Abstract
Background
The aim of this study was to describe the EEG and clinical profile of seizures in children with non-traumatic coma, compare seizure detection by clinical observations with that by continuous EEG, and relate EEG features to outcome.
Methods
This prospective observational study was conducted at the paediatric high dependency unit of Kilifi District Hospital, Kenya. Children aged 9 months to 13 years presenting with acute coma were monitored by EEG for 72 h or until they regained consciousness or died. Poor outcome was defined as death or gross motor deficits at discharge.
Results
82 children (median age 2.8 (IQR 2.0–3.9) years) were recruited. An initial medium EEG amplitude (100–300 mV) was associated with less risk of poor outcome compared to low amplitude (≤100 mV) (OR 0.2, 95% CI 0.1 to 0.7; p<0.01). 363 seizures in 28 (34%) children were observed: 240 (66%) were electrographic and 112 (31%) electroclinical. In 16 (20%) children, electrographic seizures were the only seizure types detected. The majority (63%) of electroclinical seizures had focal clinical features but appeared as generalised (79%) or focal with secondary generalisation (14%) on EEG. Occurrence of any seizure or status epilepticus during monitoring was associated with poor outcome (OR 3.2, 95% CI 1.2 to 8.7; p=0.02 and OR 4.5, 95% CI 1.3 to 15.3; p<0.01, respectively).
Conclusion
Initial EEG background amplitude is prognostic in paediatric non-traumatic coma. Clinical observations do not detect two out of three seizures. Seizures and status epilepticus after admission are associated with poor outcome.
Introduction
Acute coma is a major cause of paediatric hospital admissions in sub-Saharan Africa. Cerebral malaria (CM), acute bacterial meningitis (ABM) and viral encephalitides are common causes. These acute infectious encephalopathies are associated with high mortality and neuro-cognitive morbidity1–3 and are often accompanied by seizures.1 2 4 For example, in CM, over 80% of children have a history of convulsive seizures on admission and 60% have further seizures after admission.5 In ABM, up to 66% of the children have a history of convulsive seizures at admission.1 Recurrent and prolonged seizures are a risk factor for death and neurological sequelae in these encephalopathies.1 2 4
Seizures in comatose children may manifest as short lived convulsions, prolonged seizures or status epilepticus, or subtle or electrographic seizures. Continuous EEG observations of comatose patients in intensive care units in the developed world have demonstrated a high incidence of electrographic seizures, usually not detected clinically because the children are ventilated and paralysed.6 Also, non-epileptic events such as abnormal movements and motor posturing are common in comatose children and are often mistakenly regarded as seizures.7 The implication of such misdiagnosis is that a number of children are inadvertently treated and exposed to the potential adverse effects of antiepileptic drugs (AEDs). Further, misattribution of such events to seizures impairs our understanding of the pathophysiology of acute encephalopathies. Thus, continuous EEG monitoring could reveal more seizures and guide their treatment and prevention, help confirm the cause and role of non-epileptic events, and provide insights into the pathophysiology of acute encephalopathies.8
What is already known on this topic.
-
▶
In sub-Saharan Africa, experience with continuous EEG monitoring in childhood coma is minimal.
-
▶
There is little information about the burden of electrographic seizures and their implications for outcome.
What this study adds.
-
▶
Electrographic seizures, typically generalised, frequently occur in children with acute non-traumatic coma in sub-Saharan Africa.
-
▶
The great majority of seizures are missed by clinical observations alone in this setting.
-
▶
The implications of electrographic seizures on outcomes and motor function need to be examined and simpler tools for seizure monitoring in these children explored.
Continuous EEG monitoring is often performed with devices that have one, two or four EEG channels, such as the cerebral function analysis monitor (CFAM), which are relatively easy to use and interpret but miss an unknown proportion of seizures.9 We describe the types and characteristics of seizures in African children presenting with acute non-traumatic coma, estimate the burden of electrographic seizures as detected by 16-lead and 21-lead EEG machines, and relate EEG features to clinical features and outcome.
Methods
Setting
We conducted this study in the paediatric high dependency unit (HDU) of Kilifi District Hospital on the Kenyan coast. This hospital admits approximately 5000 children (0–13 years of age) annually, 15% of whom are initially managed in the HDU, a seven-bed facility with a typical nurse to patient ratio of 1:3. Parents or guardians stay with their children during the course of admission.
Study participants
We recruited children aged between 9 months to 13 years who presented with acute coma (Blantyre coma score (BCS) ≤2 persisting longer than 30 min after correction of hypoglycaemia and/or AED treatment) between January 2005 and December 2008.10 We excluded those with epilepsy or significant developmental delay. The study was approved by the Kenya Medical Research Institute Ethics Committee (SSC No 1249). We obtained informed consent from the parents or guardians of all patients recruited into the study.
Standard care
At admission, we provided emergency care including correction of hypoxaemia, hypoglycaemia, shock, severe electrolyte abnormalities and anaemia, based on standard guidelines.11 12 We used the WHO definition of CM: coma in a child with malaria parasitaemia in whom no other cause of illness can be found.13 ABM was considered if bacteria were identified on gram stain or isolated on culture from cerebrospinal fluid (CSF), CSF antigen tests for Haemophilus influenzae or Streptococcus pneumoniae were positive, or there was a CSF leucocyte count of at least 10 per µl and the blood to CSF glucose ratio was less than 0.67.14 Children without evident bacterial or malaria infection were classified as having unknown encephalopathy. The hospital did not have the capacity to perform tests for viruses.
We administered AEDs only for clinically evident seizures lasting at least 5 min. The first line AEDs were intravenous diazepam (0.3 mg/kg) or intramuscular paraldehyde (0.4 ml/kg) depending on venous access. A phenobarbital infusion (15 mg/kg intravenous) over 20 min was administered if the seizures were not terminated within 10 min of the second dose of the first line AED. Sodium valproate (25 mg/kg intravenous) was given if the seizures had not stopped 20 min after this. Seizures refractory to these interventions were treated with a bolus of thiopental (0.4 mg/kg intravenous) or continuous infusion of midazolam at an initial dose of 0.3 mg/kg intravenously followed by infusion at a rate of 0.1 mg/kg/h. Other than bag and mask ventilation, no other respiratory support was available. All surviving patients underwent neurological examination by SG at discharge. Gross motor deficit was indicated in a child with limb or cranial nerve paresis or paralysis. Cognitive assessments were not consistently performed at discharge. Poor outcome was defined as death or gross motor deficit at discharge.
Acquisition and analysis of EEG data
We considered alternate patients for recruitment because of the limited availability of EEG machines and staff to manage the machines. After stabilising the child and obtaining consent, we initiated EEG monitoring. We used the international 10–20 system for electrode placement using gold-plated electrodes.15 16 Two EEG machines were available for use: a 21-lead Grass-Telefactor Twin monitor (version 3.4.57; Astro-Med, Slough, UK) and a 16-lead Nervus EEG monitor (study room version 3.3.779; Taugagreining, Reykjavik, Iceland); the latter did not have ‘z’ leads. We maintained electrode impedance at less than 5 KΩ. We maintained the children on EEG monitoring for 72 h unless they regained consciousness or died before the end of that period. The nursing staff closely monitored the children and recorded clinically recognisable seizures on a specially designed chart indicating the type and site of onset of seizures and concurrent vital signs. In addition, the children's parents or guardians who were resident with the children alerted nurses if they noted any events.
Using pilot data from 16 patients, we developed a standard proforma for the documentation and analysis of EEG recordings, which included the background activity (symmetry, amplitude and frequency) at the start and end of recording, region of onset and the duration of each epileptiform episode, and transient background attenuations. We used the first 20 min of the recording to define the EEG at the start of the recording. We categorised EEG amplitudes into three bands: ‘low’ (<100 µV), ‘medium’ (100–300 µV) and ‘high’ (>300 µV) amplitudes. Background frequencies were grouped into three categories: delta, mixed theta and delta, and predominantly theta or greater frequency. We defined an EEG seizure as a distinct episode of epileptiform activity (sharp waves, spikes, sharp-slow waves, spike-slow waves and poly-spike-slow waves) over a minimum duration of 6 s, at least 9 s from another episode. Status epilepticus was defined as continuous epileptiform activity over at least 30 min or at least three distinct seizure episodes within 1 h without regaining consciousness.17 Hemispheric, unilateral or multifocal seizures were classified as focal. Clinically subtle seizures, usually characterised by unilateral twitching of facial muscles, were classified as focal clinical seizures. Seizures that were apparent both on EEG and clinically were referred to as electroclinical seizures, while those that had EEG manifestation without any clinical correlate were referred to as electrographic seizures. Transient attenuation, usually occurring after a seizure, was defined as generalised dampening of the EEG over a minimum duration of 6 s with consequent return to the preceding EEG pattern. Each recording was analysed by two of four independent raters trained by SW (consultant clinical neurophysiologist): RI (paediatric neurologist), SG (paediatric neurology trainee), GO and HG (neurophysiology technologists). The two reports were examined for congruency and disparities were reviewed by the whole team and any further disparities examined by SW. In addition, unusual EEG patterns were examined and reported by SW.
Data analysis
We analysed the data using Intercooled Stata (version 11.0; Stata, College Station, Texas, USA). We used Pearson's χ2 test to compare proportions. We examined the association between background EEG features, electroclinical and electrographic seizures, and outcome. We described the results in terms of OR and 95% CI. We applied the Kruskal–Wallis equality of population test to examine non-normally distributed continuous data. All analyses used the conventional 5% significance level.
Results
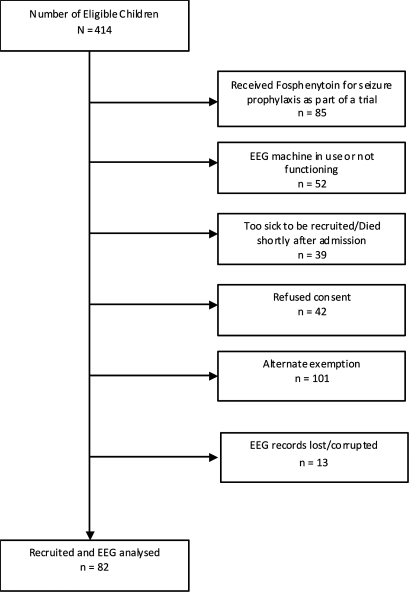
We conducted observations on 82 children (38 girls) (figure 1) with a median age of 2.8 (IQR 2.0–3.9) years. Sixty children (73%) had CM, four (5%) ABM, one (1%) sepsis (without features of meningitis) and 17 (21%) encephalopathy of unknown aetiology, probably viral encephalitis. Eligible children who were not recruited for monitoring (figure 1) had a higher prevalence of encephalopathy of unknown aetiology and ABM, and a lower prevalence of CM, compared to those who were recruited (table 1). Seventeen (21%) of the recruited children had gross motor deficits at discharge. Ten (12%) children died, compared to 81 (24%) of the eligible children who were not monitored (table 1). There was no significant association between coma status and diagnosis, and outcome (see supplementary online table 1). Sixty-two (76%) of the children who underwent monitoring had a history of convulsive seizures at or before admission. The median duration of illness at presentation was 3 (IQR 2–4) days, and the median duration from the time of admission to the start of EEG monitoring was 3.6 (IQR 2.6–7.6) h. Thirty-eight (46%) children had seizures during this intervening period of initial resuscitation, with 30 of them receiving first line AEDs and eight subsequently receiving second line AEDs. The median EEG recording time was 22 (IQR 12–44) h.
Figure 1.
Study flowchart.
Table 1.
Comparison of clinical profiles between children who were monitored and those who were not
| Monitored (n=82) | Not monitored (n=352) | p Value | |
|---|---|---|---|
| Age (months), median (IQR) | 34 (25–47) | 34 (21–46) | 0.58* |
| Sex | |||
| Male | 44 (54%) | 182 (55%) | |
| Female | 38 (46%) | 150 (45%) | 0.85 |
| History of seizures | 62 (76%) | 201 (79%)† | 0.58 |
| Coma level (BCS) | |||
| 2 | 43 (52%) | 164 (49%) | |
| 1 | 27 (33%) | 88 (27%) | |
| 0 | 12 (15%) | 80 (24%) | 0.154 |
| Diagnosis | |||
| Cerebral malaria | 60 (73%) | 185 (56%) | |
| Bacterial meningitis | 4 (5%) | 24 (7%) | |
| Unknown encephalopathy | 17 (21%) | 123 (37%) | |
| Sepsis | 1 (1%) | 0 | <0.01 |
| Died | 10 (12%) | 81 (24%) | 0.01 |
Kruskal–Wallis equality of population test.
n=253.
BCS, Blantyre coma score.
Background EEG activity
In 71 (87%) children, the initial background EEG activity was characterised by delta activity (<4 Hz) and only 11 (13%) had predominant frequencies of 4 Hz or greater. This was little changed at the end of the recording, when 68 (84%) had delta background frequency. There was no association between initial or final background EEG frequencies and outcome (table 2).
Table 2.
EEG characteristics compared with outcome
| Characteristic | Category | Good outcome | Poor outcome | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Background characteristics | ||||||
| Initial amplitude | Low | 10 | 13 | |||
| Medium | 34 | 10 | 0.2 | 0.1 to 0.7 | <0.01 | |
| High | 11 | 4 | 0.3 | 0.1 to 1.3 | 0.07 | |
| Final amplitude | Low | 23 | 16 | |||
| Medium | 29 | 11 | 0.6 | 0.2 to 1.4 | 0.21 | |
| High | 2 | 0 | 0 | 0.25 | ||
| Initial frequency | Theta+some delta | 7 | 3 | |||
| Delta | 48 | 23 | 1.1 | 0.3 to 4.8 | 0.9 | |
| Mainly theta/greater | 0 | 1 | 0.2 | |||
| Final frequency | Theta+some delta | 9 | 4 | |||
| Delta | 45 | 23 | 1.2 | 0.3 to 4.2 | 0.8 | |
| Mainly theta/greater | 0 | 0 | ||||
| Seizure profile | ||||||
| During admission | No seizure | 20 | 5 | |||
| Seizures | 35 | 22 | 2.5 | 0.8 to 7.9 | 0.1 | |
| During monitoring | No seizure | 41 | 13 | |||
| Seizures | 14 | 14 | 3.2 | 1.1 to 8.7 | 0.02 | |
| Seizure types | Electrographic seizures | 12 | 10 | 2.1 | 0.8 to 5.9 | 0.1 |
| Electroclinical seizures | 5 | 6 | 2.9 | 0.8 to 10.7 | 0.1 | |
Twenty-three (28%) children had low background amplitude at the start of recording, 44 (44%) medium amplitude and 15 (18%) high amplitude. At the end of recording, 39 (48%) children had low background wave amplitude, 40 (49%) medium amplitude and only 2 (2%) high amplitude. An initial medium amplitude was associated with lower risk of poor outcome compared to lower amplitude (OR 0.23, 95% CI 0.07 to 0.72; p<0.01) (table 2). Asymmetrical background was observed in only three children at the start of recording (one died and the other two survived without motor deficits) and in two at the end of recording (one had quadriplegia and impaired speech at discharge and the other survived without motor deficits at discharge). Transient attenuations, seen in 24 (31%) children, were mostly observed after EEG seizure episodes, and did not affect outcome.
EEG and clinical seizures
There was good inter-observer agreement on the presence of seizures on EEG between the different observers (see supplementary online table 2). A total of 363 seizures were observed in 28 (34%) children during the period of EEG monitoring, 212 (58%) of which were observed within the first 24 h of monitoring. On EEG, 247 (68%) seizures were generalised, 2 (0.6%) were of bilateral widespread multifocal origin, 38 (11%) were hemispheric (right 18 and left 20) and 65 (19%) had other focal origins. Regarding overlap in the origin of focal seizures, 39 (60%) had frontal-polar, 38 (58%) occipital, 19 (29%) temporal, 14 (22%) parietal, 10 (15%) frontal and 2 (3%) central origins, alone or in combination with other placement areas. All the seizures detected by the 21-lead EEG machine would have been detected by the 16-lead EEG machine, and 338 (93%) of all the seizures detected by both machines could have been detected with the standard placement leads used for the 2-channel CFAM (F3, F4, P3 and P4).9
Overall, 240 (66%) seizures, observed in 22 children, were electrographic. In 16 children (57% of the children who had seizures during monitoring), electrographic seizures were the only form of seizures detected during monitoring. Ten (63%) of these 16 had experienced clinically overt seizures during the preceding period of resuscitation, four (25%) of whom were given second line AEDs as a result. The frequency of electrographic seizures was similar across aetiological groups. A total of 112 (32%) seizures were electroclinical. Although 63% of these electroclinical seizures were focal clinically, on EEG they appeared mostly as generalised seizures (79%) or focal with secondary generalisation (14%) (table 3). Only five (4%) of the electroclinical seizures were actually focal clinically and on EEG. Eleven (3%) motor events characterised by clonic limb movements, observed in four children, were initially considered to be clinical seizures but had no EEG correlate and were not considered in further analysis.
Table 3.
Clinical seizure types and corresponding EEG seizure type
| EEG seizure type | |||
|---|---|---|---|
| Clinical seizure type | Generalised | Focal secondarily generalised | Focal |
| No clinical manifestation | 166 | 48 | 26 |
| Generalised | 27 | 12 | 0 |
| Focal secondarily generalised | 0 | 1 | 1 |
| Focal | 56 | 10 | 5 |
Seizures and outcome
Six (60%) of the children who died had seizures during monitoring compared to eight (47%) of those who had gross motor deficits, and 14 (25%) of those who survived without any apparent motor deficits at discharge (p=0.01; Kruskal–Wallis (KW) test) (figure 2). Children who died had a median EEG duration of seizures of 21 (IQR 0–168) min compared to 0 (IQR 0–8) min among all those who survived (p=0.06; KW test). Children who had gross motor deficits at discharge had a median seizure duration of 0 (IQR 0–452) min compared to 0 (IQR 0–1) min among those who survived without any deficits (p=0.03; KW test). Thus, the occurrence of any seizure during the period of monitoring, whether electrographic or electroclinical, was associated with an increased risk of poor outcome (OR 3.2, 95% CI 1.2 to 8.7; p=0.02) (table 2). Electrographic or electroclinical seizures were not associated with poor outcome when considered separately (table 2). Seventeen (20%) children had status epilepticus during the period of recording and were more likely to have a poor outcome compared to children who did not have any seizures during monitoring (OR 4.5, 95% CI 1.3 to 15.3; p<0.01), but not when compared to children who had seizures and no status epilepticus (OR 2.5, 95% CI 0.5 to 12.8; p=0.25).
Figure 2.
Individual patient seizure profiles. (A) Seizure profiles of children who survived without any apparent neurological sequelae at discharge; (B) seizure profiles of children who had gross motor deficits at discharge from hospital; (C) seizure profiles of children who died during admission. The y axis allows for two values: a floor indicating no seizure and a ceiling indicating a seizure. All values within the individual traces take on one or other of these two values throughout time which is measured in minutes since admission on the x axis. Proportionately, more children who died or had motor deficits at discharge had more seizures, indicated by repeated vertical lines representing repeated seizures, and horizontal lines on top of the individual graphs, representing individual prolonged seizures. Sz, seizure.
EEG during abnormal motor posturing
Twelve children exhibited abnormal motor posturing during monitoring. Seven had decerebrate posturing, three decorticate posturing, and two opisthotonus as well as decerebrate and decorticate posturing. In seven children, there were accompanying EEG background changes characterised by high amplitude (300–500 mV) slow waves (1–3 Hz) during the episodes of posturing. In one child with decerebrate posturing, EEG epileptiform activity was observed during an episode of abnormal posturing.
Discussion
We carried out a prospective study with continuous EEG in 82 children with acute non-traumatic coma. Initial medium amplitude was associated with better outcome. We detected seizures in 34% of the children during monitoring and 20% had electrographic seizures only. Clinical observations alone did not detect 66% of the seizures apparent on EEG.
Previous studies have documented electrographic seizures in 7–65% of comatose children.18–22 Most of these studies have included children of different age groups, varied aetiology including epilepsy, and conditions that are not primarily neurological. Further, in developed countries where most of these studies have been performed, children are often paralysed and ventilated, thus suppressing clinical manifestations of seizures. In one study of non-ventilated Kenyan children with CM, EEG monitoring, albeit with intermittent (6 h) recordings, revealed clinically subtle and electrographic seizures in 23% of the patients.23 This is similar to the findings in our study, where electrographic seizures were observed in 27% of children with CM and 24% of children with unknown encephalopathies. Thus, the occurrence of electrographic seizures does not appear to be modified by aetiology and it could be that there are genetic or environmental factors that influence predisposition to seizures in childhood encephalopathies irrespective of aetiology. As only four of our children had ABM and one had sepsis, it was inappropriate to calculate the prevalence of electrographic seizures in these groups.
In our study, most of the electroclinical seizures had focal clinical manifestations. However, on EEG they appeared mostly as generalised, bilateral multifocal or focal with secondary generalisation. Overall, the majority of the seizures, electroclinical or electrographic, were generalised. Focal seizures, as apparent on EEG, were mostly either hemispheric or frontal in origin even when considering CM patients only. This differs from the findings in the earlier study on children with CM from this centre in which most of the seizures on EEG appeared to originate from the posterior temporo-parietal region.23 This is a watershed region lying between the areas supplied by the middle and posterior cerebral arteries, which is vulnerable to hypoxia. In contrast, we found that most seizures were generalised in origin and the few that were focal were hemispheric, frontal or occipital in origin. Studies with concurrent MRI and cerebral blood flow measurements may help clarify the relationship between pathology and the origin of seizures.
Occurrence of any type of seizure was associated with poor outcome. Status epilepticus was associated with an even greater risk of poor outcome. Electrographic seizures alone were not independently associated with poor outcome. Experimental evidence indicates that prolonged electrographic seizures may result in memory and behavioural problems.24 It is possible that cognitive tests and prolonged follow-up in larger studies may reveal neuro-cognitive deficits in patients with electrographic seizures. Whether seizures are a cause or consequence of brain damage in acute encephalopathies can be clarified by a trial of prophylactic AEDs.
Our study has a number of limitations. We initially intended to obtain concurrent video and EEG data to clearly demonstrate discrepancies between clinical and EEG seizure detection. Unfortunately, this was not consistently possible because of technical difficulties, likely due to the high humidity and temperatures in our setting. To determine the presence or lack of a clinical correlate to an EEG seizure event, we partly relied on observations by the nurses. It is possible that some of the seizures classified as electrographic may have had subtle clinical correlates or may have been classified as such due to a lapse in observation.
Considering the study design, we were often not able to include children who were not clinically stable at admission and who were therefore more likely to die shortly after admission. Thus, a greater proportion of eligible children who were not monitored died compared to those who were monitored (table 1). These children were more likely to have a diagnosis of ABM and unknown encephalopathy (table 1), conditions which were associated with greater mortality compared to CM (see online supplementary table 3). In our study, we assessed for motor neurological sequelae at discharge. Previous studies have documented significant occurrence of neuro-cognitive sequelae many years after hospital admission for acute non-traumatic encephalopathies.25–27 Other studies have also documented resolution of neurological deficits observed at discharge.28 Thus, it would have been optimal to assess for neurological deficits over a longer period after discharge. Further, cognitive assessments may have revealed more deficits, perhaps helping to clarify their association with electrographic seizures.
All the observers involved in EEG analysis were trained in clinical neurophysiology by SW, a consultant clinical neurophysiologist, but only GO and EC were formally certified EEG technologists. SW examined all EEGs in which there was lack of agreement between two raters.
In spite of these limitations, our study highlights the significant burden of seizures missed by clinical observations alone in sub-Saharan African children with acute coma. Although continuous EEG monitoring with more leads is more sensitive for detecting seizures in comatose patients, devices with one to four EEG channels which are cheaper and can be more easily interpreted by less experienced clinicians and nurses, have potential utility in resource-poor settings. However, such use will have to be guided by appropriate training and experience to promote accuracy of analysis and reporting. Prophylactic AEDs on admission should be investigated for improving outcomes in this group of children.
Supplementary Material
Acknowledgments
Godfrey Otieno of KEMRI assisted in the design and conduct of this study. Professor Piet Kager of the Department of Infectious Diseases at the University of Amsterdam helped design the study and review the manuscript before submission. Rachel Odhiambo of KEMRI helped develop and maintain the study database.
Footnotes
Contributors SG assisted in the design and conduct of the study, analysed the data and drafted the manuscript. RI, EC, HG, SW and CN assisted in the design and conduct of the study, analysed the data and reviewed the manuscript. GF and FK assisted in the design of the study, analysed the data and reviewed the manuscript.
Funding This research was supported by the Wellcome Trust, UK, through a senior research fellowship awarded to CN (070114). SG was supported by a Wellcome Trust strategic award for training to the Kenya Medical Research Institute.
This manuscript is published with the permission of the Director of the Kenya Medical Research Institute.
Competing interests None.
Ethics approval This study was approved by the Kenya Medical Research Institute Ethics Committee (SSC No 1249).
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Pelkonen T, Roine I, Monteiro L, et al. Risk factors for death and severe neurological sequelae in childhood bacterial meningitis in sub-Saharan Africa. Clin Infect Dis 2009;48:1107–10 [DOI] [PubMed] [Google Scholar]
- 2.Anga G, Barnabas R, Kaminiel O, et al. The aetiology, clinical presentations and outcome of febrile encephalopathy in children in Papua New Guinea. Ann Trop Paediatr 2010;30:109–18 [DOI] [PubMed] [Google Scholar]
- 3.Idro R, Carter JA, Fegan G, et al. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child 2006;91:142–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol 2005;4:827–40 [DOI] [PubMed] [Google Scholar]
- 5.Crawley J, Smith S, Kirkham F, et al. Seizures and status epilepticus in childhood cerebral malaria. QJM 1996;89:591–7 [DOI] [PubMed] [Google Scholar]
- 6.Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med 2009;37:2051–6 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch LJ. Continuous EEG monitoring in the intensive care unit: an overview. J Clin Neurophysiol 2004;21:332–40 [PubMed] [Google Scholar]
- 8.Jaitly R, Sgro JA, Towne AR, et al. Prognostic value of EEG monitoring after status epilepticus: a prospective adult study. J Clin Neurophysiol 1997;14:326–34 [DOI] [PubMed] [Google Scholar]
- 9.Murdoch-Eaton D, Darowski M, Livingston J. Cerebral function monitoring in paediatric intensive care: useful features for predicting outcome. Dev Med Child Neurol 2001;43:91–6 [DOI] [PubMed] [Google Scholar]
- 10.Newton CR, Chokwe T, Schellenberg JA, et al. Coma scales for children with severe falciparum malaria. Trans R Soc Trop Med Hyg 1997;91:161–5 [DOI] [PubMed] [Google Scholar]
- 11.WHO Severe falciparum malaria. Trans R Soc Trop Med Hyg 2000;94 Suppl 1:S1–90 [PubMed] [Google Scholar]
- 12.WHO Pocket Book of Hospital Care for Children. Guidelines for the Management of Common Illnesses with Limited Resources, 2005:378. [PubMed] [Google Scholar]
- 13.WHO Guidelines for the Treatment of Malaria, 2010:194. [PubMed] [Google Scholar]
- 14.Berkley JA, Mwangi I, Ngetsa CJ, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. Lancet 2001;357:1753–7 [DOI] [PubMed] [Google Scholar]
- 15.Munday JA. Instrumentation and electrode placement. Respir Care Clin N Am 2005;11:605–15 [DOI] [PubMed] [Google Scholar]
- 16.Ernst Niedermeyer. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields: Lippincott Williams & Wilkins, Philadelphia, USA: 2004 [Google Scholar]
- 17.Sadarangani M, Seaton C, Scott JA, et al. Incidence and outcome of convulsive status epilepticus in Kenyan children: a cohort study. Lancet Neurol 2008;7:145–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahwan A, Bailey C, Shekerdemian L, et al. The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia 2010;51:1198–204 [DOI] [PubMed] [Google Scholar]
- 19.Singh RK, Stephens S, Berl MM, et al. Prospective study of new-onset seizures presenting as status epilepticus in childhood. Neurology 2010;74:636–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay SK, Hirsch LJ, Leary L, et al. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia 2006;47:1504–9 [DOI] [PubMed] [Google Scholar]
- 21.Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia 2006;47:1510–18 [DOI] [PubMed] [Google Scholar]
- 22.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol 2007;37:165–70 [DOI] [PubMed] [Google Scholar]
- 23.Crawley J, Smith S, Muthinji P, et al. Electroencephalographic and clinical features of cerebral malaria. Arch Dis Child 2001;84:247–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krsek P, Mikulecká A, Druga R, et al. Long-term behavioral and morphological consequences of nonconvulsive status epilepticus in rats. Epilepsy Behav 2004;5:180–91 [DOI] [PubMed] [Google Scholar]
- 25.Kihara M, Carter JA, Holding PA, et al. Impaired everyday memory associated with encephalopathy of severe malaria: the role of seizures and hippocampal damage. Malar J 2009;8:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter JA, Neville BG, Newton CR. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res Brain Res Rev 2003;43:57–69 [DOI] [PubMed] [Google Scholar]
- 27.Birbeck GL, Molyneux ME, Kaplan PW, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 2010;9:1173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meremikwu MM, Asindi AA, Ezedinachi E. The pattern of neurological sequelae of childhood cerebral malaria among survivors in Calabar, Nigeria. Cent Afr J Med 1997;43:231–4 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.