is specifically requested. It is important to mark the ovaries with a caudal clip so they can be identified when radiotherapy is planned and the area can be excluded (Figure 2). In Germany, the transposition of ovaries is generally funded by statutory health insurance.
Figure 2.
Transposition of the right ovary and fixation on the peritoneal wall. The trunk of the vessel was isolated and the ovary was taken out of the radiation field.
Medical therapies
GnRH agonists initially cause the release of gonadotropins (flare-up effect) and then a temporary hypogonadotropic hypogonadism. Because of this the potential toxicity of chemotherapy on the dormant ovarian tissue seems to be reduced (as in pre-puberty). The flare-up effect after GnRH agonists lasts about a week so they should only be given a week before chemotherapy. If only a few days remain until the beginning of chemotherapy, additional GnRH antagonists can be given to reduce the flare-up effect (8). The effect of the GnRH agonists is usually maintained for one to two weeks after the last chemotherapy has been given.
Multiple studies with limited significance or contradictory results have been published but now the first meta-analyses have been done. The most recent meta-analysis, which included only randomized controlled studies (n = 6) with 173 patients (control group without GnRH agonists 167 patients), showed a reduced rate of premature ovarian failure when GnRH agonists were given with an odds ratio of 0.29 (9) (Table). The criteria for premature ovarian failure (POF) in all studies was secondary amenorrhea and in some studies an increased concentration of follicle stimulating hormone (FSH).
Table. Meta-analysis of reduction of the rate of premature ovarian failure (POF) following GnRH analogues (GnRHa) given during chemotherapy.
| Study | GnRHa | Weighting | Odds Ratio | Odds Ratio | Odds Ratio | |||
| No POF | Total | No POF | Total | M-H, Random, 95-% CI | M-H, Random, 95-% CI | |||
| Badawy 2008 | 35 | 40 | 13 | 40 | 18,9 % | 14.54 [4.62, 45.78] | 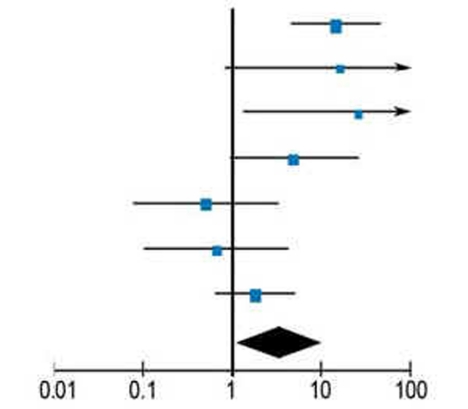 |
|
| Gilani 2007 | 15 | 15 | 10 | 15 | 8,8 % | 16.24 [0.81, 325.88] | ||
| Guiseppe 2007 | 14 | 14 | 8 | 15 | 8,8 % | 25.59 [1.29, 506.45] | ||
| Sverrisdottir 2009a | 8 | 29 | 2 | 28 | 15,6 % | 4.95 [0.95, 25.86] | ||
| Sverrisdottir 2009b | 2 | 37 | 3 | 29 | 4,3 % | 0.50 [0.08, 3.18] | ||
| Waxman 1987 | 4 | 8 | 6 | 10 | 14,2 % | 0.67 [0.10, 4.35] | ||
| ZORO 2009 | 21 | 30 | 17 | 30 | 19,5 % | 1.78 [0.62, 5.17] | ||
| Total (95-% CI) | 173 | 167 | 100 % | 3.46 [1.13, 10.57] | ||||
| No POF – all cases | 99 | 59 | (Reciprocal Odds Ratio: 0.29) | |||||
| Preferred Control | Preferred GnRHa | |||||||
Heterogeneity: Tau2 = 1.38; Chi2 = 17.68, df = 6; l2 = 66%; Test for effectiveness: Z = 2.17; M-H; Statistical test according to Mantel-Haenszel
Modified reprint from “Gonadotrophin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: a systematic review and meta-analysis”, Fertil Steril 2010; 95: 906–14: Bedaiwy MA, Abou-Setta AM, Desai N, Hurd W, Starks D, El-Nashar SA, Al-Inany HG, Falcone T.
Page No. 912, Copyright (2011), with permission from Elsevier
In the German-speaking countries two much noted prospective randomized studies of patients with Hodgkin’s lymphoma (10) and breast cancer (the ZORO study, 11) were published. In these studies no protective effect for the ovaries could be proven after giving GnRH agonists. However, the significance of these two studies is limited as patient samples were very small or poorly selected.
The risks of treatment with GnRH agonists are low. GnRH agonists can induce perimenopausal symptoms and treatment for more than 6 months can lead to bone density reduction (6% per year) which is partially reversible. There is a theoretical risk of reducing the therapeutic effect of cancer treatments for estrogen receptor positive breast cancer by using GnRH agonists. However, there is no evidence for or against this theory. The use of GnRH agonists is “off-label.”
Fertility-preserving surgery for gynecological cancers
Patients with early stages of cancer of the ovaries or uterus who wish to conceive may be able to have surgery that preserves these organs. Particularly for cervical carcinoma the fertility-preserving trachelectomy is used. For this, the tumor should be node negative and less than 2 cm in size. In a trachelectomy, the cervix is removed whilst preserving the corpus uteri. Then the vagina is joined to the corpus. In this way natural conception is possible for the majority of patients (12). Of the 200 pregnancies described in the literature, 66% resulted in live births (13). Of these, 42% were deliveries at term and 25% were premature deliveries (less than 37 weeks). Of the premature deliveries, half were before the 32nd week of pregnancy (12). Delivery is always by cesarean section. Overall, 550 cases of trachelectomy in patients with cervical carcinoma have been described. The rate of recurrence is quoted as 5% and mortality is quoted as 2% to 3% (14). This is comparable to the outcomes following radical hysterectomy.
Oocyte preservation
To prepare the oocytes for cryopreservation, different techniques are available.
Cryopreservation of unfertilized and fertilized oocytes
Comparable to the techniques used for in vitro fertilization, oocytes can be obtained by stimulation of the ovaries and follicular puncture. These can be fertilized and cryopreserved in the pronuclear phase. Following the introduction of new stimulation protocols (15), stimulation of the ovaries is now possible on any day of the patient’s cycle which means only two weeks are needed for the treatment. By using antagonists and induction of ovulation with GnRH agonists (16) ovarian hyperstimulation can be avoided which in the past often delayed chemotherapy.
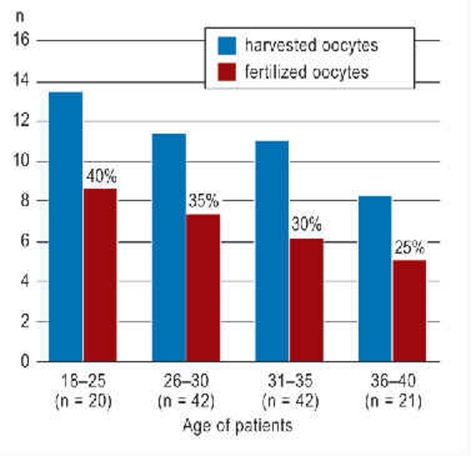
According to the registry held by the FertiPROTEKT network it is possible to cryopreserve on average, depending on the age of the patient, 5.1 to 8.6 fertilized oocytes as pronuclei prior to chemotherapy (17) (Figure 3). According to the Swiss IVF registry (18) that records the birth rate per initiated cryocycle, embryo transfer will result in a theoretical cumulative average birth rate of 40% in 18- to 25-year-old women and 25% in 36- to 40-year-old women.
Figure 3.
Number of harvested and successfully fertilized and cryopreserved oocytes in the pronuclear phase prior to cytotoxic therapy (modified according to [17]) and calculation of the theoretical birth rate after subsequent transfer according to the Swiss IVF registry (18) (percentage above the column).
If unfertilized oocytes are cryopreserved—for example if there is no partner—different freezing techniques like vitrification need to be used. In this technique, the oocytes are frozen extremely quickly in nitrogen to avoid the formation of destructive ice crystals. According to the registry in Italy, where for some time only unfertilized oocytes were allowed to be cryopreserved, the pregnancy rate is only half that of oocytes that were fertilized prior to freezing (19).
The risks of ovarian stimulation are low. According to the FertiPROTEKT network’s registry, in 205 stimulations no delay of chemotherapy was necessary as consequence of complications (20).
In hormone dependent tumors (such as breast cancer), ovarian stimulation could theoretically prove problematic as this might lead to tumor cell proliferation. So far there is no evidence for such an effect. However, since tumor cell proliferation cannot be completely ruled out, special stimulation protocols with tamoxifen or aromatase inhibitors were developed. With these, the increase in oestrogen concentration lasts only a few days and is significantly lower compared with conventional stimulation protocols (21).
Ovarian stimulation and cryopreservation is not funded by German statutory health insurance schemes. It can cost several thousand euros for obtaining and preserving the oocytes plus a few hundred euros annually for storage.
Cryopreservation of ovarian tissue
Obtaining and cryopreserving ovarian tissue is a technique that has only been available for a few years in Germany (5) and can be used at short notice prior to cytotoxic therapies. For the conservation of ovarian tissue either the whole of one ovary or 50% of one ovarian cortex is laparoscopically removed, prepared and frozen using cryoprotectants.
How much tissue is removed and cryopreserved depends on the chances of complete ovarian failure after cytotoxic therapy. If the chances are clearly over 50%, for example with radiation of the pelvis or high dose chemotherapy for Hodgkin’s lymphoma, the tissue of the whole ovary should be frozen. Cryopreservation of ovarian tissue is particularly useful for younger patients who have a high ovarian reserve and high follicular density. This technique is also useful if only 3 to 5 days remain to the beginning of chemotherapy and therefore ovarian stimulation and harvesting of follicles, which takes about two weeks, is not possible. However, one needs to consider that experience with this technique is still limited and that this technique should not be used if malignant cells are suspected to be present in the ovarian tissue (for example with leukemia).
The techniques of ovarian tissue cryopreservation, storage conditions and quality control of the process were standardized by the FertiPROTEKT network (22). To ensure high quality during the freezing process, FertiPROTEKT established central cryobanks (23). The freezing technique was checked by transplanting preserved tissue into immunodeficient mice.
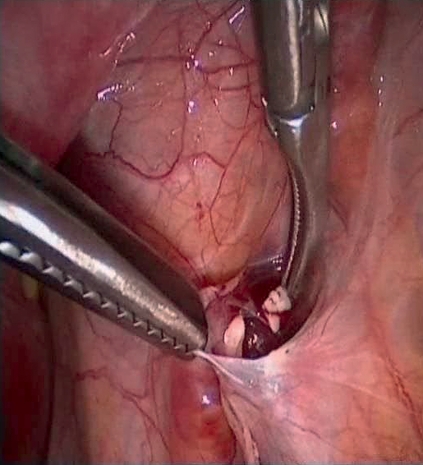
Should a patient wish to conceive despite hypergonadotropic ovarian insufficiency, tissue can be retransplanted after an appropriate period in remission. Ideally, transplantation will be into or on the remaining ovary or into a peritoneal pouch (Figure 4) near the fossa ovarica. A detailed description of the surgical techniques can be found in overview articles (24). Worldwide, there have so far been 15 live births following transplantation of ovarian tissue. In specialized centers in Copenhagen and Brussels, the pregnancy success rate per transplantation was around 30%. It is not clear if other centers have similar success rates.
Figure 4.
Transplantation of ovarian tissue into peritoneal cavity (with kind permission of R. Dittrich, Gynecological Department of Erlangen University Hospital [Dtsch Arztebl Int 2008; 105: 274–8]).
The operation to remove ovarian tissue has to be considered a low risk. In a review of 500 laparoscopies, there was one case of significant post-operative bleeding which required further surgery and delayed chemotherapy (20). The risk of retransplantation of malignant cells is considered very low provided strict quality standards are adhered to. In cases of hematological cancers or cases with a high risk of ovarian metastases, ovarian tissue should not be cryopreserved. The success rate is mostly dependent on follicular density; therefore, the age limit for cryopreservation is 35 to 37 years as up to this age follicular density is still sufficient.
Laparoscopic tissue removal can be done as an outpatient procedure but is not usually funded by German statutory health insurance carriers. The actual cryopreservation and storage of ovarian tissue is not covered by health insurance and costs a few hundred euros as a single payment for cryopreservation and a few hundred euros annually for storage.
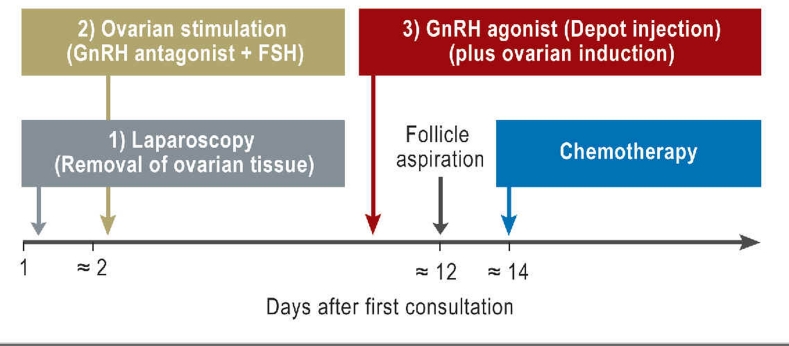
Combination of different techniques
The chances of conceiving with the help of the described techniques are limited. To improve the success rate a combination of fertility-protecting techniques should be considered (Figure 5). In a combined approach that requires a maximum of two weeks prior to chemotherapy, ovarian tissue is laparoscopically removed and gonadotropin stimulation given immediately afterwards. The number of oocytes obtained with stimulation immediately after removal of 50% of one ovary seems to be similar to that of oocytes obtained without prior removal of ovarian tissue (25). By combining these two techniques, the success rate of pregnancy can be increased and is calculated at up to 40% to 50%.
Figure 5.
Combination of three different fertility-preserving techniques to maximize chances of pregnancy (modified according to [4]).GnRH, gonadotropin releasing hormone; FSH, follicle stimulating hormone
In addition patients can receive GnRHa agonists. These are ideally given one week prior to chemotherapy. If hormonal stimulation is planned, the depot medication can be given at the same time as ovarian induction therapy.
Network FertiPROTEKT
The FertiPROTEKT network was founded in 2006 to offer fertility-preserving techniques, initially in Germany but then also in neighboring German-speaking countries, at a time when fertility-preserving techniques were still at the experimental stage. The aim was to scientifically evaluate and improve the techniques and make them part of oncological treatment protocols. Initially all university fertility clinics were included but then private fertility centers were incorporated. Today, the network comprises approximately 80 centers.
To ensure high quality counseling and therapy and to keep up to date with the rapid developments in the speciality, all centers have to attend a two-day workshop annually. A registry which includes details of all treatments given, complications, and pregnancies was also established. Standardized treatment and storage of ovarian tissue is ensured through the central cryobank. A bilingual website in German and English (www.fertiprotekt.de) is available for doctors and patients as a source of information and receives 5000 visits per month. From a scientific point of view the network has been important in the development of stimulation therapy and the establishment of recommendations. These recommendations are available on the FertiPROTEKT website and have also been published internationally with free access.
Key Messages.
Prior to any therapy which might damage the ovaries each patient up to the age of 40 should be offered advice about fertility-preserving techniques by a specialist.
Fertility-preserving techniques are generally low risk and can be used in the two weeks prior to treatment. They generally have to be funded by the patient.
The pregnancy success rate following cryopreservation of ovarian tissue or oocytes is at present difficult to estimate. Under ideal circumstances and using a combination of different techniques the success rate might be 30% to 50%.
More information is available from the network FertiPROTEKT (www.fertiprotekt.de)
Acknowledgments
Translated from the original German by Dr Ute Semrau-Boughton.
Footnotes
Conflict of interest statement\
The authors declare that no conflicts of interest exist.
References
- 1.Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117–121. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 2.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 3.Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2005;23:7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 4.von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women-a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427–435. doi: 10.1007/s00404-011-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Otte S, Friedrich M, Diedrich K, Kupka M. Preserving fertility in cancer patients. Dtsch Arztebl 2006; 103(38): A 2479-83. www.aerzteblatt.de/int/article.asp?id=58028 [Google Scholar]
- 6.Bisharah M, Tulandi T. Laparoscopic preservation of ovarian function: an underused procedure. Am J Obstet Gynecol. 2003;188:367–370. doi: 10.1067/mob.2003.38. [DOI] [PubMed] [Google Scholar]
- 7.Chambers SK, Chambers JT, Holm C, Peschel RE, Schwartz PE. Sequelae of lateral ovarian transposition in unirradiated cervical cancer patients. Gynecol Oncol. 1990;39:155–159. doi: 10.1016/0090-8258(90)90424-j. [DOI] [PubMed] [Google Scholar]
- 8.von Wolff M, Kämmerer U, Kollmann Z, Santi A, Dietl J, Frambach T. Combination of gonadotropin-releasing hormone (GnRH) agonists with GnRH antagonists before chemotherapy reduce but do not completely prevent a follicle-stimulating hormone flare-up. Fertil Steril. 2010;95:452–454. doi: 10.1016/j.fertnstert.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 9.Bedaiwy MA, Abou-Setta AM, Desai N, et al. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: a systematic review and meta-analysis. Fertil Steril. 2010;95:906–914. doi: 10.1016/j.fertnstert.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Behringer K, Wildt L, Mueller H, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma .Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol. 2010;21:2052–2060. doi: 10.1093/annonc/mdq066. [DOI] [PubMed] [Google Scholar]
- 11.Gerber B, von Minckwitz G, Stehle H, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29(17):2334–2341. doi: 10.1200/JCO.2010.32.5704. [DOI] [PubMed] [Google Scholar]
- 12.Plante M, Gregoire J, Renaud MC, Roy M. The vaginal radical trachelectomy: An update of a series of 125 cases and 106 pregnancies. Gynecol Oncol. 2011;121:290–297. doi: 10.1016/j.ygyno.2010.12.345. [DOI] [PubMed] [Google Scholar]
- 13.Jolley JA, Battista L, Wing DA. Management of pregnancy after radical trachelectomy: case reports and systematic review of the literature. Am J Perinatol. 2007;24:531–539. doi: 10.1055/s-2007-986680. [DOI] [PubMed] [Google Scholar]
- 14.Marchiole P, Benchaib M, Buenerd A, Lazlo E, Dargent D, Mathevet P. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): a comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH) Gynecol Oncol. 2007;106:132–141. doi: 10.1016/j.ygyno.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 15.von Wolff M, Thaler CJ, Frambach T, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–1365. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Bodri D, Guillén JJ, Galindo A, Mataró D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: findings of a large retrospective cohort study. Fertil Steril. 2009;91:365–371. doi: 10.1016/j.fertnstert.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Lawrenz B, Jauckus J, Kupka M, Strowitzki T, von Wolff M. Efficacy and safety of ovarian stimulation before chemotherapy in 205 cases. Fertil Steril. 2010;94:2871–2873. doi: 10.1016/j.fertnstert.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Schweizer IVF-Register. Jahresbericht 2008, p. 17. http//www.sgrm.org/wb/pages/fivnat-kommission/statistiken_reports.php.
- 19.Scaravelli G, Vigiliano V, Mayorga JM, Bolli S, De Luca R, D’Aloja P. Analysis of oocyte cryopreservation in assisted reproduction: the Italian National Register data from 2005 to 2007. Reprod Biomed Online. 2010;21:496–500. doi: 10.1016/j.rbmo.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Lawrenz B, Jauckus J, Kupka M, Strowitzki T, von Wolff M. Fertility preservation in > 1000 patient's - patients characteristics, spectrum, efficacy and risks of applied preservation techniques. Arch Gynecol Obstet. 2011;283:651–656. doi: 10.1007/s00404-010-1772-y. [DOI] [PubMed] [Google Scholar]
- 21.Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 22.FertiPROTEKT official website of the “Netzwerk für Fertilitätsprotektion bei Chemo- und Strahlentherapien”. www.fertiprotekt.de. Responsible for content: Prof. Dr. Michael von Wolff, launched: January 2007, last revised: March 2009.
- 23.Montag M, Tolba R, Schulz M, Sadek F, van der Ven H. Untersuchungen zum Einfluss des Mediums auf den Transport von Ovarialgewebe im Rahmen der Fertilitätsprotektion. J Reprod & Endokrinol. 2007;5 [Google Scholar]
- 24.Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 25.Huober-Zeeb C, Lawrenz B, Popovici RM, et al. Improving fertility preservation in cancer: Ovarian tissue cryobanking followed by ovarian stimulation can be efficiently and safely combined. Fertil Steril. 2011;95:342–344. doi: 10.1016/j.fertnstert.2010.07.1074. [DOI] [PubMed] [Google Scholar]