Abstract
The authors conducted a time-series analysis to examine seasonal variation of mortality risk in association with particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5) and chemical species in Xi’an, China, using daily air pollution and all-cause and cause-specific mortality data (2004–2008). Poisson regression incorporating natural splines was used to estimate mortality risks of PM2.5 and its chemical components, adjusting for day of the week, time trend, and meteorologic effects. Increases of 2.29% (95% confidence interval: 0.83, 3.76) for all-cause mortality and 3.08% (95% confidence interval: 0.94, 5.26) for cardiovascular mortality were associated with an interquartile range increase of 103.0 μg/m3 in lagged 1–2 day PM2.5 exposure. Stronger effects were observed for the elderly (≥65 years), males, and cardiovascular diseases groups. Secondary components (sulfate and ammonium), combustion species (elemental carbon, sulfur, chlorine), and transition metals (chromium, lead, nickel, and zinc) appeared most responsible for increased risk, particularly in the cold months. The authors concluded that differential association patterns observed across species and seasons indicated that PM2.5-related effects might not be sufficiently explained by PM2.5 mass alone. Future research is needed to examine spatial and temporal varying factors that might play important roles in modifying the PM2.5–mortality association.
Keywords: chemical components, excess relative risk, mortality, PM2.5, time series, Xi’an
Population-based research has indicated an association between ambient fine particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5) and cardiopulmonary mortality and morbidity (1–4). Most of the studies focused on the effects of PM2.5, but very few considered the differential toxicity of PM2.5 based on its component species. Since ambient PM2.5 consists of species from various sources, including traffic emission, biomass combustion, and crustal origination, the PM2.5 species originating from different sources and their mixtures may have greater or lesser toxicity. Nevertheless, recent epidemiologic and toxicologic findings have suggested the importance of examining particulate matter species-associated biologic responses and possible underlying mechanisms across the range of cardiopulmonary outcomes (5). The US National Research Council highlighted research priorities characterizing the toxicity and health risks associated with particulate matter components and supporting the regulation of most toxic particulate matter species and prioritization of particulate emission-control targets (6).
Several North American nationwide time-series analyses illustrated inconsistent seasonal and annual patterns of cardiopulmonary mortality and morbidity associated with ambient PM2.5 across locations (7, 8). A few studies further examined the association of mortality and morbidity outcomes with seasonal and annual averages of PM2.5 species, trying to explain the differential city-to-city PM2.5 health risk (9–12). Secondary particulates such as sulfate and nitrate, as well as trace elements like elemental carbon, organic carbon, and sulfur, have been found most responsible for increased mortality and hospital admission. Transition metals of PM2.5 species, including zinc, nickel, chromium, and iron, also have been of significant toxicity concern. However, controversy remains regarding the specific PM2.5 species associated with adverse health effects across locations and seasons. Differences in sources, chemical characteristics, and meteorology might have contributed to the variations.
In Asia, although air quality has improved in many urban areas despite increased combustion of fossil fuels, many cities remain highly polluted. Consistent with evidence from the West, Asian studies suggested that exposure to air pollution is associated with increased occurrence of adverse health outcomes (13). Ongoing research efforts support analyses examining variability in air pollution sources and the geographic, meteorologic, and population characteristics of Asian populations (14–16). Importantly, research on the effects of PM2.5 species in typical Asian areas might explain the difference in effects observed between Western and Eastern populations.
As the largest and most populated country in Asia, China experienced overall worsening of air quality in the past decades, along with its rapid economic development (17). Considering the various source-apportioned PM2.5 pollution patterns across geographic locations in China (18), investigators find that assessment of differential toxicity and the health impact of PM2.5 mass and species in the Chinese population is of special interest, which would fill the knowledge gaps between Western and Asian studies. Such endeavor would also have significant public health and air quality management implications for the Chinese population. The objective of our time-series analysis was to assess the acute effects of PM2.5 and the major chemical species on all-cause and cause-specific mortality in Xi’an, by using day-to-day PM2.5 mass and species data.
MATERIALS AND METHODS
Study site and population
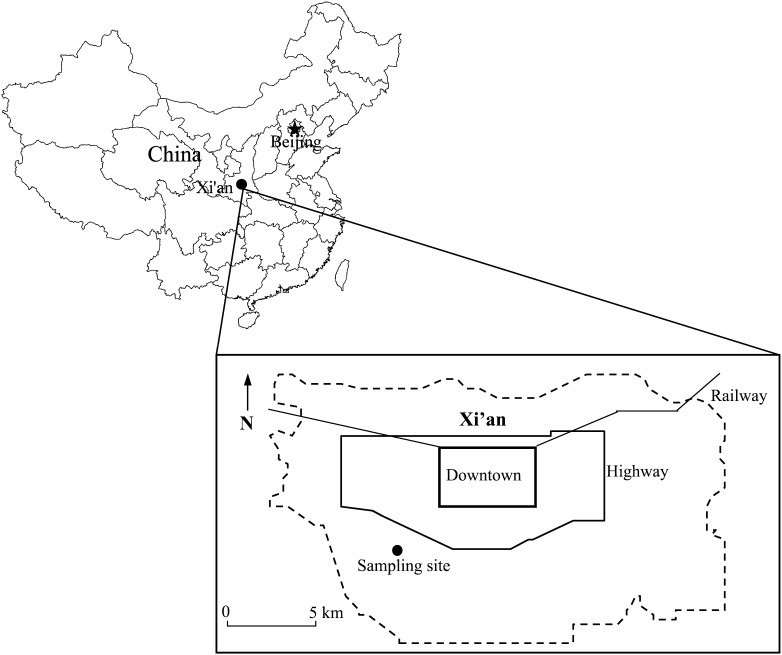
The city of Xi’an is located in the Loess Plateau of northwestern China (Figure 1), with 5.4 million urban residents according to the China City Statistical Yearbook published in 2005. Each year, between November 15 and March 15, space heating is used in all cities north of the Yangtze River in China, including Xi’an, thus commonly defined as the “heating period.” Xi'an is the largest historical city in northwestern China and among the most severely polluted Chinese cities (19). The ambient particulates in Xi’an result mainly from coal combustion space heating in winter and fugitive dust and dust storms in spring, and vehicle emissions contribute to elevated carbonaceous and secondary aerosol species in the city’s high PM2.5 levels (20, 21).
Figure 1.
Location of air sampling site in Xi’an, China.
Data collection
We obtained daily mortality data for urban residents from the Xi’an Center for Disease Control and Prevention for the period between January 1, 2004, and December 31, 2008 (5 years of data). The mortality data were for all causes in all age groups, age-specific groups (0–44 years, 45–64 years, ≥65 years), sex-specific groups (female, male), and all-ages cause-specific groups (cardiovascular and respiratory diseases). The International Classification of Diseases, Tenth Revision (ICD-10), codes of mortality were as follows: all natural causes (ICD-10 codes A00–R99), respiratory diseases (ICD-10 codes I00–I98), and cardiovascular diseases (ICD-10 codes I00–I99). We also obtained cause-specific death counts for coronary disease, stroke, and chronic obstructive pulmonary disease (COPD). We removed the death counts on December 31 and January 1 of each year, because the daily death counts appeared to be much higher than the average death counts on other days, which likely resulted from the recording of unspecified death counts that had accumulated throughout the year.
PM2.5 monitoring was conducted at a site located in an urban residential area approximately 10 miles (1 mile = 1.6 km) south of downtown Xi’an, which had no major industrial activities or local fugitive dust sources (Figure 1) (21). PM2.5 mass and species analyses were conducted at the laboratories of the Institute of Earth Environment, Chinese Academy of Sciences, in Xi’an. For these analyses, we obtained daily average concentrations of PM2.5 mass between January 1, 2004, and December 31, 2008 (5 years of data). Two subsets of PM2.5 samples were further analyzed for elements and anions, which were selected mainly on the basis of the results of health effects assessed in previous studies (7, 9, 22): organic carbon, elemental carbon, sulfur, potassium, calcium, iron, zinc, chlorine, lead, manganese, bromine, cadmium, nickel, chromium, and the water-soluble anions ammonium, sulfate, and nitrate. Two sub-data sets included one for daily average concentrations of organic carbon, elemental carbon, and other elements from January 1, 2006, to December 31, 2008 (3 years of data), and one for water-soluble anion data from January 1, 2006, to December 31, 2006 (1 year of data). To adjust for the effects of weather on mortality, we obtained information on the daily averaged temperature and the relative humidity for the study period from the China State Meteorology Bureau.
Statistical methods
Because daily counts of mortality data follow a Poisson distribution, we used Poisson regression models to evaluate the associations between mortality and exposure to PM2.5 and to specific species. Natural spline functions (23, 24) of calendar time, temperature, and relative humidity were used to adjust for seasonality and long-term trends and to control for the potential confounding effects of weather. Degrees of freedom of natural spline functions were determined by the Akaike Information Criterion (25), generalized cross-validation, and the literature (16, 26). If there was overdispersion in the variance, we applied a partial autocorrelation function (PACF) to guide the selection of degrees of freedom until the absolute values of the sum of PACF for lags up to 30 days reached a minimum. Analyses were also adjusted for year and day of the week as dummy variables to control for different baseline mortality rates for each year and each day of the week. Residuals of each model were examined to check whether there were discernible patterns and autocorrelation by means of residual plots and PACF plots, respectively.
We first assessed the mortality risk in association with exposure to PM2.5 mass on the same day (lag 0) and up to 6 prior days (lag days 0–6), using an individual-lag model. We fit the following individual-lag model to obtain the estimated pollution-associated relative rate of increased mortality:
 |
where E(Yt) is the expected number of deaths at day t; β represents the log-relative rate of mortality associated with a unit increase of air pollutants; Zt-n indicates the pollutant concentrations at day t to n (n = 0, 1, …, 6) to represent concentrations at various lag days; day of the week and year represent the effects of the day of the week and the time over years; and ns(time, df) denotes the natural spline function of calendar time, whereas ns(temperature, df) and ns(relative humidity, df) denote the natural spline functions of temperature and humidity, respectively.
Because most of the significant associations estimated in the individual-lag model occurred across lag days 0–6, we applied a constrained distributed-lag model to investigate the association between mortality risk and cumulative exposures to PM2.5 and major species of the previous week, accounting for the daily effect in the prior week. Our distributed-lag model constrains lag-specific regression coefficients to be a step function by including variables that are averages of the same day’s and previous 6 days’ concentrations.
Finally, because central space heating is operated for a specific set of months each year in Xi’an, we further stratified the whole-year analysis into the heating period (November 15–March 15) and the nonheating period (March 16–November 14). We examined the excess relative risk of PM2.5 and selected species on all causes of mortality for the whole year, as well as for the heating and nonheating periods, using the individual-lag model and the distributed-lag model. We conducted separate regression analyses of the distributed-lag model for each period and examined the heterogeneity of effect estimates across periods.
All results were presented as the percentage change of excess relative risk of mortality and its 95% confidence interval in association with each 10-μg/m3 or interquartile-range increase in PM2.5 and major species. All analyses were performed by using R, version 2.12.1, statistical software available on the Comprehensive R Archive Network (CRAN) at http://cran.r-project.org.
RESULTS
Table 1 provides a descriptive summary of PM2.5 mass and species concentrations, as well as meteorologic conditions, in Xi’an for a whole year and for the heating and nonheating periods between 2004 and 2008. The average PM2.5 concentration during the heating period was approximately 60% higher than that observed during the nonheating period. Most PM2.5 species, except for calcium and iron, had somewhat higher concentrations during the heating period. However, most proportions of major species did not vary significantly across periods, except for nickel (Web Figure 1, the first of 5 Web figures and 3 Web tables that are posted on the Journal’s Web site (http://aje.oxfordjournals.org/)). In Xi’an, the carbonaceous species elemental carbon and organic carbon contributed approximately 5% and 16% to PM2.5 mass, whereas the secondary particulate species sulfate, nitrate, and ammonium contributed approximately 22%, 9%, and 6%. We observed the highest correlations among all species between PM2.5 and sulfate, nitrate, and ammonium (correlation coefficients (r) ranging between 0.65 and 0.75) and between PM2.5 and organic and elemental carbon, sulfur, chlorine, potassium, manganese, and lead (r ranging between 0.65 and 0.8) (results shown in Web Tables 1 and 2).
Table 1.
Summary Statistics of PM2.5 and Component Species Concentrations (μg/m3), Meteorologic Conditions in Xi’an, China, 2004–2008
| Whole Year |
Heating |
Nonheating |
||||
| Mean (SD) | IQR | Mean (SD) | IQR | Mean (SD) | IQR | |
| 2004–2008 | ||||||
| Temperature, °C | 15.3 (9.8) | 16.9 | 3.9 (4.3) | 6.3 | 20.9 (6.2) | 9.9 |
| Relative humidity, % | 60.6 (17.4) | 28.0 | 58.7 (17.5) | 25.0 | 61.5 (17.4) | 28.0 |
| PM2.5 | 176.7 (103.8) | 111.8 | 235.8 (125.1) | 169.3 | 147.0 (75.5) | 88.1 |
| 2006–2008 | ||||||
| Organic carbon | 28.0 (18.7) | 18.2 | 40.7 (20.8) | 27.4 | 21.7 (13.7) | 11.0 |
| Elemental carbon | 8.8 (5.2) | 6.6 | 10.8 (5.6) | 7.5 | 7.8 (4.8) | 5.8 |
| Sulfur | 5.1 (3.4) | 4.3 | 6.7 (4.2) | 5.6 | 4.3 (2.6) | 3.6 |
| Potassium | 1.8 (1.7) | 1.5 | 2.5 (2.0) | 1.6 | 1.5 (1.3) | 1.3 |
| Calcium | 2.5 (3.3) | 2.4 | 2.1 (2.1) | 2.0 | 2.7 (3.8) | 3.0 |
| Iron | 1.6 (1.7) | 1.2 | 1.5 (1.0) | 1.0 | 1.7 (2.0) | 1.3 |
| Zinc | 1.4 (1.2) | 1.2 | 1.8 (1.3) | 1.6 | 1.3 (1.1) | 1.0 |
| Chlorine | 1.3 (1.5) | 1.5 | 2.3 (1.9) | 2.7 | 0.8 (1.0) | 0.9 |
| Lead | 0.49 (0.37) | 0.40 | 0.68 (0.46) | 0.57 | 0.40 (0.27) | 0.29 |
| Manganese | 0.11 (0.08) | 0.09 | 0.13 (0.08) | 0.10 | 0.10 (0.08) | 0.08 |
| Bromine | 0.04 (0.05) | 0.04 | 0.05 (0.04) | 0.05 | 0.03 (0.05) | 0.03 |
| Cadmium | 0.03 (0.05) | 0.03 | 0.03 (0.04) | 0.04 | 0.03 (0.05) | 0.03 |
| Nickel | 0.01 (0.04) | 0.01 | 0.01 (0.03) | 0.01 | 0.01 (0.05) | 0.01 |
| Chromium | 0.01 (0.01) | 0.01 | 0.02 (0.01) | 0.01 | 0.011 (0.01) | 0.01 |
| 2006 only | ||||||
| Ammonium | 11.2 (9.1) | 11.7 | 14.5 (10.8) | 15.8 | 9.6 (7.7) | 10.7 |
| Sulfate | 38.1 (27.0) | 34.6 | 44.8 (31.3) | 44.7 | 34.8 (24.0) | 30.7 |
| Nitrate | 16.2 (12.9) | 16.2 | 20.5 (14.2) | 21.3 | 14.0 (11.6) | 12.5 |
Abbreviations: IQR, interquartile range; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter; SD, standard deviation.
Table 2 summarizes the daily death counts from 2004 to 2008 averaged for the whole year and for the heating and nonheating periods in Xi’an. Death counts due to cardiovascular diseases and respiratory diseases accounted for approximately 46% and 10% of all-cause mortality, respectively, whereas deaths in the ≥65-year group accounted for more than 70% of all-cause mortality.
Table 2.
Summary Statistics of Daily Mortality Counts in Xi’an, China, 2004–2008
| Death Counts, mean (SD) |
|||
| Whole Year | Heating | Nonheating | |
| All causes | |||
| All ages | 25.8 (9.6) | 28.6 (10.4) | 24.4 (8.9) |
| 0–44 years | 2.5 (1.9) | 2.7 (1.9) | 2.5 (1.9) |
| 45–64 years | 5.3 (3.1) | 5.6 (3.1) | 5.1 (3.0) |
| ≥65 years | 18.0 (7.6) | 20.3 (8.3) | 16.8 (6.9) |
| Sex | |||
| Female | 10.2 (4.8) | 11.5 (5.2) | 9.6 (4.5) |
| Male | 15.6 (6.4) | 17.1 (6.9) | 14.8 (6.1) |
| Cardiovascular | 11.8 (5.8) | 13.9 (6.4) | 10.8 (5.1) |
| Coronary | 6.2 (3.6) | 7.5 (4.0) | 5.6 (3.1) |
| Stroke | 4.5 (2.7) | 5.2 (2.9) | 4.2 (2.6) |
| Respiratory | 2.6 (1.9) | 3.1 (2.2) | 2.4 (1.8) |
| COPD | 0.9 (1.0) | 1.2 (1.2) | 0.8 (0.9) |
Abbreviations: COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Mortality effects estimated by the individual-lag model
Table 3 presents the percent change of adjusted excess relative risk of all-cause and cause-specific mortality, per 10-μg/m3 and interquartile-range increases in PM2.5 concentrations averaged over a 1–2 day lag, estimated by an individual-lag model. In the whole-year analyses, all-cause mortality and cardiovascular mortality were significantly increased by 0.20% (95% confidence interval (CI): 0.07, 0.33) and 0.27% (95% CI: 0.08, 0.46), per 10-μg/m3 increase in PM2.5 concentration, with adjustment for weather and time-varying effects. Sensitivity analyses showed that, per 10-μg/m3 increase, the risk estimates for PM2.5 on all-cause mortality remained significant and robust when extremely high values were removed (data shown in Web Table 3).
Table 3.
Excess Relative Risk of Mortality per 10 μg/m3 and IQR Increases in PM2.5 Concentration Averaged for Lag 1–2 Days Estimated by Individual-Lag Model, in Xi’an, China, 2004–2008
| Mortality | Per 10 μg/m3 |
Per IQR |
||
| Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | |
| All causes | ||||
| All ages | 0.20 | 0.07, 0.33 | 2.29 | 0.83, 3.76 |
| 0–44 years | 0.23 | −0.18, 0.63 | 2.55 | −2.02, 7.33 |
| 45–64 years | 0.25 | −0.04, 0.53 | 2.80 | −0.43, 6.14 |
| ≥65 years | 0.18 | 0.03, 0.34 | 2.09 | 0.35, 3.85 |
| Sex | ||||
| Female | 0.25 | 0.05, 0.45 | 2.83 | 0.53, 5.19 |
| Male | 0.17 | 0.00, 0.34 | 1.92 | 0.06, 3.83 |
| Cardiovascular | 0.27 | 0.08, 0.46 | 3.08 | 0.94, 5.26 |
| Coronary | 0.39 | 0.14, 0.65 | 4.48 | 1.53, 7.52 |
| Respiratory | 0.19 | −0.20, 0.59 | 2.17 | −2.24, 6.77 |
Abbreviations: CI, confidence interval; IQR, interquartile range of PM2.5 averaged for lag 1–2 days: 103.0 μg/m3; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter.
Excess relative risk is adjusted for temperature, relative humidity, day of the week, and time trend.
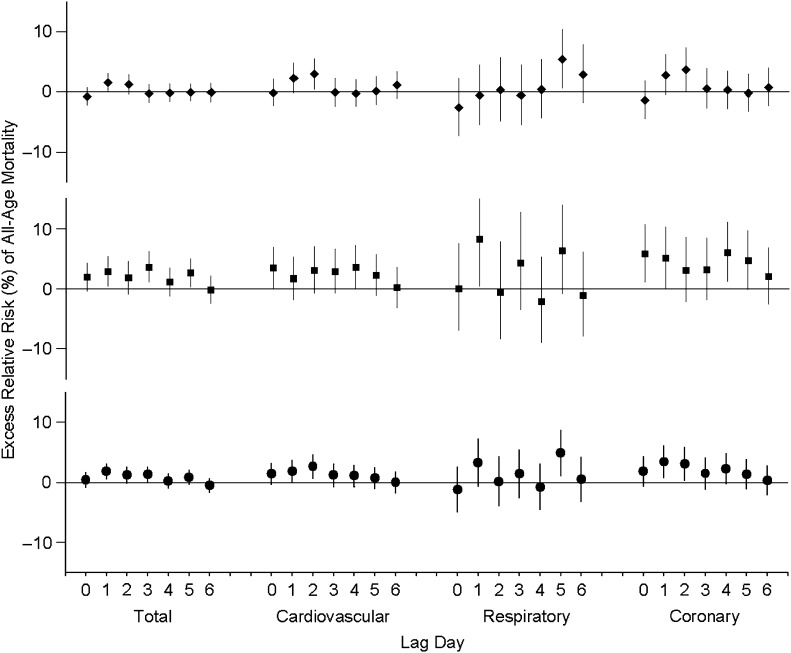
Individual-lag-day effects of PM2.5 exposure on all-cause, cardiovascular, and respiratory mortality in a whole year and during the heating and nonheating periods are presented in Figure 2. Increased all-cause mortality was found to be associated with exposure to PM2.5 for the whole year. Likewise, seasonal variation of individual-lag effects for major PM2.5 chemical species is presented in Web Figures 1–5. Specifically, increased all-cause and cardiovascular mortality risks were associated with exposures to secondary aerosols (sulfate, nitrate, ammonium) and combustion species (elemental carbon and sulfur) with lags of 0–3 days for the whole year and with greater risks observed during the heating period. However, the confidence intervals of the effect estimates for sulfate, nitrate, and ammonium are also large, which likely was due to small sample sizes (1 year of daily data). Further, several elements (bromine, chlorine, chromium, nickel, zinc, and lead) showed stronger associations with increased mortality at various lag days in the heating period.
Figure 2.
Excess relative risk, by percentage, of all-age mortality per IQR increases in PM2.5 between lag 0 and lag 6 days estimated by individual-lag model, Xi’an, China, 2004–2008. IQR, interquartile range; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter. Excess relative risk is adjusted for temperature, relative humidity, day of the week, and time trend. ♦, estimate for nonheating period; ▪, estimate for heating period; •, estimate for whole year. Bars, 95% confidence interval.
Mortality effects estimated by the distributed-lag model
Table 4 presents the adjusted mortality risk estimated by the distributed-lag model, per interquartile-range increases in PM2.5 exposure averaged for the prior week. A distributed-lag model may underestimate the acute exposure effect that occurred in recent days, such as the same day and 1 or 2 days, by summing up the cumulative effects. Distributed-lag model results estimated an increase of 1.83% (95% CI: 0.29, 3.39) for all-cause mortality and a greater increase of 3.46% (95% CI: 0.78, 6.20) in the cold months, per interquartile-range increases in PM2.5 concentrations. We also observed greater mortality risk in the elderly (≥65 years), in males, and in cardiovascular groups, in the heating period.
Table 4.
Excess Relative Risk of Mortality per IQR Increases in PM2.5 Estimated by Constrained Distributed-Lag Model, in Xi’an, China, 2004–2008
| Mortality | Whole Year |
Heating |
Nonheating |
|||
| Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | |
| All causes | ||||||
| All ages | 1.83 | 0.29, 3.39 | 3.46* | 0.78, 6.20 | 0.28 | −1.74, 2.34 |
| 0–44 years | 1.88 | −2.95, 6.95 | −2.50 | −10.4, 6.09 | 2.44 | −3.83, 9.12 |
| 45–64 years | 2.52 | −0.91, 6.07 | 3.21 | −2.73, 9.51 | 0.00 | −4.33, 4.53 |
| ≥65 years | 1.61 | −0.23, 3.48 | 4.28** | 1.08, 7.58 | 0.06 | −2.37, 2.55 |
| Sex | ||||||
| Female | 2.54 | 0.10, 5.05 | 2.83 | −1.35, 7.18 | 2.54 | −0.73, 5.92 |
| Male | 1.35 | −0.63, 3.36 | 3.88** | 0.43, 7.45 | −1.15 | −3.70, 1.46 |
| Cardiovascular | 2.97 | 0.69, 5.29 | 4.90 | 1.00, 8.95 | 1.72 | −1.34, 4.87 |
| Coronary | 4.83 | 1.67, 8.09 | 8.72** | 3.26, 14.46 | 1.90 | −2.33, 6.31 |
| Respiratory | 1.63 | −3.02, 6.49 | 3.34 | −4.56, 11.90 | −0.03 | −6.25, 6.60 |
Abbreviations: CI, confidence interval; IQR, interquartile range of PM2.5 averaged for lag 0–6 days: 78.9 μg/m3 (whole year), 104.0 μg/m3 (heating period), and 59.7 μg/m3 (nonheating period); PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter.
* P = 0.05–<0.10; **P < 0.05 (significant difference existed between heating and nonheating periods).
Excess relative risk is adjusted for temperature, relative humidity, day of the week, and time trend.
In sequential period-stratified, distributed-lag model analyses of PM2.5 species-associated risk for various mortalities in all age groups (Tables 5–8), in general, secondary aerosols (sulfate and ammonium), combustion species (elemental carbon, sulfur, and chlorine), and transition metals (chromium, lead, nickel, and zinc) appeared most responsible for increased risk, particularly in the cold months.
Table 5.
Excess Relative Risk of All-Age, All-Cause Mortality per IQR Increases in Selected PM2.5 Species Estimated by Constrained Distributed-Lag Model, in Xi’an, China, 2004–2008
| PM2.5 Species | Whole Year |
Heating |
Nonheating |
|||
| Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | |
| Organic carbon | 2.21 | −0.41, 4.90 | 1.68 | −2.98, 6.56 | 2.27 | −0.03, 4.62 |
| Elemental carbon | 0.13 | −2.50, 2.84 | 6.15* | 2.87, 9.53 | −0.02 | −3.34, 3.40 |
| Sulfur | 1.41 | −0.92, 3.79 | 6.79* | 2.65, 11.09 | −0.06 | −2.61, 2.55 |
| Bromine | 0.75 | −1.39, 2.93 | 3.57 | −1.33, 8.72 | −0.06 | −1.98, 1.90 |
| Chlorine | 1.54 | −1.37, 4.54 | 6.35 | 1.72, 11.19 | 3.83 | 0.66, 7.11 |
| Chromium | 0.07 | −1.18, 1.33 | 2.92* | 0.66, 5.24 | −1.20 | −2.73, 0.35 |
| Potassium | −0.18 | −2.41, 2.10 | 0.45 | −2.19, 3.17 | −2.51 | −5.30, 0.36 |
| Nickel | 0.32 | −0.18, 0.83 | 1.58 | 0.75, 2.41 | 0.71 | −0.13, 1.56 |
| Lead | −0.65 | −2.76, 1.52 | 3.23 | −1.07, 7.71 | 0.56 | −2.02, 3.22 |
| Zinc | −0.44 | −2.49, 1.66 | 2.17 | −1.54, 6.02 | 1.98 | −0.14, 4.14 |
| Ammonium | 2.96 | −1.97, 8.14 | 5.05* | −1.99, 12.61 | −6.09 | −13.40, 1.84 |
| Sulfate | 4.26 | −0.49, 9.24 | 5.17* | −2.03, 12.90 | −5.13 | −12.26, 2.58 |
| Nitrate | 3.17 | −1.91, 8.52 | 1.68 | −2.98, 6.56 | −1.44 | −7.40, 4.90 |
Abbreviations: CI, confidence interval; IQR, interquartile range; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter.
* P < 0.05 (significant difference existed between heating and nonheating periods).
Excess relative risk is adjusted for temperature, relative humidity, day of the week, and time trend.
Table 8.
Excess Relative Risk of All-Age Coronary Mortality per IQR Increases in Selected PM2.5 Species Estimated by Constrained Distributed-Lag Model, in Xi’an, China, 2004–2008
| PM2.5 Species | Whole Year |
Heating |
Nonheating |
|||
| Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | |
| Organic carbon | 7.14 | 1.74, 12.83 | 9.23 | −0.42, 19.81 | 7.93 | 3.00, 13.1 |
| Elemental carbon | 9.03 | 3.28, 15.10 | 17.85** | 10.75, 25.39 | 5.35 | −1.85, 13.07 |
| Sulfur | 2.40 | −2.33, 7.35 | 14.59** | 5.84, 24.06 | 0.13 | −5.15, 5.70 |
| Bromine | 1.06 | −3.22, 5.54 | 6.84 | −3.06, 17.75 | −1.19 | −5.05, 2.83 |
| Chlorine | 10.88 | 4.78, 17.33 | 19.65 | 9.79, 30.40 | 10.32 | 3.49, 17.60 |
| Chromium | 0.59 | −1.97, 3.22 | 3.77 | −0.85, 8.60 | 0.57 | −2.62, 3.85 |
| Potassium | 6.50 | 1.89, 11.32 | 4.46 | −0.79, 9.98 | 5.02 | −0.98, 11.39 |
| Nickel | −0.84 | −1.93, 0.27 | 1.47** | −0.09, 3.05 | −1.14 | −3.12, 0.87 |
| Lead | 0.16 | −4.09, 4.60 | 12.39** | 3.32, 22.25 | 1.23 | −4.15, 6.90 |
| Zinc | −0.68 | −4.81, 3.62 | 10.17** | 2.57, 18.34 | −0.73 | −5.06, 3.81 |
| Ammonium | 4.09 | −6.30, 15.62 | 3.14 | −11.11, 19.67 | −7.97 | −22.72, 9.59 |
| Sulfate | 5.30 | −4.75, 16.41 | 2.04 | −12.36, 18.81 | −2.51 | −17.68, 15.45 |
| Nitrate | 5.06 | −5.62, 16.95 | 5.35 | −11.03, 24.74 | −2.32 | −14.44, 11.5 |
Abbreviations: CI, confidence interval; IQR, interquartile range; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter.
* P < 0.05 (significant difference existed between heating and nonheating periods).
Excess relative risk is adjusted for temperature, relative humidity, day of the week, and time trend.
Table 6.
Excess Relative Risk of All-Age Cardiovascular Mortality per IQR Increases in Selected PM2.5 Species Estimated by Constrained Distributed-Lag Model, in Xi’an, China, 2004–2008
| PM2.5 Species | Whole Year |
Heating |
Nonheating |
|||
| Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | |
| Organic carbon | 1.77 | −2.03, 5.71 | 3.43 | −3.41, 10.76 | 2.28 | −1.13, 5.80 |
| Elemental carbon | 3.00 | −0.96, 7.11 | 10.17** | 5.26, 15.30 | 1.53 | −3.45, 6.78 |
| Sulfur | 0.52 | −2.89, 4.05 | 7.76* | 1.66, 14.23 | 0.96 | −2.91, 4.99 |
| Bromine | 0.92 | −2.21, 4.15 | 1.96 | −5.06, 9.49 | −0.07 | −2.90, 2.83 |
| Chlorine | 4.16 | −0.12, 8.61 | 10.04 | 3.22, 17.32 | 6.29 | 1.51, 11.30 |
| Chromium | 0.22 | −1.64, 2.11 | 3.78** | 0.42, 7.25 | −0.76 | −3.05, 1.58 |
| Potassium | 0.91 | −2.36, 4.28 | 0.33 | −3.49, 4.30 | −0.30 | −4.48, 4.07 |
| Nickel | 0.07 | −0.69, 0.85 | 2.04** | 0.90, 3.20 | 0.00 | −1.35, 1.37 |
| Lead | −1.26 | −4.34, 1.92 | 5.26 | −1.12, 12.05 | 2.32 | −1.59, 6.38 |
| Zinc | −0.99 | −3.96, 2.08 | 4.48 | −0.89, 10.14 | 1.32 | −1.79, 4.53 |
| Ammonium | 3.29 | −1.97, 8.14 | 2.55 | −7.91, 14.2 | −8.03 | −19.02, 4.45 |
| Sulfate | 4.22 | −0.49, 9.24 | 2.22 | −8.43, 14.11 | −4.78 | −15.82, 7.71 |
| Nitrate | 6.32 | −1.91, 8.52 | 4.91 | −7.11, 18.48 | −0.27 | −9.52, 9.92 |
Abbreviations: CI, confidence interval; IQR, interquartile range; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter.
* P = 0.05–<0.10; **P < 0.05 (significant difference existed between heating and nonheating periods).
Excess relative risk is adjusted for temperature, relative humidity, day of the week, and time trend.
Table 7.
Excess Relative Risk of All-Age Respiratory Mortality per IQR Increases in Selected PM2.5 Species Estimated by Constrained Distributed-Lag Model, in Xi’an, China, 2004–2008
| PM2.5 Species | Whole Year |
Heating |
Nonheating |
|||
| Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | Excess Relative Risk, %a | 95% CI | |
| Organic carbon | 4.72 | −3.02, 13.07 | 0.68 | −12.06, 15.26 | 3.67 | −3.24, 11.07 |
| Elemental carbon | 3.41 | −4.46, 11.92 | 8.43 | −0.96, 18.71 | 5.28 | −4.95, 16.61 |
| Sulfur | 12.65 | 5.20, 20.64 | 19.48 | 6.76, 33.70 | 7.17 | −1.01, 16.03 |
| Bromine | 3.26 | −3.26, 10.23 | 11.84 | −2.81, 28.71 | 0.61 | −5.36, 6.97 |
| Chlorine | 6.63 | −2.12, 16.17 | 14.99 | 1.01, 30.91 | 3.68 | −5.45, 13.71 |
| Chromium | −2.04 | −5.58, 1.63 | −0.14 | −6.26, 6.37 | −2.61 | −7.10, 2.10 |
| Potassium | 3.89 | −2.73, 10.95 | 9.15* | 1.44, 17.45 | −8.40 | −16.42, 0.38 |
| Nickel | −0.52 | −2.05, 1.04 | 1.82* | −0.71, 4.43 | −1.90 | −4.53, 0.81 |
| Lead | 5.32 | −1.08, 12.14 | 13.15 | 0.28, 27.67 | 1.78 | −5.86, 10.04 |
| Zinc | 1.08 | −5.21, 7.78 | 1.90 | −8.66, 13.68 | 4.29 | −2.34, 11.38 |
| Ammonium | 10.33 | −3.47, 26.10 | 19.54 | −1.13, 44.53 | −1.09 | −21.11, 24.00 |
| Sulfate | 9.95 | −3.10, 24.75 | 17.28 | −3.33, 42.28 | −0.73 | −20.04, 23.25 |
| Nitrate | 7.65 | −6.26, 23.62 | 18.66 | −4.23, 47.02 | −0.06 | −15.91, 18.77 |
Abbreviations: CI, confidence interval; IQR, interquartile range; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter.
* P < 0.05 (significant difference existed between heating and nonheating periods).
Excess relative risk is adjusted for temperature, relative humidity, day of the week, and time trend.
DISCUSSION
PM2.5 species can vary spatially and temporally (27, 28), and previous studies also observed seasonal variation and differential sources contributing to risk heterogeneity (29, 30). In this time-series approach, we confirmed significant mortality risk with exposure to PM2.5. We also observed that seasonal variation of PM2.5 and chemical species was associated with mortality risk in Xi’an. In addition, secondary aerosols (sulfate, nitrate, and ammonium) and combustion species (elemental carbon and sulfur) in PM2.5 were found to be significantly associated with increased mortality risk, and the magnitude of increased mortality risk was even larger during the heating period. Exposure to the elements nickel, zinc, chromium, and lead was also found to be associated with increased risk for cardiovascular disease mortality during the cold months in Xi’an.
In these time-series analyses, we observed elevated risks of 2.29% (95% CI: 0.29, 3.39) for all-cause mortality and 3.08% (95% CI: 0.69, 5.29) for cardiovascular mortality per interquartile-range increase of 103.0 μg/m3 with a 2-day lag of PM2.5 concentrations in Xi’an. Considering that there was an approximately 10-fold higher level of PM2.5 observed in this study, we found that the magnitude of mortality risk of PM2.5 observed is comparable to that found in Western populations, which tend to have much lower PM2.5 exposure levels. In a study in 6 California counties, Ostro et al. (31) reported a 1.6% (95% CI: 0.0, 3.1) increase in cardiovascular mortality, for an interquartile-range increase of 14.6 μg/m3 with a 3-day lag for PM2.5 exposure. A case-crossover analysis conducted in 25 US communities showed an association between PM2.5 and all-cause mortality of 1.12% (95% CI: 0.29, 2.14) and stroke mortality of 1.36% (95% CI: 0.02, 2.04) increases, per 10-μg/m3 increase of PM2.5 (32). A recent analysis by Zanobetti and Schwartz (33) reported an increased cardiovascular mortality risk of 0.85% (95% CI: 0.46, 1.24) per 10-μg/m3 increase in PM2.5 exposure at lag 0–1 days. Although study methods might have influenced the analysis results, disparities in findings between Chinese and Western populations provide evidence of the differential toxicity of PM2.5 with different components across locations and seasons, as well as on various health outcomes.
We observed strong effects of the secondary aerosol anions sulfate, nitrate, and ammonium, as well as the components of biomass combustion that produce sulfur, potassium, chlorine, and bromine species, on all-cause and cardiorespiratory mortality. Likewise, the associations in the heating period were stronger but were reduced or near null in the nonheating period. Although similar mortality effects of secondary aerosols were confirmed in several recent studies (10, 31), findings on various health outcomes remain inconsistent. Hoek et al. (34) reported that sulfate and nitrate were associated with all-cause mortality and that nitrate was also associated with cardiovascular mortality. Mar et al. (35) found increased all-cause and cardiovascular mortality associated with sulfate at the regional scale. In contrast, Fairley (36) indicated that sulfate was associated with respiratory mortality, but no significant associations were found with cardiovascular mortality. However, secondary pollutants, which are related to multiple sources and have undergone complicated atmospheric processing, could vary widely across seasons and geographic locations as regional-scale transport species in China and worldwide (37–39). In a large population-based study across the United States, proportions of sulfate alone or in combination with other species are reported to be major modifiers of the PM2.5 mass–mortality association (9).
For the combustion and traffic markers elemental and organic carbon (38, 40, 41), we observed significant association between elemental carbon and all-cause and cardiovascular diseases mortality, mostly in the heating period. Yet, our findings were consistent with those of previous studies in which the seasonal variation of elemental carbon effects varied with locales and sources. Ostro et al. (31) observed an association between elemental carbon and all-cause mortality in the cold period (October–March) but not in the whole year. Zhou et al. (10) reported that elemental carbon had higher concentrations and stronger effects in the warm period (April–September) in Detroit, while showing higher levels in the cold period (October–March) in Seattle. Ito et al. (11) demonstrated that elemental carbon was significantly associated with cardiovascular mortality in the warm period and nearly significant in the cold period in New York City.
For the transition metals, nickel, zinc, chromium, and lead were found mostly associated with all-cause and cardiorespiratory mortality in the heating period. Chromium, nickel, and zinc were reported from industrial and combustion processes and found to be associated with daily mortality in a multicity analysis conducted in Canada (42). Recent studies provided further evidence that exposure to nickel was related to acute cardiovascular outcome changes (7, 43, 44). For lead, Mar et al. (35) found it was negatively associated with all-cause mortality; however, Ostro et al. (31) did not observe a mortality effect of lead.
In Xi’an, water-soluble and carbonaceous aerosols from coal combustion and vehicle emissions were found to be the dominant species in fine particulate matter in the cold months (45). In the current study, we observed approximately 20%–50% concentration increases of major chemical species in PM2.5 in the cold months, whereas the proportions of most species remained similar across periods. The overall stronger mortality effects of combustion and secondary aerosols observed in the heating period may not be interpreted as independent influences of coal combustion and traffic alone. Potential seasonal influence and effect modifiers for PM2.5 and species-associated health effects warrant further investigation.
An important strength of our study is the application of the distributed-lag model in assessing cumulative effects, rather than using models that may estimate the effects of a single day or several days at a particular lag period alone. In our study, the key results of PM2.5 and species-associated mortality effects, obtained by both the individual-lag model and the distributed-lag model, are largely in agreement with each other. The distributed-lag model approach better summarized the cumulative effects in association with exposure to PM2.5 and species in proceeding days and allowed us to assess the heterogeneity of effects between cold and warm periods.
Our results added to previous findings in several ways. First, the relatively high level of PM2.5 mass and species observed in Xi’an increased the study power to detect small changes and statistically significant associations. Second, with 5 years of daily PM2.5 mass and 1–3 years of subsets of species data, we were able to assess the seasonal distribution of PM2.5 species and the associated mortality effect over time, which has been rarely studied in the Chinese population, and more importantly to provide evidence filling in knowledge gaps in PM2.5-associated effects between Western and Eastern populations. Third, our results were consistent with previous findings on combustion-derived species associated with cardiorespiratory mortality and morbidity risk (10, 11, 46, 47). However, the association patterns over time were not always consistent with previous findings, which suggested that PM2.5 toxicity may be largely determined by its component species from various sources of the local environment and, thus, the overall PM2.5-related effects might be modified by some of its species that differ across locations. Finally, the distinct seasonal pattern of PM2.5 species mortality effects observed in our study provided an important rationale to further examine how time-varying factors, such as personal activity and weather conditions, may modify the mortality effects of PM2.5 and species (48).
Limitations in our study should be noted when interpreting the results. First, exposure data were obtained from a single monitoring station that might not well represent population exposure levels, particularly in Chinese cities with a dense urban population. Ito et al. (49) also found that secondary species tend to have higher monitor-to-monitor temporal correlations, whereas local combustion-sourced species, such as elemental carbon and nickel, had lower correlations. Thus, the interpretation of our results could be limited by the uncertainties with regard to the influence of relative exposure errors on the observed associations across the species.
In summary, we observed a significant mortality association with exposure to PM2.5 and species from combustion, vehicle emission, and industry process sources. The differential seasonal association pattern across species indicated that PM2.5-related effects are not sufficiently explained by mass alone. Although understanding the biologic causal mechanism of the PM2.5 mortality effect remains challenging, our study, along with the related work of others, provides evidence that primary PM2.5 species, as well as time-varying factors, might play important roles in modifying the PM2.5–mortality association. More studies examining source-specific health evidence of PM2.5 will be critical for developing a comprehensive air control strategy at local and regional scales.
Supplementary Material
Acknowledgments
Author affiliations: College of Environmental Sciences and Engineering and Center for Environment and Health, Peking University, Beijing, China (Wei Huang, Yebin Tao, Lingzhen Dai, Tong Zhu); Institute of Earth and Environment, Chinese Academy of Sciences, Xi’an, Shanxi Province, China (Junji Cao); Department of Biostatistics, University of Medicine and Dentistry of New Jersey, Piscataway, New Jersey (Shou-En Lu); and Xi’an Center for Disease Control and Prevention, Xi’an, Shanxi Province, China (Bin Hou, Zheng Wang).
Drs. Wei Huang and Junji Cao are equally contributing first authors to the work.
This study was supported by the China State Ministry of Environmental Protection (grants 201009032 and 200809109) and the Ministry of Science and Technology (grant 2008AA062503). Partial support for Dr. Shou-En Lu was received from the National Institute of Environmental Health Sciences (grant 2P30ES005022-21).
The authors thank Dr. Thomas J. Smith of the Harvard School of Public Health for his review and comments on an earlier version of this paper.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- ICD-10
International Classification of Diseases, Tenth Revision
- PACF
partial autocorrelation function
- PM2.5
particulate matter less than 2.5 μm in aerodynamic diameter
References
- 1.Laden F, Schwartz J, Speizer FE, et al. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities Study. Am J Respir Crit Care Med. 2006;173(6):667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostro B, Broadwin R, Green S, et al. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect. 2006;114(1):29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 4.Samoli E, Peng R, Ramsay T, et al. Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA Study. Environ Health Perspect. 2008;116(11):1480–1486. doi: 10.1289/ehp.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Rajagopalan S, Pope CA, III, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 6.Research Priorities for Airborne Particulate Matter: IV. Continuing Research Progress. Washington, DC: National Research Council; 2004. [Google Scholar]
- 7.Bell ML, Ebisu K, Peng RD, et al. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179(12):1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng RD, Dominici F, Pastor-Barriuso R, et al. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161(6):585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 9.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19(5):680–689. doi: 10.1097/ede.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Ito K, Lall R, et al. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect. 2011;119(4):461–466. doi: 10.1289/ehp.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K, Mathes R, Ross Z, et al. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2010;119(4):467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson RW, Fuller GW, Anderson HR, et al. Urban ambient particle metrics and health: a time-series analysis. Epidemiology. 2010;21(4):501–511. doi: 10.1097/EDE.0b013e3181debc88. [DOI] [PubMed] [Google Scholar]
- 13.Health Effects Institute. Outdoor Air Pollution and Health in the Developing Countries of Asia: A Comprehensive Review. Boston, MA: Health Effects Institute; 2010. [Google Scholar]
- 14.Lee JT, Shin D, Chung Y. Air pollution and daily mortality in Seoul and Ulsan, Korea. Environ Health Perspect. 1999;107(2):149–154. doi: 10.1289/ehp.99107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piver WT, Ando M, Ye F, et al. Temperature and air pollution as risk factors for heat stroke in Tokyo, July and August 1980–1995. Environ Health Perspect. 1999;107(11):911–916. doi: 10.1289/ehp.99107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CM, Vichit-Vadakan N, Kan H, et al. Public Health and Air Pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect. 2008;116(9):1195–1202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang W, Liu W, Qian J, et al. Modeling and simulation of a transcutaneous energy transmission system used in artificial organ implants. Artif Organs. 2009;33(12):1069–1074. doi: 10.1111/j.1525-1594.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- 18.Chan CK, Yao X. Air pollution in mega cities in China. Atmos Environ. 2008;42(1):1–42. [Google Scholar]
- 19.Zhang XY, Cao JJ, Su H, et al. Particulate pollution control in Xi’an. In: United Nations Development Program, China International Center of Economic and Technology Exchange, editor. Urban Air Pollution Control in China. Beijing, China: China Science & Technology Press; 2001. [Google Scholar]
- 20.Cao JJ, Lee SC, Zhang XY, et al. Characterization of airborne carbonate over a site near Asian dust source regions during spring 2002 and its climatic and environmental significance. J Geophys Res. 2005;110(D3):D03203. (doi:10.1029/2004JD005244) [Google Scholar]
- 21.Cao JJ, Wu F, Chow JC, et al. Characterization and source apportionment of atmospheric organic and elemental carbon during fall and winter of 2003 in Xi’an, China. Atmos Chem Phys. 2005;5(3):3127–3137. [Google Scholar]
- 22.Cao Z, Yang Y, Lu J, et al. Atmospheric particle characterization, distribution, and deposition in Xi’an, Shaanxi Province, central China. Environ Pollut. 2011;159(2):577–584. doi: 10.1016/j.envpol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Samet JM, Katsouyanni K, Pia T. Air pollution and health: a combined European and North American Approach (APHENA) Epidemiology. 2006;17(suppl 6):S19–S20. [Google Scholar]
- 24.Wood SN. Generalized Additive Models: An Introduction With R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 25.Hurvich CM, Simonoff JS, Tsai CL. Smoothing parameter selection in nonparametric regression using an improved Akaike Information Criterion. J R Stat Soc Series B Stat Methodol. 1998;60(2):271–293. [Google Scholar]
- 26.Bell ML, McDermott A, Zeger SL, et al. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA. 2004;292(19):2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martuzevicius D, Grinshpun SA, Reponen T, et al. Spatial and temporal variations of PM(2.5) concentration and composition throughout an urban area with high freeway density—the Greater Cincinnati Study. Atmos Environ. 2004;38(8):1091–1105. [Google Scholar]
- 28.Weber R, Bergin M, Kiang CS, et al. Short-term temporal variation in PM2.5 mass and chemical composition during the Atlanta Supersite Experiment, 1999. J Air Waste Manag Assoc. 2003;53(1):84–91. doi: 10.1080/10473289.2003.10466123. [DOI] [PubMed] [Google Scholar]
- 29.Samet JM, Dominici F, Zeger SL, et al. The National Morbidity, Mortality, and Air Pollution Study. Part I: Methods and Methodologic Issues. Cambridge, MA: Health Effects Institute; 2000. (Health Effects Institute research report no. 94 (part. 1)) [PubMed] [Google Scholar]
- 30.Dominici F, McDermott A, Daniels M, et al. Revised Analyses of Time-Series Studies of Air Pollution and Health. Boston, MA: Health Effects Institute; 2003. Mortality among residents of 90 cities; pp. 9–24. (Health Effects Institute special report) [Google Scholar]
- 31.Ostro B, Feng WY, Broadwin R, et al. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2007;115(1):13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17(3):279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 33.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoek G, Brunekreef B, Verhoeff A, et al. Daily mortality and air pollution in the Netherlands. J Air Waste Manag Assoc. 2000;50(8):1380–1389. doi: 10.1080/10473289.2000.10464182. [DOI] [PubMed] [Google Scholar]
- 35.Mar TF, Norris GA, Koenig JQ, et al. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108(4):347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairley D. Daily mortality and air pollution in Santa Clara County, California: 1989–1996. Environ Health Perspect. 1999;107(8):637–641. doi: 10.1289/ehp.99107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang XY, Cao JJ, Li LM, et al. Characterization of atmospheric aerosol over Xi’an in the south margin of the Loess Plateau, China. Atmos Environ. 2002;36(26):4189–4199. [Google Scholar]
- 38.Viana M, Querol X, Alastuey A, et al. Identification of PM sources by principal component analysis (PCA) coupled with wind direction data. Chemosphere. 2006;65(11):2411–2418. doi: 10.1016/j.chemosphere.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YH, Su H, Zhong LJ, et al. Regional ozone pollution and observation-based approach for analyzing ozone-precursor relationship during the PRIDE-PRD2004 campaign. Atmos Environ. 2008;42(25):6203–6218. [Google Scholar]
- 40.Hammond DM, Dvonch JT, Keeler GJ, et al. Sources of ambient fine particulate matter at two community sites in Detroit, Michigan. Atmos Environ. 2008;42(4):720–732. [Google Scholar]
- 41.Zheng M, Salmon LG, Schauer JJ, et al. Seasonal trends in PM2.5 source contributions in Beijing, China. Atmos Environ. 2005;39(22):3967–3976. [Google Scholar]
- 42.Burnett RT, Brook J, Dann T, et al. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal Toxicol. 2000;12(suppl 4):15–39. doi: 10.1080/08958370050164851. [DOI] [PubMed] [Google Scholar]
- 43.Lippmann M, Ito K, Hwang JS, et al. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114(11):1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanobetti A, Franklin M, Koutrakis P, et al. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:58. doi: 10.1186/1476-069X-8-58. (doi:10.1186/1476-069x-8-58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen ZX, Cao JJ, Arimoto R, et al. Chemical characteristics of fine particles (PM1) from Xi’an, China. Aerosol Sci Technol. 2010;44(6):461–472. [Google Scholar]
- 46.Ostro B, Lipsett M, Reynolds P, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118(3):363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metzger KB, Tolbert PE, Klein M, et al. Ambient air pollution and cardiovascular emergency department visits. Epidemiology. 2004;15(1):46–56. doi: 10.1097/01.EDE.0000101748.28283.97. [DOI] [PubMed] [Google Scholar]
- 48.Ito K, Xue N, Thurston G. Spatial variation of PM2.5 chemical species and source-apportioned mass concentrations in New York City. Atmos Environ. 2004;38(31):5269–5282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.