Summary
Neurofibromatosis type 1 (NF-1) is one of the most common inherited diseases and as an autosomal dominant genetic disorder results from NF-1 gene mutation with 100% penetration and wide phenotypic variability.
The disease can involve a wide variety of tissues derived from all three embryonic layers. NF-1 vasculopathy has been described primarily in peripheral arteries, but arteries supplying the CNS may also be involved. Of those, extracranial vertebral involvement is the commonest and most important. A series of four patients with NF-1 and vascular disease of the vertebral artery is described with a review of the pathophysiology, vascular phenotypes, their management and the pertinent literature.
Key words: vertebral aneurysms, neurofibromatosis type 1, vertebro-vertebral fistula, endovascular treatment
Introduction
Neurofibromatosis type 1 (NF-1) is a common genetic disorder with a prevalence of about one patient in 3 - 4000. NF-1 primarily affects tissues derived from the neural crest but can also involve non neural crest-derived tissues including bone, brain and blood vessels1,2.
The requisites for diagnosis are the presence of two or more of the following criteria: six or more "café-au-lait" spots, two or more neurofibromas of any type, or one plexiform neurofibroma; axillary or inguinal freckling; two or more Lisch nodules; optic pathway gliomas; distinctive bone lesions such as sphenoid dysplasia or thinning of the long bone cortex, with or without pseudoarthrosis and, finally, a firstdegree relative diagnosed with NF-1 3. NF-1 can go along with three patterns of vascular lesions: arterial stenosis or occlusion, dysplastic changes with aneurysm formation and ruptured arteries causing arteriovenous fistulae. Of these three, stenosis of the renal artery is the most frequent4, but occlusion of the cerebral arteries can also occur and may result in "moyamoyalike" disease1,5,6.
Aneurysmal formation and fistulae in the head and neck region typically affect the vertebral arteries rather than the carotid arteries, these parachordal or vertebra-vertebral fistulas being the consequence of an arterial rupture into a vein7-9.
During the last 20 years we have seen seven patients with NF-1 and associated vascular lesions. One pediatric patient harboured a stenosis of the distal ICA and proximal MCA resulting in a pseudo Moya Moya, one patient had an extradural cervical ICA fusiform aneurysm and one patient had extensive venous lakes within a facial plexiform neurofibroma. The other four patients harboured vascular lesions that were located at the vertebral artery and that we present here as a series of rare cases.
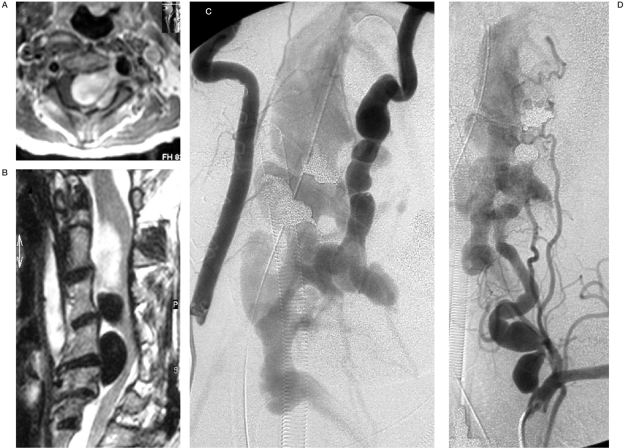
Figure 1.
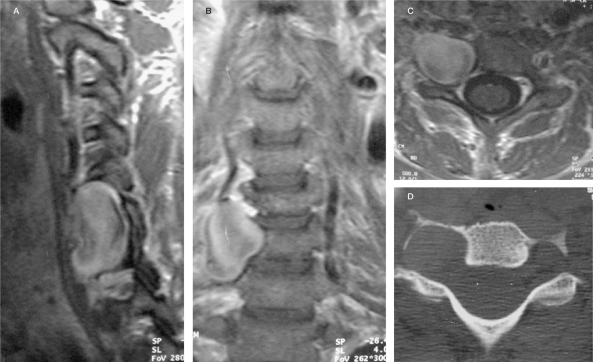
Case 1. A) Sagittal T2-W MRI (A) revealing large flow void epidural structures at C2-5, compatible with venous pouches and a partially thrombosed superior portion, causing severe cord compression. B) Axial T1-W MRI post Gd enhancement showing extension of the epidural venous pouches along the left neural foramen with nerve root compressions. C,D) Right and left vertebral angiograms demonstrating AV shunting from the LVA at C4 level, compatible with VVAVF. Dysplastic change and tortuousity of the LVA proximal and distal to the fistula are also noted with indirect collaterals from the left ascending cervical artery and thyrocervical trunk due to a "sump effect".
Case Reports
Case 1
A 49-year-old woman complained of neck pain with torcicollis for two years prior to her first admission and had progressive swelling of her left hand for four weeks. Neurofibromatosis type I was previously diagnosed by the presence of cutaneous neurofibromas and multiple pigmented skin lesions resembling café-au-lait spots. On clinical examination, there was a left paresis and paresthesia involving the dermatomes and myotomes levels C5 to T1 and hypereflexia. During hospitalization, her neurological status deteriorated quickly, with evolving tetraparesis. MRI showed large hyposignal T1/hyposignal T2 epidural lesions compatible with flow void structures at the left side of C2-4 levels, causing severe spinal cord compression. An intense enhancing hyposignal T1/hypersignal T2 lesion was also noted above the aforementioned flow void structures at the C1-2 level. Due to rapid worsening, a laminectomy was performed to decompress a supposed neurofibroma.
During surgery, an arterialized vein causing spinal cord compression was found and the procedure was stopped. Postoperative angiography disclosed a vertebrovertebral arteriovenous fistula (VVAVF) fed by a dysplastic left vertebral artery at C4 level draining into a complex network of venous channels including the epidural and vertebral venous plexus, internal and external jugular veins. Endovascular treatment of the fistula using bare GDC coils in the proximal portion of the venous pouch and the parent artery was done in another institution resulting in a major reduction of flow through the fistula. Although at the end of the procedure a residual shunt was visible, it was thought to spontaneously thrombose over the following weeks. Following the intervention, the patient had an uneventful recovery and regained her motor function in spite of the partial treatment. Two months later, the patient was hospitalized again following a sudden onset of neck pain accompanied by an audible bruit at the left side of the neck. A new MRI showed increased size of the epidural venous pouches and cord compression.
Because of the rapid clinical deterioration, she was referred to our institution for further endovascular treatment. Occlusion of the left vertebral artery distal to the fistula was achieved by a cross-over technique via the right vertebral artery employing histoacryl diluted with lipiodol at one part to three injected between the previous placed coils, This led to complete occlusion of the vertebral artery fistula, but a small shunt was visible via the thyrocervical artery that could also be occluded with glue. Control angiography performed one week later confirmed complete occlusion of the fistula. The patient's deficits improved and the neck pain disappeared. On two year follow-up, she remains asymptomatic without any sign of fistula recurrence.
Case 2
A 45-year-old man, previously diagnosed NF-1, complained of a sudden onset of a cervical audible bruit, retroauricular and cervical pain with progressive paraparesis of the legs for six months. T2 weighted MRI sequences demonstrated a hyposignal intensity cervical lesion, extending through the right C5-6 neural foramen into the epidural space with cord compression. There was also hypersignal T2 change of the cord, compatible with compressive myelopathy. The right vertebral angiography showed vertebrovertebral arteriovenous fistula at the C5 level. The lesion was embolized after selective catheterization of the vertebral artery with an 8F shuttle catheter with a latex detachable gold-valve balloon Number 9 (Nycomed, Suresnes, France) mounted on a MiniTorquer (Nycomed, Suresnes France), completely closing the fistula and preserving the right vertebral artery by selectively placing the balloon within the fistulous point. This procedure was done under heparinization.
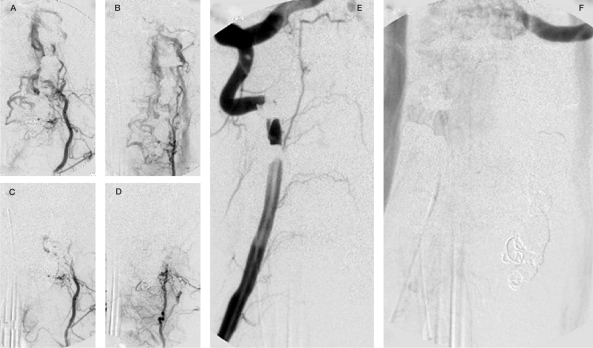
Figure 2.
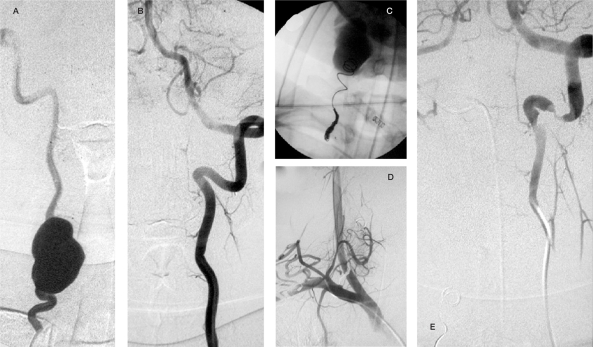
Case 1 (continued),A,B) Left cervical arteries angiogram: collateral contribution to the fistula throughout deep cervical and thyrocervical trunk. C,D) The control after glue and particle embolization. E,F) The right vertebral angiographic control after completed left vertebral occlusion with no remaining fistula.
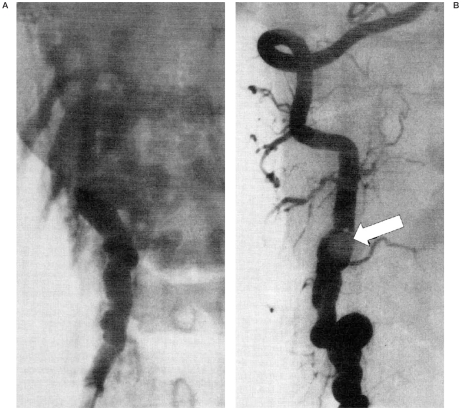
Figure 3.
Case 2. Right vertebral angiogram AP view pre and post gold valve balloon (GVB) embolization demonstrating dysplastic change of the RVA with early filling of the epidural venous plexus at C4 level which completely disappeared after deployment of one No. 9 GVB that enabled preservation of the RVA.
Figure 4.
Case 3. Right vertebral angiogram, AP view, pre-embolization (A) and left vertebral angiogram (B) lateral view pre-embolization both demonstrate an AV shunt at the left C3-4 level, with venous blood draining into dilated epidural pouches and then further draining into the jugular veins. There is complete obliteration of the VVAVF after GDC embolization and sacrifice of the LVA (C, right VA angiogram, AP view).
After embolization the patient had total remission of his symptoms and good clinical evolution (figure 6). The patient was put on aspirin. Follow-up angiography two years later demonstrated persistent occlusion of the fistula and an asymptomatic spontaneous occlusion of the parent artery.
Figure 5.
Case 4. On T2W MRI sequences enlargement of transverse foramen due to a mass presumably representing a large aneurysm (high signal due to low flow and turbulence) can be seen that demonstrates contrast enhancement (A-C: T1 weighted images after contrast) and bone erosion on CT (D). Adjacent structures are compressed.
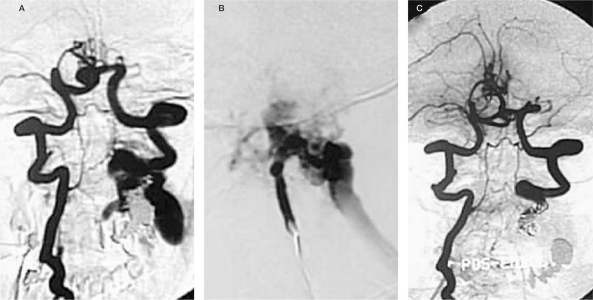
Figure 6.
Case 4 . Angiography (A: right vertebral artery injection, B: left vertebral artery injection) reveals a large fusiform shaped aneurysm at C5-6 level of the RVA with good supply to the posterior circulation through the LVA. After GDC embolization contrast stagnation in the aneurysm was noted immediately after sacrifice of the proximal RVA. Post embolization angiograms (D,E) confirmed complete obliteration of the aneurysm.
Case 3
A 48-year-old man presented with a two year history of cervical bruit and right cervical pain radiating to the right arm. A pulsatile left cervical mass was noted at the initial physical examination. His MRI in T1 and T2 W sequences revealed multiple hyposignal intense structures at the left epidural spaces from C2 to C4 levels and at the left side of the neck, compatible with flow-void structures, suggestive of a vascular lesion. The left and right vertebral angiograms showed a vertebrovertebral arteriovenous fistula at left C3-4 level, draining into multiple epidural pouches further draining into the left internal jugular vein. During fluoroscopy, dysplasia of the left sphenoid bone was also detected and physical examination after the procedure revealed café-au-lait spots and subcutaneous neurofibromas, confirming the diagnosis of NF-1. Due to the good supply to the posterior fossa from the right vertebral artery, the decision to sacrifice the left vertebral artery was taken using bare GDC coils.
After crossing with a two-tip microcatheter from the right vertebral artery to the left, coils were placed into the most proximal portion of the venous pouch and subsequently into the distal parent artery while retrieving the microcatheter. After this distal embolization, the left vertebral artery was catheterized and the proximal portion of the parent artery was occluded while the first coil was anchored in the distal coil package. This procedure was done under heparinization.
Following embolization, no residual flow was noted. The patient's symptoms totally disappeared a few days after embolization and he was discharged from the hospital returning to his normal life. MRI follow-up performed one year later demonstrated complete and stable occlusion of the fistula.
Case 4
A 14-year-old female patient, a known case of NF-1, presented with progressive cervical and right arm pain associated with paresthesis at dermatomes C5 and C6, suggestive of radicular compression. A plain film of the cervical spine showed enlargement of the right C5-C6 transverse foramen and the CT scan and MRI demonstrated that the abnormality was due to a vertebral aneurysm. She was referred to the neuroradiology department and an angiogram confirmed the presence of a right vertebral aneurysm at the C5-C6 level. Due to the symptoms and risk of rupture the patient was treated by sacrifice of the right vertebral artery using bare coils in the proximal part of the parent artery after good collateral to the posterior fossa was demonstrated from the left vertebral artery.
The patient recovered from her paresthesia at one month after the procedure and besides no recurrence of the aneurysm after eight years of clinical, angiographic and MRI follow-up she still has some radicular symptoms attributable to the persisting mass effect of the thrombosed aneurysm.
Discussion
General considerations and genetics on NF-1
NF-1 is characterized by autosomal dominant inheritance with complete penetrance but extremely variable expression10 from simple skin macules and neurofibromas to aggressive multiple tumour presentations or complex vascular lesions11. There is no evidence of locus heterogeneity nor have any homozygotes been found2: individuals with NF-1 are heterozygote for an NF-1 mutation2,10. Familial NF-1 cases are responsible for half of all NF-1 cases 4 while the other half represents new mutations.
The NF-1 gene was isolated in the proximal portion of the long arm of chromosome 17. It encodes a protein, neurofibromin12,13, that is expressed in all tissues during organogenesis, but is found just in a few specific cells in adult individuals13-15. The functions of human neurofibromin are still unclear, but it seems to be involved in the control of cellular growth and differentiation13.
Neurofibromin expression has been recognized in endothelial and smooth muscle cells of blood vessels in renal and cerebral arteries and in the aorta16. Its function in vascular cells is not known but neurofibromin expression is related to a down-regulating of cellular growth and its loss is therefore associated with cell proliferation (tumoral mechanism).
A defect in neurofibromin may therefore be linked to vasculogenesis in NF-1 by stimulation of the proliferation of endothelial and smooth muscle cells. By the same mechanism, the integrity of the endothelial layer can be compromised following a breakdown function promoting the spread of cells and stenosis or increase the fragility of the vessel wall16. Other hypotheses for vascular abnormalities in NF-1 include the presence of a dysplastic process due to abnormal function of neurofibromin altering the vascular histogenesis17-19 or the modification of the normal process of vascular maintenance and repair20.
Interestingly, the vascular disease in NF-1 does not affect all arteries globally in an NF-1 patient, although the whole endothelial system and all vascular cells have the same constitutional mutation of the NF-1 gene. Hamilton assumes that a 'second hit' mutation of the normal NF-1 allele may be needed to promote vascular changes or a somatic mutation at another locus may be necessary for development of NF-1 vascular lesions. Environmental factors probably contribute, such as local hemodynamic injury, diet, smoking, exercise, stress and other recognized factors of vascular lesions20.
Genotype-phenotype correlations in NF-1 are rare and familial correlations are low even for some of the more common general manifestations21,22. However, some evidence of this association has recently been described, such as the concordance between twin pairs for specific malformations and tumors, clusters of findings, or certain recurrent tumor configurations21, which proves the role of non-random genetic mutation factors in the pathogenesis of NF-1. NF-1 gene is a prototype of a histogenesis control gene, i.e. a gene that functions in at least two phases of an organism's life: in coordinating embryologic histogenesis and in wound-healing histogenesis4,17,19.
Pathophysiological concepts in NF-1 vasculopathy
Vascular involvement was first described in 190523 but it was only in 1945 that Reubi first reported the results of his observation of the histological aspects of renal arteries in NF-1 affected patients24 based on the location and size of the arteries involved and the depth of wall extension by the lesion. The histological classification was further expanded by Salyer and Salyer and currently has four categories: a pure intimal type, an advanced intimal type, the intimal aneurysmal type, and the nodular or epithelial type20,24,25. They believed that these alterations result from Schwann cells proliferation in the vascular walls but could not demonstrate this association.
In 1974, Greene reported pathologic findings from the kidney of a patient with multiple vascular lesions studied by electron microscopy revealing the nature of cells comprising the nodules in the pathological arteries as smooth muscle cells. Neither tumors nor Schwann cells were found. He postulated two basic groups of vascular lesions associated with NF-1: dysplasia of the vessel wall with smooth muscle cell proliferation, which is similar to Reubi's descriptions26 and a perivascular involvement, either causing retraction or infiltration of the vessel wall by neurofibromatous tissues27. Both processes may lead to stenosis and occlusive symptoms or arterial dysplasia, aneurysmal formation or arterial rupture.
Deans reported three cases of parachordal fistulas in NF-1 and suggested two pathological mechanisms: the first theory stated that dysplastic smooth muscle or neurofibromatous proliferation in the arterial wall leads to an aneurysm that may subsequently rupture into adjacent veins, while the second postulation is related to mesodermal dysplasia that may lead to congenital fistulae 26,28. The role of mesodermal involvement in NF-1 may be inferred by the characteristic distribution of the vasculopathy among the cervicocranial vessels. Both in our experience (four of seven cases with vertebral artery involvement as stated in the Introduction) and in the literature, the cervical vertebral artery is particularly prone to vascular lesions in NF-1. Since this cervical part of the vertebral artery is the only craniocervical vessel to be derived from the mesoderm while all other vessels are derived from neural crest cells, one may presume mesodermal dysplasia as the cause for the parachordal fistulae which is further underlined by the fact that the (neural crest derived) intracranial portion of the vertebral artery is never involved in the vascular diseases present in NF-129.
NF-1 vascular symptoms and the vertebral artery:
- Extracranial Vertebral Aneurysm (EVA):
Extracranial vertebral aneurysms are rare and can be traumatic or spontaneous in origin. They are usually associated with cervical trauma and may occur in the setting of fibromuscular dysplasia, Ehler-Danlos Syndrome, or infections and inflammatory disease. In an NF-1 context, there are 11 case reports in the literature (See Table 1) to which we have added one. In these reports, patients typically presented with radicular compressive symptoms (n=4), or, when ruptured, hematomas of the neck and thorax (n=4), vascular lesions were asymptomatic in three patients. In the cases presented there was predominance of female gender, the left side was more frequently (88%) affected than the right, and there were encountered in slighty older patients (mean age 44 years old). The most common clinical symptom of EVA in NF-1 is upper radiculopathy followed by acute rupture symptoms, cervical mass and ischemic embolic syndromes, but three asymptomatic cases were also found. Four cases were located between C1-C2 while eight cases were found in the lower cervical segments (C3 - T1).
Table 1.
Spectrum of NF-1 Vasculopathy in Vertebral Artery and Neck Region: Vertebral Aneurysms.
| Authors | Age/ sex |
Clinical symptoms | Type lesion |
Level | Main vessels(s) |
Treatment |
|---|---|---|---|---|---|---|
| Schubiger, 1978[32] | 50/M | Radiculopathy | VA | C2-C6 | LVA | Surgical removal |
| Pentecost, 1981[33] | 1/F | Periferal nerve and plexal impairment and neuropathy |
VA | T1 | LVA | None |
| Detwiler, 1987[34] | 52/F | Neck mass, Cervical Pain, Myelopathy |
VA | C2 | LVA | Embolization - Balloons |
| Negoro, 1990[35] | 47/M | Cervical Mass Pain | VA | C1 | LVA | Embolization - Balloons |
| Schievink, 1991[36] | 43/F | No symptoms | VA | C7 | LVA | None |
| Muhonen, 1991[23] | 52/F | Acute neck mass and pain | VA | C2 | LVA | Balloons |
| Ohkata, 1994[37] | 48/F | Radiculopathy | VA | C4-C6 | LVA | Surgical trapping |
| Uranishi, 1995[3370] | 60/F | Radiculopathy, Cerebellar infaction |
VA | LVA | - | |
| Horsley,1997[38] | 56/F | Neck Mass, Kyphosis | VA | C5-C7 | LVA | Embolization - balloons |
| Hoffman, 1998[39] | 59/M | No symptoms | VA | C6 | RVA | None |
| Magara,1998[40] | 28/M | Neck Mass, Hemothorax | VA | C6-C7 | LVA | Surgical ligation |
| Miyazaki, 2004[41] | 52/F | Neck Pain, Monoparesis LUL and sonnolence Thoraxic haematoma |
VA | C5-C7 | LVA | Tried Balloon and surgery not successful Patient died. |
| Case 4, 2007 | 14/F | Radiculopathy | VA | C5-C6 | RVA | Embolization - Balloons |
- Parachordal Fistulas:
The parachordal fistulas are arteriovenous shunts located in different areas along the neuroaxis following the notochord and fed by metameric arteries of the craniocervical junction or segmental arteries in the paraspinal region at different levels from the basisphenoid from thoracic, lumbar and sacral regions. In NF-1, there is a clear predominance of occurrence in neck region. The symptoms of lesions at cervical level are mainly caused by compression by epidural engorged venous dilatation. Bruit and tinnitus are most commonly reported but cervical myelopathy and radicular pain have more clinical impact. Among 29 cases collected from literature (Table 1) and three cases added in this report, the mean age of onset of symptoms was 50 years. These fistulas frequently occurred in women (72%), on the left side (60%), unilaterally (93%) and principally involving the vertebral artery (91% are truly VVAVF)30.
Figure 7.
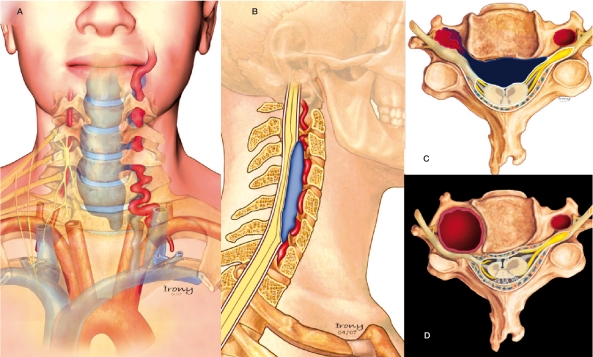
Schematic Illustration of the pathophysiology of the presented cases. In the first three patients, a vertebrovertebral arteriovenous fistula was present. In these drawings, the dysplastic artery ruptured to a venous vertebral compartment and major important reflux to the epidural venous plexus causing spinal cord and radicular compression can be appreciated (AC). D) Illustration of the vertebral aneurysm with irregular and thick walls of the RVA associated with adjacent bone erosion from the pulsatility and enlargement of the transverse foramen causing compression of the exiting nerve root as was present in patient 4.
The initial clinical presentation of compressive symptoms is commonly associated with another differential diagnosis (neurofibroma, bone dysplasia) however in NF-1, vascular disease should be always considered to prevent unnecessary procedures and increase risks for the patient. To date, five patients have been reported presenting with neck mass or acute symptoms or radiculomyelopathy who underwent urgent decompressive surgery for "tumor compression" and the suspicion of a vascular lesion aroused during the procedure and confirmed afterwards by proper angiography.
Table 2.
Spectrum of NF-1 Vasculopathy in Vertebral Artery and Neck Region: Vertebral Aneurysms.
| Authors | Age/ sex |
Clinical symptoms | Type lesion |
Level | Main vessels(s) |
Treatment |
|---|---|---|---|---|---|---|
| Penfield, l946[42] | Neck Pain/Paraplegia | VVAVF | VA | |||
| Calbucci, 1977 [30] | Neck Pain | VVAVF | VA | |||
| Kawazaki, l977[43] | 32/M | Neck Pain, Bruit, myelophaty |
Occipito-Vertebral Fistula |
C2-C3 | Left Occipital Artery | None |
| Shibui, l977[30] | 27/F | Bruit, Tetraplegia | VVAVF | C5 | Right Vertebral Artery | Surgical Ligation |
| Latchaw, l980[44] | 34/F | Bruit, Neck pain, Hemiparesis |
VVAVF | C2-C4 | LVA, Occipital, Thyrocervical trunk |
Surgical Resection |
| Deans, l982[26] | 53/F | Bruit, Hemiparesia | VVAVF | C2-C4 | LVA, Occipital | Surgical Ligation |
| Deans, l982[26] | 45/F | Bruit, Radiculopathy, Monoparesis Left limb, Neck shoulder pain |
VVAVF | C4-C5 | LVA | Embolization - Coils |
| Deans, l982[26] | 58/F | Acute neck mass and cervical pain, |
Maxilo-jugular fistula |
Left Maxillary Artery | Partial embolization with Gianturco coils and subsequently surgery |
|
| Murata, l983[45] | 46/F | Tinnitus, Myelopathy | VVAVF | C2-C6 | LVA | Surgical Ligation |
| Hiekata, l984[46] | 60/F | Bruit, VB insufficiency | VVAVF | C7 | VA, Ascending cervical, Deep cervical, Thyrocervical trunk |
|
| Kamiyama, 1985 [47] | 56/F | Bruit, Tinnitus | VVAVF | C5-C6 | LVA | None |
| Parkinson, l986[48] | 54/F | Bruit, Tinnitus | VVAVF | CI | LVA | Surgical Ligation |
| Takahashi, l986[36] | 39/F | Bruit, Myelopathy | VVAVF | Cl-C4 | VA | Embolization - Detachble Balloons. |
| Kubokura, l987[49] | 38/F | Bruit, Neck Pain | VVAVF | C3 | LVA, Occipital, Deep cervical, Thyrocervical trunk |
Surgical and Baloon embolization |
| Westacott, l988[50] | 40/F | Bruit, Radiculopathy Tetraparesis |
VVAVF | C2-C3 | RVA | Surgical Ligation |
| Westacott, l988[50] | 46F | Neck Pain, Radiculopathy | VVAVF | C2-C7 | LVA | None |
| Hasegawa, l989[51] | 47/M | Bruit, Suboccipital Pain, Tetraplegic after atlantoaxial dislocation and fistula evolution |
VVAVF | Cl-C2 | Both VA's. | Ballons and Surgery |
| Wada, l989[52] | 24/F | Bruti, Neck pain, Hemiparesis |
VVAVF | C3-C4 | LVA, Occipital, Thyrocervical trunk |
Surgery and embolization |
| Johnson, l990[53] | 11/F | Bruit, Tinnitus | VVAVF | C2 | RVA, Ascending pharyngeal | Embolization - Balloons and Coils |
| Shievink, 1991 [36] | 28/F | Bruit, Cervical mass. | VVAVF | C4 | RVA, Rdeep cervical, External carotid |
Surgical ligation and resection |
| Cluzel, l994[28] | 48/M | Radiculopathy | Thyro-cervical- vertebral Fistula |
C5-C7 | L subclavian artery, LVA, Thyrocervical trunk, ascending cervical |
None |
| Cluzel, l994[28, 54] | 25/M | Paraesthesia | VVAVF | C4-C6 | RVA | Embolization - Balloons |
| Koenigsberg, l997[54] | 34/F | Neck pain, Radiculopathy, Gait impairment, sphincter incontinence and kyphosis |
VVAVF | C5-C7 | RVA, ascending cervical | Embolization - Balloons |
| Yilmaz, l997[55] | 28/M | Neck and cervical mass | Occipito-jugular fistula |
CI | Occipital artery | Coil and glue embolization |
| Ushikoshi, l999[30] | 40/F | Neck mass and Occipital Hematoma |
VVAVF | CI | LVA | Embolization - Particles and Coils (Transvenous) |
| Xiaoping, 2000[59] | 38/F | Subacute onset of left hemiparesis, neck pain, and urinary retention |
VVAVF | C3-C4 | LVA | - |
| Roth, 2000[56] | 36/F | Neck Pain and sudden severe cervical hematoma |
VVAVF | C4-C7 | LVA, RVA | Embolization Coil and after Surgical Ligation |
| Kahara, 2002[57] | 38/M | Radicular Pain | VVAVF | C3-C4 | RVA | Embolizations - Coils |
| Tanaka, 2002[58] | 20/M | Acute Neck Mass | Occipito-vertebral Fistula |
C2 | LVA, L ascending pharyngeal, Bilateral Occipital |
Embolization - Coils and Glue |
| Sidhartha, 2003 | 36/F | Tetraparesis | VVAVF | C5 Bilateral |
Bilateral VA, Right Ascending cervical |
Embolization - Coils |
| Case 1,2007 | 49/F | Radiculopathy, Myelopathy | VVAVF | C4 | LVA, Ascending cervical | Embolization - Coils and glue |
| Case 2,2007 | 45/M | Bruit, retro auricular and cervical pain, Myelopathy |
VVAVF | C5 | RVA | Balloon |
| Case 3,2007 | 48/M | Cervical bruit and cervical pain and radiculopathy |
VVAVF | C3 | LVA, Ascending cervical | Embolization - Coils |
Management and treatment strategies
The angiogram is the gold standard examination to diagnose these aneurysms and fistulas and define the appropriate treatment and management strategy. However, MRI is important to evaluate the possibility of a vascular lesion, to analyze the perilesional anatomy and the spinal cord prior to the procedure, exclude or confirm other associated tumoral lesions and for follow-up. MRI is also essential to analyze the vessel wall, venous compartments and thrombosis before and after treatment.
Considering the pathologic changes in the arterial wall in NF-1 patients, the optimal treatment for aneurysmal or arteriovenous fistula is sacrifice of the affected artery. The literature and our series, after careful angiographic analysis to be undertaken prior to therapeutic intervention, have shown that this strategy is feasible and effective. The most important parameter is the collateral flow from the non-involved vertebral artery as well as from the anterior circulation through the posterior communicating arteries. At the present time, other alternatives, like stent grafts or stents with coils, are still experimental, even though they have been used to reconstruct vessels in some traumatic cases, when the vessel wall was normal, in a disease with underlying vascular wall damage like NF-1 and because of the fusiform configuration of EVAs, parent vessel occlusion is the logical proposition.
Treatment strategies for EVAs described in the literature vary between surgery and endovascular management for parent arterial occlusion. The initial reports were treated by surgery (n = 2) and the following cases including our case were treated through the endovascular route by parent artery sacrifice using detachable balloons or coils (n=7) or conservatively (n=3).The mortality (n=2) was related to ruptured and profuse bleeding in spite of the treatment. The clinical outcome of treated patients was recovery partially (n = 1) or fully (n = 8) of the symptoms with no repermeabilization of the aneurysms on imaging follow-up.
The endovascular technique can deal properly with VVAVFs. The regional vascular anatomy and flow dynamics are important to plan the management. The exact location of AVF on the segmental part of the artery of its intersegmental course may impact the potential preserve of the patency of the affected artery. A "sump effect" of regional arteries which opacify the shunt zone without representing a true direct supply can be used as alternative "pathways" when habitual ways cannot be used31. In the cases reviewed managed endovascularly (n=17), the embolism materials were diverse (balloons, glue, bare and gianturco coils) but no matter what was used the goal was common: to occlude the fistulous site with or without the parent vessel. But as seen in the experience with case 2 of this series, even if the vertebral artery was preserved during the treatment, the follow-up demonstrated spontaneous thrombosis of the parent artery afterwards, thus reinforcing the commented treatment planning.
According to the nature of the NF-1 vasculopathy, in the future it will be very important to identify the role of the NF gene and neurofibromin in vascular phenotypic expression and vessel wall proliferation, as well as to recognize and propose a gene therapy method, feasible and efficient, applicable in NF-1 patients before the onset of vascular disorders. Even though techniques to control the intimal hyperplasia that occurs in age-related vasculopathy and atherosclerosis are being developed and could also be appropriate for NF-120. While these genetic and molecular advances are not currently available, attention should be paid to the management of lesions in the cervical region in NF-1 and further to suspected aneurysms or arteriovenous fistulas, an appropriate radiological approach has to be iplemented regarding the essential role of MRI, MR angiography and angiography in these diseases.
References
- 1.Riccardi VM. Neurofibromatosis update. Neurofibromatosis. 1989;2(5-6):284–291. [PubMed] [Google Scholar]
- 2.Riccardi VM. Neurofibromatosis. Neurol Clin. 1987;5(3):337–349. [PubMed] [Google Scholar]
- 3.Jentarra G, Snyder SL, Narayanan V. Genetic aspects of neurocutaneous disorders. Semin Pediatr Neurol. 2006;13(1):43–47. doi: 10.1016/j.spen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Riccardi VM. Neurofibromatosis: Phenotype, Natural History and Pathogenesis. 2nd ed. Baltimore, MD: Johns Hopkins University Press; 1992. [Google Scholar]
- 5.Fujimura T, et al. Neurofibromatosis 1 associated with an intracranial artery abnormality, moyamoya disease and bilateral congenital large hairy pigmented macules. Br J Dermatol. 2004;150(3):611–613. doi: 10.1046/j.1365-2133.2004.05819.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology. 2005;64(3):553–555. doi: 10.1212/01.WNL.0000150544.00016.69. [DOI] [PubMed] [Google Scholar]
- 7.Beaujeux RL, et al. Endovascular treatment of vertebral arteriovenous fistula. Radiology. 1992;183(2):361–367. doi: 10.1148/radiology.183.2.1561336. [DOI] [PubMed] [Google Scholar]
- 8.Merland JJ, et al. Endovascular treatment of vertebral arteriovenous fistulas in twenty-two patients. Ann Vasc Surg. 1986;1(1):73–78. doi: 10.1016/S0890-5096(06)60706-1. [DOI] [PubMed] [Google Scholar]
- 9.Vinchon M, et al. Vertebral arteriovenous fistulas: a study of 49 cases and review of the literature. Cardiovasc Surg. 1994;2(3):359–369. [PubMed] [Google Scholar]
- 10.Friedman Epidemiology of Neurofibromatosis Type 1. American Journal of Medical Genetics (Semin Med Genet) 1999;89:1–6. [PubMed] [Google Scholar]
- 11.Delis KT, Gloviczki P. Neurofibromatosis type 1: from presentation and diagnosis to vascular and endovascular therapy. Perspect Vasc Surg Endovasc Ther. 2006;18(3):226–237. doi: 10.1177/1531003506296488. [DOI] [PubMed] [Google Scholar]
- 12.Riccardi VM. Skin, blood, nerve cells, and heritabilit. New lessons from neurofibromatosis type 1. Arch Dermatol. 1995;131(8):944. [PubMed] [Google Scholar]
- 13.Li Yoc, Huntsman Breidenbach H. Genomic organization of the neurofibromatosis 1 gene (NF-1) Genomics. 1995;25:9–18. doi: 10.1016/0888-7543(95)80104-t. [DOI] [PubMed] [Google Scholar]
- 14.Li F, et al. Neurofibromin is a novel regulator of RAS-induced signals in primary vascular smooth muscle cells. Hum Mol Genet. 2006;15(11):1921–1930. doi: 10.1093/hmg/ddl114. [DOI] [PubMed] [Google Scholar]
- 15.Ismat FA, et al. The neurofibromin GAP-related domain rescues endothelial but not neural crest development in NF-1 mice. J Clin Invest. 2006;116(9):2378–2384. doi: 10.1172/JCI28341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton KK, Xu J, Gutmann DH. Expression of the neurofibromatosis I gene product, neurofibromin, in blood vessel endothelial cells and smooth muscle. Neurobiol Dis. 1995;2(1):13–21. doi: 10.1006/nbdi.1995.0002. [DOI] [PubMed] [Google Scholar]
- 17.Riccardi VM. Histogenesis control genes: embryology, wound-healing, and NF-1. Teratology. 2000;62(1):4. doi: 10.1002/1096-9926(200007)62:1<4::AID-TERA2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Riccardi VM. Histogenesis control genes and neurofibromatosis 1. Eur J Pediatr. 2000;159(7):475–476. doi: 10.1007/s004310051312. [DOI] [PubMed] [Google Scholar]
- 19.Riccardi VM. The vasculopathy of NF-1 and histogenesis control genes. Clin Genet. 2000;58(5):345–347. doi: 10.1034/j.1399-0004.2000.580502.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SJ, Friedman JM. Insights into the pathogenesis of neurofibromatosis 1 vasculopathy. Clin Genet. 2000;58(5):341–344. doi: 10.1034/j.1399-0004.2000.580501.x. [DOI] [PubMed] [Google Scholar]
- 21.Lubinsky MS. Non-random associations and vascular fields in neurofibromatosis 1: a pathogenetic hypothesis. Am J Med Genet A. 2006;140(19):2080–2084. doi: 10.1002/ajmg.a.31303. [DOI] [PubMed] [Google Scholar]
- 22.Easton DP, MA, Huson SM, Ponder BA. An analysis of variation in expression of neurofibromatosis (NF) type 1 (NF-1): Evidence for modifying genes. Am J Hum Genet. 1993;53:305–313. [PMC free article] [PubMed] [Google Scholar]
- 23.Muhonen MG, Godersky JC, VanGilder JC. Cerebral aneurysms associated with neurofibromatosis. Surg Neurol. 1991;36(6):470–475. doi: 10.1016/0090-3019(91)90163-4. [DOI] [PubMed] [Google Scholar]
- 24.Reubi F. Neurofibromatose et lesions vasculaires. Schweiz Med Wochenschr. 1945;75:463–465. [Google Scholar]
- 25.Saylor The vascular lesions of neurofibromatosis. Angiology. 1974;25:510–519. doi: 10.1177/000331977402500803. [DOI] [PubMed] [Google Scholar]
- 26.Deans WR, et al. Arteriovenous fistula in patients with neurofibromatosis. Radiology. 1982;144(1):103–107. doi: 10.1148/radiology.144.1.6806851. [DOI] [PubMed] [Google Scholar]
- 27.Greene JF, Fitzwater JE, Burgess J. Arterial lesions associated with neurofibromatosis. Am J Clin Pathol. 1974;62(4):481–487. doi: 10.1093/ajcp/62.4.481. [DOI] [PubMed] [Google Scholar]
- 28.Cluzel P, et al. Vertebral arteriovenous fistulae in neurofibromatosis: report of two cases and review of the literature. Neuroradiology. 1994;36(4):321–325. doi: 10.1007/BF00593272. [DOI] [PubMed] [Google Scholar]
- 29.Giuffre R, Sherkat S. The vertebral artery: developmental pathology. J Neurosurg Sci. 1999;43(3):175–189. [PubMed] [Google Scholar]
- 30.Ushikoshi S, et al. Vertebral arteriovenous fistula that developed in the same place as a previous ruptured aneurysm: a case report. Surg Neurol. 1999;51(2):168–173. doi: 10.1016/s0090-3019(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 31.Lasjaunias P, Berestein A, Ter brugge K. Surgical Neuroangiography. Paris: Springer; 2003. [Google Scholar]
- 32.Schubiger O, Yasargil MG. Extracranial vertebral aneurysm with neurofibromatosis. Neuroradiology. 1978;15(3):171–173. doi: 10.1007/BF00329063. [DOI] [PubMed] [Google Scholar]
- 33.Pentecost M, et al. Aneurysms of the aorta and subclavian and vertebral arteries in neurofibromatosis. Am J Dis Child. 1981;135(5):475–477. doi: 10.1001/archpedi.1981.02130290071024. [DOI] [PubMed] [Google Scholar]
- 34.Detwiler K, Godersky JC, Gentry L. Pseudoaneurysm of the extracranial vertebral artery. Case report. J Neurosurg. 1987;67(6):935–939. doi: 10.3171/jns.1987.67.6.0935. [DOI] [PubMed] [Google Scholar]
- 35.Negoro M, et al. Extracranial vertebral artery aneurysm with neurofibromatosis. Endovascular treatment by detachable balloon. Neuroradiology. 1990;31(6):533–536. doi: 10.1007/BF00340136. [DOI] [PubMed] [Google Scholar]
- 36.Schievink WI, Piepgras DG. Cervical vertebral artery aneurysms and arteriovenous fistulae in neurofibromatosis type 1: case reports. Neurosurgery. 1991;29(5):760–765. doi: 10.1097/00006123-199111000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Ohkata N, et al. A case of multiple extracranial vertebral artery aneurysms associated with neurofibromatosis. No Shinkei Geka. 1994;22(7):637–641. [PubMed] [Google Scholar]
- 38.Horsley M, Taylor TK, Sorby WA. Traction-induced rupture of an extracranial vertebral artery aneurysm associated with neurofibromatosis. A case report. Spine. 1997;22(2):225–227. doi: 10.1097/00007632-199701150-00019. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann KT, et al. Giant aneurysm of the vertebral artery in neurofibromatosis type 1: report of a case and review of the literature. Neuroradiology. 1998;40(4):245–248. doi: 10.1007/s002340050576. [DOI] [PubMed] [Google Scholar]
- 40.Magara T, et al. Massive mediastinal bleeding due to spontaneous rupture of the vertebral artery in von Recklinghausen disease. Jpn J Thorac Cardiovasc Surg. 1998;46(9):906–909. doi: 10.1007/BF03217843. [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki T, et al. Extracranial vertebral artery aneurysm ruptured into the thoracic cavity with neurofibromatosis type 1: case report. Neurosurgery. 2004;54(6):1517–1520. doi: 10.1227/01.neu.0000125547.31328.69. discussion 1520-1521. [DOI] [PubMed] [Google Scholar]
- 42.Penfield W. Notes on Operative Technic in Neurosurgery. Ann Surg. 1946;124(2):383–385. [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki M, et al. A case of spontaneous extracranial vertebral A.V.M. with neurofibromatosis (author’s transl) No Shinkei Geka. 1977;5(8):877–882. [PubMed] [Google Scholar]
- 44.Latchaw RE, et al. Combined embolization and operation in the treatment of cervical arteriovenous malformations. Neurosurgery. 1980;6(2):131–137. [PubMed] [Google Scholar]
- 45.Murata T, et al. Case of cervical AVM associated with neurofibromatosis presenting unilateral pulsating exophthalmos. Neurol Med Chir (Tokyo) 1983;23(10):807–813. doi: 10.2176/nmc.23.807. [DOI] [PubMed] [Google Scholar]
- 46.Hiekata T, et al. Surgical treatment of thoracoabdominal aortic aneurysms: report of four cases and review of the literature. Nippon Kyobu Geka Gakkai Zasshi. 1984;32(3):400–407. [PubMed] [Google Scholar]
- 47.Kamiyama K, et al. Neurofibromatosis associated with intra- and extracranial aneurysms and extracranial vertebral arteriovenous fistula. No Shinkei Geka. 1985;13(8):875–880. [PubMed] [Google Scholar]
- 48.Parkinson D, Hay R. Neurofibromatosis. Surg Neurol. 1986;25(1):109–113. doi: 10.1016/0090-3019(86)90128-x. [DOI] [PubMed] [Google Scholar]
- 49.Kubokura T, et al. Neurofibromatosis with extracranial vertebral arteriovenous fistulae. Case report. Neurol Med Chir (Tokyo) 1987;27(12):1173–1179. doi: 10.2176/nmc.27.1173. [DOI] [PubMed] [Google Scholar]
- 50.Westacott S, et al. MRI diagnosis of vertebral arteriovenous malformations in neurofibromatosis. Br J Neurosurg. 1988;2(3):385–389. doi: 10.3109/02688698809001010. [DOI] [PubMed] [Google Scholar]
- 51.Hasegawa H, et al. Bilateral vertebral arteriovenous fistulas and atlantoaxial dislocation associated with neurofibromatosis - case report. Neurol Med Chir (Tokyo) 1989;29(1):55–59. doi: 10.2176/nmc.29.55. [DOI] [PubMed] [Google Scholar]
- 52.Wada K, et al. Neurofibromatosis with spinal paralysis due to arteriovenous fistula. Arch Orthop Trauma Surg. 1989;108(5):322–324. doi: 10.1007/BF00932324. [DOI] [PubMed] [Google Scholar]
- 53.Johnson CE, Russell EJ, Huckman MS. Resolution of spinal epidural vascular pseudotumor following balloon occlusion of a postoperative vertebral arteriovenous fistula. Neuroradiology. 1990;31(6):529–532. doi: 10.1007/BF00340135. [DOI] [PubMed] [Google Scholar]
- 54.Koenigsberg RA, et al. Cervical vertebral arteriovenous fistula balloon embolization in a patient with neurofibromatosis type 1. Surg Neurol. 1997;47(3):265–273. doi: 10.1016/s0090-3019(96)00254-6. [DOI] [PubMed] [Google Scholar]
- 55.Yilmaz M, et al. Management of a large arteriovenous fistula in the face: a case of neurofibromatosis type 1. Ann Plast Surg. 1997;39(3):308–313. doi: 10.1097/00000637-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 56.Roth TC, et al. Complex vertebral arteriovenous fistula and ruptured aneurysm in neurofibromatosis: a therapeutically challenging case. Skull Base Surg. 2000;10(1):3541. doi: 10.1055/s-2000-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kahara V, et al. Vertebral epidural arteriovenous fistula and radicular pain in neurofibromatosis type I. Acta Neurochir (Wien) 2002;144(5):493–496. doi: 10.1007/s007010200071. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka T, et al. Combination of intravascular surgery and surgical operation for occipital subcutaneous arteriovenous fistula in a patient with neurofibromatosis type I. No Shinkei Geka. 2002;30(3):309–313. [PubMed] [Google Scholar]
- 59.Xiaoping M, et al. Arteriovenous fistula in neurofibromatosis. Neurology. 2000;55:288. doi: 10.1212/wnl.55.2.288. [DOI] [PubMed] [Google Scholar]