Summary
We positioned the following self-expanding stents certified for intracranial application: 16 Neuro form (Boston Scientific), three INX (Medtronic), one Leo (Balt). 6F calibre femoral introducers and guiding catheters were used for stent placement changing to 5F calibre introducers and guiding catheters (Envoy, Cordis) for the Neuroform 2 and 3 stents. All procedures were carried out under general anaesthesia and heparinization. Our pharmacological protocol consisted of adjunctive treatment with anti-aggregants during the interventional procedure and for the following six months, without premedication. From November 2000 to August 2006 we treated 28 patients (27 F/1M) with giant wide-necked aneurysms and one dissecting basilar artery aneurysm requiring the placement of 29 stents.
We successfully positioned 20 stents: 11 stents combined with coils (8 immediate; 3 late) with complete exclusion of the aneurysm from the circulation in seven cases and subtotal exclusion in four; nine stents not followed by embolization with complete exclusion of the aneurysm from the circulation in six cases and subtotal exclusion in three.
Stenting was not possible in nine cases due to extreme vessel tortuosity and the poor flexibility of release systems for the first stents. No late stent occlusion or subarachnoid haemorrhage were encountered after treatment.
Key words: brain aneurysms, intracranial stenting, interventional neuroradiology
Introduction
Coil embolization of brain aneurysms is a well-established alternative to surgery in the treatment of ruptured, unruptured and asymptomatic aneurysms1-7. The progressive reduction of peri-procedural complications and the results of multicentric studies like ISAT and ISUIA have made endovascular intervention the first choice treatment in many hospitals8-10. When aneurysm morphology and location are appropriate the aneurysm can be completely excluded from the circulation by microcatheterization and coil embolization11,12. However, fusiform aneurysms, giant lesions (maximum diameter >10 mm) and wide-necked aneurysms13-16 remain a challenge. Wide-necked aneurysms have a maximum neck diameter > 4 mm or a dome/neck ratio <2 17 entailing a high risk of coil migration during the embolization procedure. This complication increases the likelihood of thrombo-embolism until the artery supplying the aneurysm has been completely occluded.
In 1997 Moret et Al. first described the "remodelling" technique in the treatment of widenecked aneurysms18. The technique consists in placement of a balloon across the neck of the aneurysm and inflating it as a means of protection during the release of each coil 19-24. Another option is the use of three-dimensional coils serving as containers for subsequently deployed coils 25,26. Between 1997 and 1998 a number of authors described their first experiences of intracranial stent-assisted coil placement27-32. The stent is positioned across the neck of the aneurysm to prevent coil migration through its mesh. In addition to ongoing improvements to this technique the embolizing agent Onyx was subsequently injected after stent placement. The use of one technique rather than another in different institutions is usually dictated by personal experience, preference and ease of handling. We opted for stenting combined when necessary with coil embolization. We report our experience and the reasons behind our choice of technique.
Material and Methods
All stents were certified for intracranial use. Neuroform stents (Boston Scientific) were positioned in 16 patients33, INX stents (Medtronic) in three and Leo stents (BALT) in one patient. INX stents were used from 2000 to 2001 in the initial period of stenting at our hospital. The Leo stent was used when maximum length was required.
We used 6F femoral introducers and guiding catheters changing to Envoy 5F-Cordis catheters with an internal diameter of 0.056 inch with Neuroform stents 2 and 3.
All procedures were carried out in the angiography suite in the Neuroradiology Unit of Bellaria Hospital using a monoplane angiogram (Philips) from 2000 to 2001 and a biplane device (General Electric) from 2002 to date.
All patients underwent endovascular treatment under general anaesthesia and the following medical management protocol. Before the procedure i.v. heparin was administered to obtain coagulation times between 250 and 350 s, and a 1000 mg bolus of lysine acetylsalicilyc acid (Aspegic). After the procedure and for the following 48 h, enoxaparin (Clexane) 4 x 2 was associated with ticlopidine (Tiklid) 250 x 2, acetyl salicylic acid (Ascriptin) 0.3 x 1, ranitidine (Zantac) one 150 mg tab daily.Anti-aggregant therapy was administered at these doses for seven days after the procedure. Ticlopidine (Tiklid) was subsequently reduced to 250 x 1 and continued alongside the other drugs at the same doses for seven days. Ticlopidine (Tiklid) was then suspended and acetyl salicylic acid (Ascriptin) 0.3 x 1, ranitidine (Zantac) one 150 mg tab daily continued for six months. No premedication was given prior to Neuroform stent insertion.
Haemochrome was measured on the tenth day of treatment to disclose possible low platelet levels. In Italy clopidogrel bisulfate (Plavix) is not given to patients without cardiopathy. When possible clopidogrel bisulfate (Plavix) can replace ticlopidine (Tiklid) at a dose of 75 mg daily.
From November 2000 to April 2006 28 patients (27F/1M) with wide-necked aneurysms, including one dissecting basilar artery aneurysm, were referred to our Unit. Twelve of these patients had multiple aneurysms. Placement of 29 intracranial stents was indicated. Two intracranial stents were indicated in one patient (case 18) with a bilateral giant aneurysm of the carotid siphon.
Right stent placement failed in this case due to extreme vessel tortuosity.
Results
Twenty stents were positioned successfully (see Tables A and B). The following treatment strategies were adopted:
| Case | Age Gender |
Aneurysm's morphology and location |
Treatment | End of Procedure Control |
Last Follow Up |
|---|---|---|---|---|---|
| 1 | 43-year-old woman |
Small wide-necked aneurysm of the right carotid siphon |
Neuroform stent + coils |
Stent patent, aneurysm excluded from the circulation |
After 8 months: unchanged |
| 2 | 63-year-old woman Fig. 1 |
Wide-necked aneurysm of the basilar artery apex |
Neuroform stent + coils |
Stent patent, aneurysm partially excluded from the circulation |
After 3 months: unchanged |
| 3 | 53-year-old woman |
Giant aneurysm of the basilar artery apex |
INX stent + coils |
Stent patent, aneurysm excluded from the circulation |
Stent patent, aneurysm excluded from the circulation |
| 4 | 60-year-old woman |
Giant aneurysm of the right carotid siphon + wide-necked right carotid ophthalmic aneurysm |
Neuroform stent to cover both ostia + coils |
Stent patent, aneurysms partially excluded from the circulation |
After 2 months: stent patent, greater partial exclusion from the circulation |
| 5 | 52-year-old woman Fig. 2 |
Large intracavernous aneurysm of the left carotid siphon |
Neuroform stent + coils |
Stent patent, aneurysm partially excluded from the circulation (90%) |
After 4 months: unchanged |
| 6 | 50-year-old woman |
Giant aneurysm of the left carotid siphon |
Neuroform stent + coils |
Stent patent, aneurysm excluded from the circulation |
No further follow-up visits: recent procedure |
| 7 | 68-year-old woman Fig.3 |
Wide-necked aneurysm of the basilar artery |
Neuroform stent + coils |
Stent patent, aneurysm partially excluded from the circulation |
After 3 months: unchanged |
| 8 | 48-year-old woman |
Wide-necked aneurysm of the basilar artery |
INX stent + coil embolization after 1,3 and 43 months |
After 43 months: stent patent, aneurysm excluded from the circulation |
No further follow-up visits |
| 9 | 48-year-old woman Fig.4 |
Giant aneurysm of the left carotid siphon |
LEO stent + coil embolization after 2 and 13 months |
After 12 months: stent patent, aneurysm excluded from the circulation |
After 17 months: unchanged |
| 10 | 61-year-old woman Fig. 5 |
Giant aneurysm of the left carotid- ophthalmic artery |
Neuroform stent + coil embolization |
Stent patent, aneurysm excluded from the circulation |
After 8 months: unchanged |
| Case | Age Gender |
Aneurysm's morphology and location |
Treatment | End of Procedure Control |
Last Follow Up |
|
|---|---|---|---|---|---|---|
| 11 | 62-year-old woman |
Wide-necked aneurysm proximal to the origin of the anterior communicating artery |
Neuroform stent + coil embolization after 1 month |
Stent patent, aneurysm excluded from the circulation |
After 8 months): unchanged |
|
| 12 | 57-year-old woman |
Left carotid siphon aneurysm partially vascularized after surgery |
Coil embolization end of procedure control: loop of one coil migrated into the siphon, recovery impossible treatment (after 1 month): placement of a Neuroform stent |
Stent patent, loop of the coil adhering to the arterial wal |
No further follow-up visits |
|
| 13 | 40-year-old woman |
Aneurysm of the left postero-inferior cerebellum |
Neuroform stent |
Stent patent, aneurysm partially recanalized |
After 8 months: unchanged |
|
| 14 | 53-year-old woman |
Aneurysm in the right carotid siphon embolized with coils on 16-11-2004, with neck revascularization |
Neuroform stent |
Stent patent | MR angiography after 5 days: unchanged No further follow-up visits: recent procedure |
|
| 15 | 55-year-old woman |
Aneurysm with undefined neck in the left carotid siphon |
Neuroform stent |
Stent patent | After 24 months: stent patent, aneurysm excluded from the circulation |
|
| 16 | 53-year-old woman |
Two wide-necked aneurysms in the right carotid siphon |
Neuroform stent |
Stent patent | After 5 months: stent patent, aneurysms excluded from the circulation |
|
| 17 | 49-year-old woman |
Small wide-necked aneurysm in the right carotid siphon |
Neuroform stent |
Stent patent | After 37 months: stent patent, aneurysm excluded from the circulation |
|
| 18 | 55-year-old woman |
Wide-necked aneurysm in the left carotid siphon |
INX stent |
Stent patent | After 29 months: stent patent, aneurysm excluded from the circulation |
|
| 19 | 44-year-old woman |
Dissecting basilar artery aneurysm |
Neuroform stent |
Stent patent | After 34 months: stent patent, aneurysm excluded from the circulation |
|
| 20 | 36-year-old woman |
Wide-necked aneurysm in the left carotid siphon |
Neuroform stent |
Stent patent | No further follow-up visits: recent procedure |
|
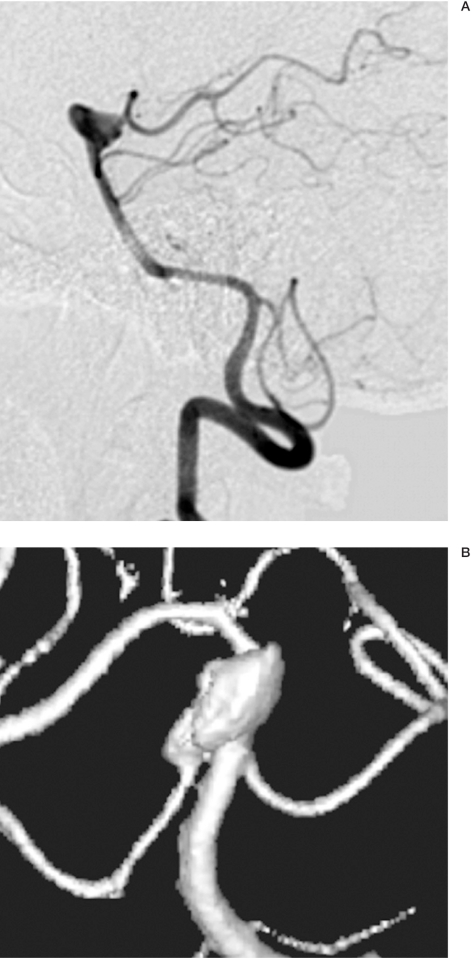
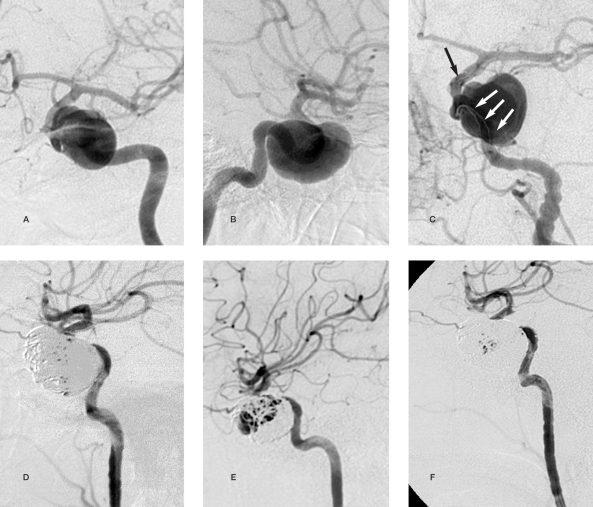
Figure 1.
Case 2:63-year-old woman A,B) An aneurysm of the basilar artery apex can be seen originating from the sac of the superior cerebellar arteries and the right posterior cerebral artery. C) Placement of a Neuroform2 stent with proximal end in the basilar artery and the distal end in the right posterior cerebral artery. D,E) partial embolization of the aneurysm with 6 coils for a total of 115 cm. The proximal portion of the lesion was not embolized to avoid occluding the origin of the superior cerebellar arteries. The posterior cerebral artery maintains stent patency. F) MR angiography follow-up after six months shows the aneurysm partially thrombosed and the origin of right posterior cerebral artery.
Figure 2.
Case 5:52-year-old woman. A) 3D reconstructed anterior oblique views: intracavernous aneurysm of the left carotid siphon. B) A Neuroform2 stent is positioned in the left carotid siphon across the aneurysm ostium followed by coil embolization. After placement of the fifth coil thrombosis developed in the stent apex. C) Immediate local thrombolytic treatment was given with injection of 700,000 units of urokinase until complete resolution and disappearance of the thrombus. D) At the end of the procedure the aneurysm appears 90% occluded. Two loops of the last coil lie in the siphon between the stent and the arterial wall without affecting local blood flow.
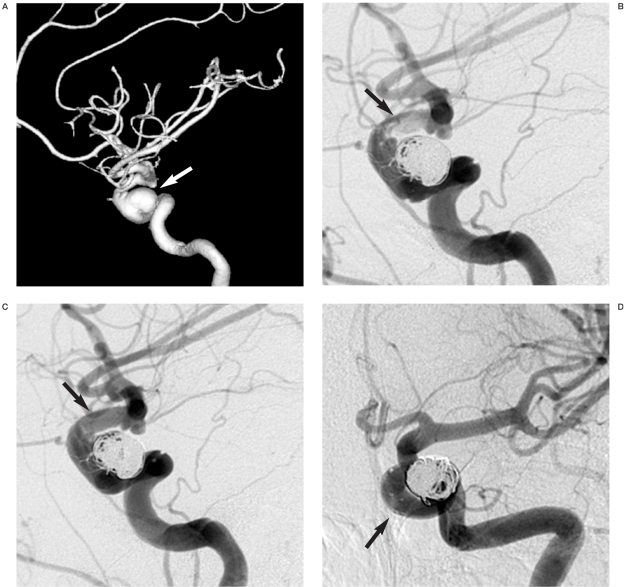
Figure 3.
Case 7: 68-year-old woman. A) Pancisternal subarachnoid haemorrhage. B) Acute angiography disclosed a moderate in size and wide neck aneurysm of the basilar artery apex giving rise to the superior cerebellar and posterior cerebral arteries. A Neuroform stent was positioned with its distal tip in the left posterior cerebral artery. C) During release of the first coil the proximal tip of the stent migrated into the aneurysm to lie superior to the right posterior cerebral artery. D,E) Lateral and anteroposterior views: at the end of the procedure the aneurysm is partially excluded from the circulation (80%) preserving patency of the superior cerebellar and posterior cerebral arteries. F,G) MR angiography follow-up after three months shows the stent is patent with a flow signal in the superior cerebellar and posterior cerebral arteries. The superior portion of the aneurysm is completely excluded from the circulation.
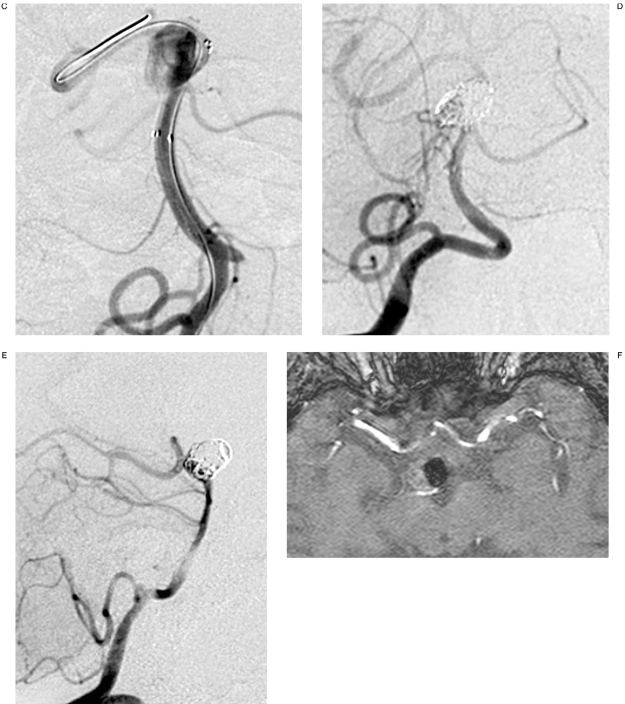
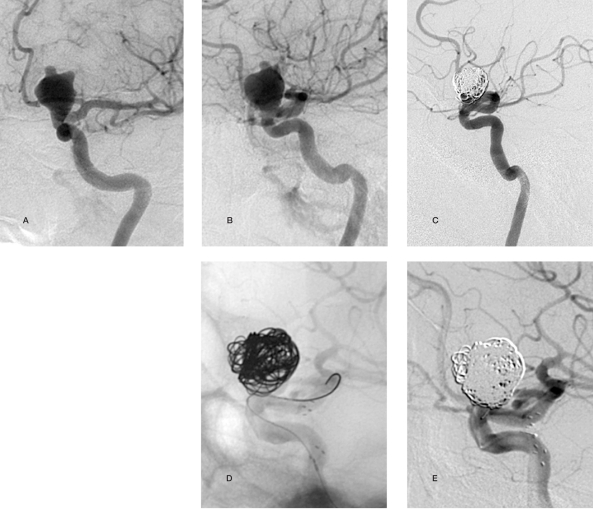
Figure 4.
Case 9: 48-year-old woman. A,B) Lateral and anteroposterior views: giant aneurysm of the left carotid siphon. C) Placement of a LEO stent. D) Two months later there are no signs of aneurysm occlusion. The lesion is completely excluded from the circulation after coil embolization. E) Coil compaction is detected after ten months with leakage of contrast medium into the medial portion of the aneurysm. F) Coil embolization three months after the last follow-up with complete exclusion of the aneurysm from the circulation.
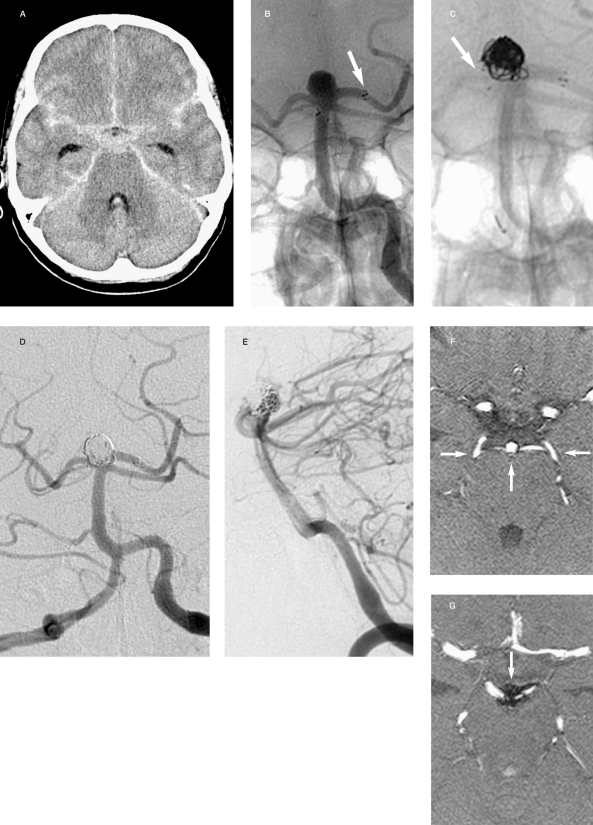
Figure 5.
Case 10: 61-year-old woman. A,B) Anteroposterior and lateral views: giant aneurysm of the left carotid-ophthalmic artery with a neck diameter of 6 mm. C) At the end of embolization the proximal portion of the aneurysm remains perfused as no stable position was found to position the coils which tended to protrude into the carotid lumen. D,E) After six months the aneurysm is unchanged. A Neuroform2 stent is positioned to assist coil embolization resulting in complete exclusion of the aneurysm from the circulation.
Immediate post-stent embolization in eight patients with complete exclusion of the aneurysm from the circulation in four and subtotal exclusion in four. One giant aneurysm presented subtotal exclusion from the circulation after coil embolization so that embolization was completed after Neuroform stent placement.
Late post-stent embolization in three patients with complete exclusion of the aneurysm from the circulation;
Stent placement without embolization in nine patients with complete exclusion from the circulation of five aneurysms and one dissecting pseudo-aneurysm. These lesions had already been treated by embolization in two cases and surgery in one, all of which revascularized.
The following disease-related complications were encountered in our series:
- Vasospasm (3 cases);
- Delayed ischaemia without neurological deficits (1 case).
The following tehnique-related complications were encountered in our series:
- Coil migration through the stent's mesh (1 case);
- Acute thrombosis within the stent successfully treated with urokinase (1 case);
Stent migration into the aneurysm during deployment of the microcatheter with coils (1 case) (figure 3C);
Stent detachment from the release system and distal arterial migration in the left popliteal artery (1 case).
In patients with technique-related complications, the outcomes were good, without clinical consequence.
Nine stents could not be positioned due to extreme vessel tortuosity and the limited flexibility of early stent release systems. The following treatment strategies were adopted:
- 3D coil embolization (Micrus endovascular) in three patients with subtotal exclusion;
- Surgery in six patients.
Long-term follow-up monitoring was done with:
- MR angiography if coils were present in the aneurysmal sac,
- CT angiography if only the stent was present,
- Angiography if aneurysm revascularization was suspected.
Follow-up has lasted more than 24 months in six patients. We are not aware of any cases of late stent occlusion or SAH after treatment.
Discussion
The most important factors in the choice of aneurysm management are efficacy and patient safety. When the aneurysm is amenable, patient safety is ensured by the simplest, shortest and least invasive procedure.
The remodelling technique carries several disadvantages including possible balloon-induced injury to the arterial wall or vasospasm, longer execution times due to recurrent intermittent vessel occlusion, the risk of delayed coil migration, and the concomitant use of two catheters, one for coils, the other for the balloon, with double femoral access or a single large diameter access (at least 7F). Luzardo et al. recently described improved single access in 48 patients using a new 6F guiding catheter with an internal diameter of 0.070 inch (Envoy 6F, Cordis)35. Despite this, the technique appears cumbersome, especially considering the reported outcomes: literature reports give rates of aneurysm exclusion from the circulation varying from 67 to 83%, with technical failure rates of around 23%36.
Three-dimensional coil embolization is a reliable method. Leonardi et al. described their experience using 3D Micrus coils (BALT) to treat wide-necked aneurysms 37. The procedure was effective for lesions with a dome/ neck ratio of 1.5, but unreliable for the embolization of very wide-necked aneurysms. In patients 10,11, 12, 13, 14 in our series, the first choice treatment was 3D coil embolization: results were unsatisfactory and intracranial stents were subsequently positioned in these cases. Stenting became a more attractive technique after the introduction of self-expanding stents devised for intracranial deployment. Before this balloon-expanded coronary stents not certified for intracranial insertion had been used. These stents had limited flexibility and navigability in the intracranial arteries, especially the distal stretches, whereas the new self-expanding stents have a narrower release system calibre offering greater flexibility and arterial navigability, and reducing arterial wall injury. In addition to preventing coil migration, stents also have therapeutic advantages. The stent mesh aids endothelial growth, favouring coil compaction and reducing blood inflow into the aneurysm, thereby leading to stasis and hence thrombosis as demonstrated in experimental and clinical studies 38-43.
All these factors led to the development of the overlapping technique, i.e. overlapping two stents across the aneurysm neck to reduce the amplitude of the stent mesh, thereby enhancing the therapeutic properties of the stent44,45. On the other hand, the overlap of two stents and the resulting reduction in vascular lumen also increases the likelihood of thrombus formation within the stent. This drawback and the greater technical complexity of the procedure led us to prefer single stent placement across the aneurysm neck for the following reasons:
Stability over time. A wire mesh is positioned across the aneurysm neck.
Relatively simple procedure.
Possibility of dividing up the procedure in difficult cases. The stent is positioned during the first treatment session to reduced inflow into the aneurysm. Coil embolization is performed at a later date. An interventional procedure should not last too long as the longer catheters remain in the vasculature, the greater the risk of complications even when the patient is under total heparinization.
Occlusion of the aneurysm with the placement of a single stent.
After stent placement in patients presenting marked stasis of contrast medium we opted not to proceed to coil embolization, especially in small aneurysms with a greater risk of perforation. Aware of the correlation between aneurysm growth and rupture and internal flow within the lesion, stent characteristics are extremely important to curb inflow46.
We have used only certified material devised for intracranial use both for patient safety and for legal reasons. Boston Scientific Neuroform stents were positioned in the majority of our patients. This is a self-expanding nitinol stent with a 3F maximum calibre over-the-wire release system. The stent is divided into segments joined in two places making it more flexible and adaptable to the vascular curvature and distal arterial navigation. The device has an open cell design characterized by mesh widening along the external margin of sharp arterial bends, reducing the risk of occluding arterial collaterals and particularly suited to the treatment of aneurysms in the T bifurcations. Its low radial force cannot dilate arteries with a calibre narrower than that of the stent47-49, and it also has low thrombogenicity50. The enhanced flexibility of the Neuroform2 51 and especially the more recent Neuroform3 stents allows them to be deployed using smaller calibre guiding catheters. This has facilitated intracranial arterial navigation reducing the risk of vessel wall injury and occlusion, namely in the vertebral arteries. It was thus possible to use 5F calibre femoral introducers, thereby facilitating arterial compression at the end of the procedure.
Coils were the only embolizing material injected in our series. Despite many literature reports, we did not use Onyx for the following reasons:
-
-
it requires balloon assistance with the risk of arterial wall injury;
-
-
two concomitant microcatherters are required, one for the balloon the other for the Onyx, entailing double femoral access;
-
-
the procedure itself lasts longer and is further prolonged by repeated arterial occlusions.
The decision not to administer anti-aggregant pre-medication prior to Neuroform stent insertion was based on the fact that correct stent placement could not be guaranteed and patients with ruptured or unruptured aneurysms would in any case be exposed to the risks of anti-aggregant drugs. By contrast, adjunctive treatment with anti-aggregants during the interventional procedure and for the following six months is essential. This protocol yielded excellent results without complications even treating acute cases, namely the dissecting pseudo-aneurysm of the basilar artery 52 and more recently the giant aneurysm of the basilar artery apex associated with massive subarachnoid haemorrhage refractory to surgical management. Excellent outcomes have also been reported by Katsaridis et al. using a similar therapeutic protocol 53.
Conclusions
Self-expanding stents are a reliable means of treating wide-necked intracranial aneurysms. The devices can be adapted to the individual patient's needs, and are relatively simple to position given the complexity of these lesions.
References
- 1.Murayama Y, Vifluela F, et al. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90(2):207–214. doi: 10.3171/jns.1999.90.2.0207. [DOI] [PubMed] [Google Scholar]
- 2.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 3.Wanke I, Doerfler A, et al. Endovascular treatment of unruptured intracranial aneurysms. Am J Neuroradiol. 2002;23(5):756–761. [PMC free article] [PubMed] [Google Scholar]
- 4.Derdeyn CP, Barr JD, et al. The International Subarachnoid Aneurysm Trial: a position statement from the executive committe of the American Society of Interventional and Therapeutic Neuroradiology. Am J Neuroradiol. 2003;24:1404–1408. [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston SC, Wilson CB, et al. Endovascular and surgical treatment of ruptured posterior circulation cerebral aneurysms: comparison of risks. Ann Neurol. 2000;48:11–19. doi: 10.1002/1531-8249(200007)48:1<11::aid-ana4>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Lempert TE, Malek AM, et al. Endovascular treatment of ruptured posterior circulation cerebral aneurysms: clinical and angiographic outcomes. Stroke. 2000;31:100–110. doi: 10.1161/01.str.31.1.100. [DOI] [PubMed] [Google Scholar]
- 7.Claiborne SC, Higashida RT, et al. Recommendations for the endovascular treatment of intracranial aneurysms: a statement for healthcare professionals from the Committee on Cerebrovascular Imaging of the American Heart Association Council on Cardiovascular Radiology. Stroke. 2002;33:2536–2544. doi: 10.1161/01.str.0000034708.66191.7d. [DOI] [PubMed] [Google Scholar]
- 8.International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 9.International Study of Unruptured Intracranial Aneurysms Investigators Unruptured intracranial aneurysms. natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Kim DJ, et al. Stent-assisted coil embolization of intracranial wide-necked aneurysms. Neuroradiology. 2005;47:680–689. doi: 10.1007/s00234-005-1402-8. [DOI] [PubMed] [Google Scholar]
- 11.Henkes H, Fisher S, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54:268–285. doi: 10.1227/01.neu.0000103221.16671.f0. [DOI] [PubMed] [Google Scholar]
- 12.Henkes H, Kirsch M, et al. Coil treatment of a fusiform upper basilar trunk aneurysm with a combination of Kissing neuroform stents, Trispan-, 3D- and fibered coils, and permanent implantation of the microguidewires. Neuroradiology. 2004;46:464–468. doi: 10.1007/s00234-004-1192-4. [DOI] [PubMed] [Google Scholar]
- 13.Phatouros CC, Sasaki TYJ, et al. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery. 2000;47:107–115. doi: 10.1097/00006123-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Jou LD, Quick CM, et al. Computational approach to quantifyng hemodynamic forces in giant cerebral aneurysms. Am J Neuroradiol. 2003;24:1804–1810. [PMC free article] [PubMed] [Google Scholar]
- 15.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 16.Vallee JN, Aymard A, et al. Endovascular treatment of basilar tip aneurysms with Guglielmi detachable coils: predictors of immediate and long term results with multivariant analysis: 6-year experience. Radiology. 2003;226:867–870. doi: 10.1148/radiol.2263011957. [DOI] [PubMed] [Google Scholar]
- 17.Higashida RT, Halbach VV, et al. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. Am J Neuroradiol. 2005;26:1751–1756. [PMC free article] [PubMed] [Google Scholar]
- 18.Moret J, Cognard C, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results: apropos of 56 cases. J Neuroradiol. 1997;24(1):30–44. [PubMed] [Google Scholar]
- 19.Cottier JP, Pasco A, et al. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. Am J Neuroradiol. 2001;22:345–351. [PMC free article] [PubMed] [Google Scholar]
- 20.Mericle RA, Wakhloo AK, et al. Temporary balloon protection as an adjunct to endosaccular coiling of wide-necked cerebral aneurysms: technical note. Neurosurgery. 1997;41:975–978. doi: 10.1097/00006123-199710000-00045. [DOI] [PubMed] [Google Scholar]
- 21.Lefkowitz MA, Gobin YP, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysma: II, clinical results. Neurosurgery. 1999;45:531–537. doi: 10.1097/00006123-199909000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Nelson PK, Levy DI. Balloon-assisted coil embolization of wide-necked aneurysms of the internal carotid artery: medium-term angiographic and clinical follow-up in 22 patients. Am J Neuroradiol. 2001;22:19–26. [PMC free article] [PubMed] [Google Scholar]
- 23.Levy DI, Ku A. Balloon-assisted coil placement in wide-necked aneurysms: technical note. J Neurosurg. 1997;86:724–726. doi: 10.3171/jns.1997.86.4.0724. [DOI] [PubMed] [Google Scholar]
- 24.Aletich VA, Debrun GM, et al. The remodelling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: experience at the University of Illinois at Chicago. J Neurosurg. 2000;93:388–396. doi: 10.3171/jns.2000.93.3.0388. [DOI] [PubMed] [Google Scholar]
- 25.Cloft HJ, Joseph GJ, et al. Use of three-dimensional Guglielmi detachable coils in the treatment of wide-necked cerebral aneurysms. Am J Neuroradiol. 2000;21:1312–1314. [PMC free article] [PubMed] [Google Scholar]
- 26.Malek AM, Higashida RT, et al. Treatment of an intracranial aneurysm using a new three-dimensional-shape Guglielmi detachable coil: technical case report. Neurosurgery. 1999;44:1142–1144. doi: 10.1097/00006123-199905000-00125. discussion 1144-1145. [DOI] [PubMed] [Google Scholar]
- 27.Higashida RT, Smith W, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysms of the basilar artery. J Neurosurg. 1997;87(6):944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 28.Lylik P, Ceratto R, et al. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery. 1998;43(2):385–388. doi: 10.1097/00006123-199808000-00132. [DOI] [PubMed] [Google Scholar]
- 29.Sekhon LH, Morgan MK, et al. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm. Neurosurgery. 1998;43(2):380–383. doi: 10.1097/00006123-199808000-00127. [DOI] [PubMed] [Google Scholar]
- 30.Mericle RA, Lanzino G, et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysms: technical case report. Neurosurgery. 1998;43(5):1229–1234. doi: 10.1097/00006123-199811000-00130. [DOI] [PubMed] [Google Scholar]
- 31.Bracard S, Anxionnat R, et al. Combined endovascular stenting and endosaccular coiling for the treatment of a wide-necked intracranial vertebral aneurysm. Interventional Neuroradiology. 1999;5:245–249. doi: 10.1177/159101999900500307. [DOI] [PubMed] [Google Scholar]
- 32.Tenjin H. Morpholgic and haemodinamic chenges after stent placement for experimental carotid aneurysm. Interventional Neuroradiology. 2004;10(sup 1):153–154. doi: 10.1177/15910199040100S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isalberti M, Costa A, et al. Trattamento endovascolare di 15 pazienti con stent intracranici Neuroform® Rivista di. Neuroradiologia. 2003;16(sup 1):127. [Google Scholar]
- 34.Cotroneo E, Gigli R. Efficacia e limiti degli stents intracranici nella terapia degli aneurismi intracavernosi a colletto largo - dati preliminari. Rivista di Neuroradiologia. 2001;14(sup 3):227–230. [Google Scholar]
- 35.Luzardo GD, Ross IB, Gal G. Balloon-assisted coiling through a single 6F guiding catheter. Am J Neuroradiol. 2006;27:190–191. [PMC free article] [PubMed] [Google Scholar]
- 36.Wanke I, Doerfler A, et al. Treatment of wide-necked intracranial aneurysms with a self-expanding stent system: initial clinical experience. Am J Neuroradiol. 2003;24:1192–1199. [PMC free article] [PubMed] [Google Scholar]
- 37.Leonardi M, Simonetti L, et al. 3D Micrus coil cage in wide-necked aneurysms. Interventional Neuroradiology. 2003;9:141–152. doi: 10.1177/159101990300900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanzino G, Wakhloo AK, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91(4):538–546. doi: 10.3171/jns.1999.91.4.0538. [DOI] [PubMed] [Google Scholar]
- 39.Kessler IM, Mounayer C, et al. The use of balloon-expandable stents in the management of intracranial arterial disease: a 5-year single-center experience. Am J Neuroradiol. 2005;26:2342–2348. [PMC free article] [PubMed] [Google Scholar]
- 40.Horowitz MB, Purdy PD. The use of the stents in the management of neurovascular disease: a review of historical and present status. Neurosurgery. 2000;46:1335–1342. doi: 10.1097/00006123-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Ohta M, Hirabayashi M, et al. Impact of the stent design on intra-aneurysmal flow. Interventional Neuroradiology. 2004;10(Sup 2):85–94. doi: 10.1177/15910199040100S216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanninen R, Manninen H, Ronkainen A. Broad-based intracranial aneurysms: thrombosis induced by stent placement. Am J Neuroradiol. 2003;24:263–266. [PMC free article] [PubMed] [Google Scholar]
- 43.Musacchio M, Mont’Alverne F, et al. Application of combined stenting and coiling for endovascular treatment of intracranial aneurysms. Rivista di Neuroradiologia. 2003;16:1163–1166. [Google Scholar]
- 44.Benndorf G, Herbon U, et al. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: case report. Am J Neuroradiol. 2001;22:1844–1848. [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira E, Birnbaum L, Mader G. Overlapping stent-supported coil embolization of wide-neck basilar tip aneurysm: technical case report. Neuroradiology. 2005;47:153–157. doi: 10.1007/s00234-004-1304-1. [DOI] [PubMed] [Google Scholar]
- 46.Castellan L, Causin F, Perini S. Trattamento endovascolare degli aneurismi intracranici mediante stent. Rivista di Neuroradiologia. 2003;16(sup 2):110–113. [Google Scholar]
- 47.Alfke K, Straube T, et al. Treatment of intracranial broad-neck aneurysms with a new self-expanding stent and coil embolization. Am J Neuroradiol. 2004;25:584–591. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao QP, Li TL, et al. Combined Neuroform intracranial stent and bioactive matrix dethachable coil for embolization of a broad-necked persistent primitive trigeminal artery aneurysm. Interventional Neuroradiology. 2005;11:63–68. doi: 10.1177/159101990501100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiorella D, Albuquerque FC, et al. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6–16. doi: 10.1227/01.neu.0000097194.35781.ea. [DOI] [PubMed] [Google Scholar]
- 50.Simionato F, Scomazzoni F, Righi C. GDC embolization of three intracranial wide-neck aneurysms using a novel self-expandable nitinol microstent. Rivista di Neuroradiologia. 2003;16:175–177. [Google Scholar]
- 51.Handa A, Abdo G, et al. Efficacy and limitations of the Neuroform stent system for intracranial aneurysms. Interventional Neuroradiology. 2004;10(Sup 2):62–78. doi: 10.1177/15910199040100S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leonardi M, Raffi L, et al. Endovascular treatment of basilar artery dissection by stent deployment; a case report. Interventional Neuroradiology. 2004;10:315–319. doi: 10.1177/159101990401000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katsaridis V, Papagiannaki C, Violaris C. Embolization of acutely ruptured and unruptured wide-necked cerebral aneurysms using the Neuroform2 stent without pretreatment with antiplatelets: A single center experience. Am J Neuroradiol. 2006;27:1123–1128. [PMC free article] [PubMed] [Google Scholar]