Summary
Surgical procedures designed to restore vascular patency for a recurrent stenosis following carotid endarterectomy (CEA) are burdened with technical difficulties as well as with the possibility of serious neurological complications. An endovascular approach employing transluminal percutaneous angioplasty and stenting (PTAS) is a promising solution to these problems.
We aimed to evaluate the incidence of carotid artery restenosis following CEA, and to evaluate the safety and efficacy of treating post-CEA restenosis with an endovascular technique (PTAS).
One hundred and two patients who underwent CEA for symptomatic and asymptomatic stenosis were included in the analysis. Clinical and sonographic follow-up examinations identified carotid artery restenosis in 16 patients, who fulfilled our criteria for endovascular treatment. Carotid PTAS was performed on symptomatic patients with a stenosis over 60% of the artery lumen (n=7) and in asymptomatic patients with a stenosis over 80% (n=9). The post-PTAS patients were evaluated by duplex sonography every three months over a 24 month follow-up period for evidence of restenosis.
The cumulative incidence of post-CEA carotid restenosis qualifying for PTAS was 9.3% during an average 12-month follow-up interval. The average time from CEA to carotid PTAS was 11 months. All 16 endovascular procedures were technically successful. All of the carotid arteries were widely patent following PTAS. There were no immediate perioperative complications. One patient died two days after carotid PTAS from a cerebral hemorrhage. Thirteen of the 16 patients remained asymptomatic and had no sonographic evidence of significant restenosis during the 24-month post-PTAS follow-up period. One patient developed a symptomatic 80% restenosis proximal to the stent six months after carotid PTAS. Another patient developed an asymptomatic 60% restenosis proximal to the stent at 24 months. One patient was lost to follow-up.
Following CEA, there is a significant risk of developing a symptomatic or high-grade carotid artery restenosis requiring correction. Endovascular treatment (PTAS) of a recurrent stenosis after CEA is a safe and effective alternative to repeat carotid surgery.
Key words: carotid endarterectomy, restenosis, percutaneous angioplasty, stenting
Introduction
Progression of atherosclerosis in the cervical internal carotid artery (ICA) can lead to stenosis with a subsequent drop in cerebral perfusion, transient ischemic attacks (TIA) and stroke. Carotid endarterectomy (CEA) can reduce the risk of stroke in patients with symptomatic ≤70% stenosis of the cervical ICA1. However, recurrent stenosis is being found in a substantial number of patients (up to 37%) during the three-year period after CEA2-5.
Patients with post-CEA asymptomatic restenosis ≥ 80% and symptomatic stenosis ≥ 60% require a therapeutic intervention to reduce the risk of stroke. A repeat CEA in such patients, however, may be either difficult or dangerous6,7.
Despite the risks, some vascular surgeons recommend a repeat CEA; however, endovascular treatment with percutaneous transluminal angioplasty and stenting (PTAS) might be a preferable approach for these patients7-9,11.
In this study, we present our institutional experience with the incidence of recurrent carotid artery stenosis following CEA, and its endovascular treatment using carotid PTAS.
Materials and methods
The institutional review board approved this retrospective study. Clinical charts of 274 consecutive patients with stenosis of the cervical ICA, who underwent standard CEA without patching in our University Hospital between January 2001 and December 2002 were reviewed. The Hospital serves as the primary tertiary care center for a densely populated metropolitan area of about 3.5 million people.
One-hundred-seventy-six patients had neurological symptoms and a ≥ 60% cervical ICA stenosis as determined with carotid ultrasonography. The remaining 98 patients were asymptomatic with a ≥ 80% cervical ICA stenosis. Following endarterectomy, all patients were routinely scheduled for follow-up carotid ultrasound every three months, but only 172 patients participated in follow-up ultrasound examinations at our institution during the 24-month post-CEA period.
Color-coded duplex ultrasound
The carotid ultrasonographic studies were performed using a broadband 7.5 MHz (range 5 to 9 MHz) linear probe (Siemens Elegra, Erlangen, Germany) according to standards previously described12. Carotid arteries were assessed in transverse and longitudinal views using grey-scale and color Doppler imaging. A sample volume, adjusted to the size of the artery lumen, was placed at the site of the highest velocity acceleration in the ICA. Angle corrected peak-systolic, end-diastolic and mean blood flow velocities were measured by automatic, or in case of weak Doppler signal, manual tracing of velocity waveforms obtained from the ICA and the distal segment of the common carotid artery (CCA). The site of highest velocity was selected based on the presence of color aliasing artefact. Flow velocities were used to calculate velocity ratios: VICA/VCCA. The degree of the ICA stenosis was determined based on grey-scale images, velocity and velocity ratios criteria. At least one from the following criteria had to be met for detection of a 60% ICA narrowing:
1) measurements of the diameters of the most narrowed segment relative to the distal disease free segment of the ICA based on grey scale images. Calculations of the degree of narrowing were performed similar to angiographic NASCET criteria 1, 2) peak-systolic velocity (PSV) in the narrowed segment greater than 125 cm/s but less than 290 cm/s, 3) end-diastolic velocity (EDV) in the narrowed segment less than 125 cm/s but more than 65 cm/s, 4) VICA/VCCA end-diastolic velocity ratio more than 2.4. Criteria for a 80% stenosis were: 1) narrowing of greater than 80% based on measurements from grey scale images as above, 2) PSV in the narrowed segment greater than 290 cm/s, 3) EDV in the narrowed segment greater than 125 cm/s 4) VICA/VCCA ratio more than 4.5. Patients in our Institution are qualified for carotid surgery based on these specific ultrasonographic criteria. A previously performed validation of these criteria in our department comparing sonographic results with angiographic and intraoperative findings demonstrated excellent agreement. A surgically eligible carotid stenosis had to be confirmed in at least two consecutive examinations, performed by experienced sonographers. The same sonographic criteria were used to determine the degree of carotid restenosis during the post-CEA follow-up period. Symptomatic patients with a recurrent ICA stenosis equal to 60% and more and asymptomatic patients with a greater or equal to 80% restenosis were qualified for PTAS.
PTAS of recurrent carotid stenosis
PTAS was performed without a cerebral protection system. Anti-platelet treatment was started three days before the procedure by administering ticlopidine in a dose of 250 mg twice daily and 75 mg of aspirin once a day. This therapy was continued throughout the postoperative period. During the endovascular procedure each patient received 5000 units of heparin in an I.V. bolus, and then was given a low molecular weight heparin (Enoxparine) subcutaneously in a twenty-four hour dose of 40 mg. The procedure started with selective catheterization of the common carotid artery using 6F introducers with a hemostatic Balt valve. For the patients in whom common femoral and brachial access was used, introducers were 60 - 90 cm long, while in patients with direct common carotid access the introducer was 10 cm long. In most patients, catheters were introduced through the common femoral artery. In two patients, the catheter was inserted through the brachial artery. Direct common carotid puncture (surgically exposed) was required in two patients. An initial selective carotid digital subtraction angiogram (DSA) was obtained. With fluoroscopic and roadmap guidance, the stenosis was traversed with an appropriate 0.014 guide wire. If deemed necessary, the stenosis was predilated with a 3 to 5 mm diameter balloon angioplasty catheter. A self-expanding stent (Smart or Precise Stent, Cordis or Wallstent, Boston Scientific) (6 mm diameter and 30 mm length or 7 mm diameter and 40 mm length) was then deployed across the stenotic segment. If necessary, the stented segment was post-dilated with a 5 to 6 mm diameter balloon angioplasty catheter. At the end of the procedure the effectiveness of the PTAS was evaluated by a final DSA to determine degree of stent expansion, the diameter of the common carotid artery, the diameter of the internal carotid artery and the blood flow through the intracranial circulation. All patients underwent sonographic evaluation every three months following PTAS for a minimum of a 12-month follow-up period.
Statistical analysis
The data were analyzed using statistical software (SYSTAT® for Windows, SYSTAT, Evanston, IL, USA). Descriptive data are provided in the tables. The cumulative incidence of a recurrent stenosis was calculated, which is defined here as the probability that an individual patient after PTAS will develop a recurrent stenosis over the average time of follow-up observation. Multivariate linear regression analysis was employed to determine whether the degree of restenosis, presence of clinical symptoms and the time after PTAS had an effect on the long-term results of PTAS after adjusting for age. Probability level less than 0.1 was considered significant for predictive models.
Results
A significant cervical ICA restenosis was found in 16 out of 172 patients (cumulative incidence: 9.3%), who underwent CEA and who were followed-up at our institution for a minimum of 12 months. Seven patients experienced recurrent transient ischemic attacks (TIAs) and had a ≥ 60% carotid restenosis, whereas the remaining nine patients had an asymptomatic ≥ 80% carotid restenosis. In nine patients the stenosis was very focal, measuring <10 mm in length, while in seven patients the stenosis was longer (up to 25 mm). The stenosis was concentric in 14 cases and eccentric in two.
All 16 patients underwent technically successful PTAS with no immediate periprocedural complication. PTA was performed prior to stent placement in 12 patients using 3 to 5 mm diameter balloon angioplasty catheters. In four patients, the stents were deployed without predilatation. Stents measuring 7 mm in diameter and 40 mm in length were placed across the carotid bifurcation from the CCA to the cervical ICA in 14 cases. In two patients stents measuring 6 mm in diameter and 30 mm in length were deployed in the cervical ICA distal to the carotid bifurcation (Table 1).
Table 1.
Patients characteristics and outcomes measures after endovascular treatment of recurrent carotid stenosis.
| Patient | Elapsed time, (months) |
TIA | Restenosis (%) |
Percutaneous access |
Position of stent |
Ultrasound results during follow-up |
|---|---|---|---|---|---|---|
| 1 | 3 | Yes | 65 | CFA | ICA | Not studied |
| 2 | 9 | Yes | 80 | CFA | BCICA | no stenosis |
| 3 | 12 | No | 85 | CFA | BCICA | no stenosis |
| 4 | 9 | Yes | 70 | CCA | BCICA | no stenosis |
| 5 | 15 | No | 90 | CFA | BCICA | no stenosis |
| 6 | 24 | Yes | 75 | BA | BCICA | no stenosis |
| 7 | 9 | No | 95 | CFA | BCICA | no stenosis |
| 8 | 3 | No | 90 | CFA | BCICA | no stenosis |
| 9 | 12 | No | 85 | CFA | BCICA | no stenosis |
| 10 | 21 | Yes | 65 | CCA | BCICA | 80% CCA narrowing |
| 11 | 12 | No | 90 | CFA | ICA | no stenosis |
| 12 | 21 | Yes | 90 | BA | BCICA | no stenosis |
| 13 | 24 | No | 85 | CFA | BCICA | 60% CCA narrowing |
| 14 | 15 | Yes | 85 | CFA | BCICA | no stenosis |
| 15 | 18 | No | 85 | CFA | BCICA | no stenosis |
| 16 | 18 | No | 90 | CFA | BCICA | no stenosis |
|
TIA - transient ischemic attack; CFA - common femoral artery; BA - brachial artery CCA - common carotid artery; ICA - internal carotid artery; BCICA - bifurcation of the common and internal carotid arteries | ||||||
In 11 patients the stents were dilated after deployment with a 5 - or 6 mm diameter balloon angioplasty catheter to achieve precise stent apposition to the arterial wall and to minimize any residual stenosis defined as less 15%. In five patients, after placement the stent was well apposed to the arterial wall without a significant residual stenosis on DSA and post-deployment angioplasty was unnecessary (figures 1 and 2)
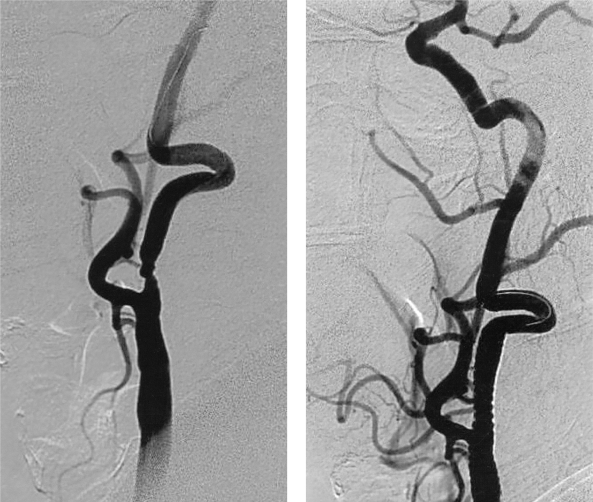
Figure 1.
A) Restenosis of the internal carotid artery in a long segment - 12 months after endarterectomy, B) PTAS with a 6 x 30 stent in the left internal carotid artery.
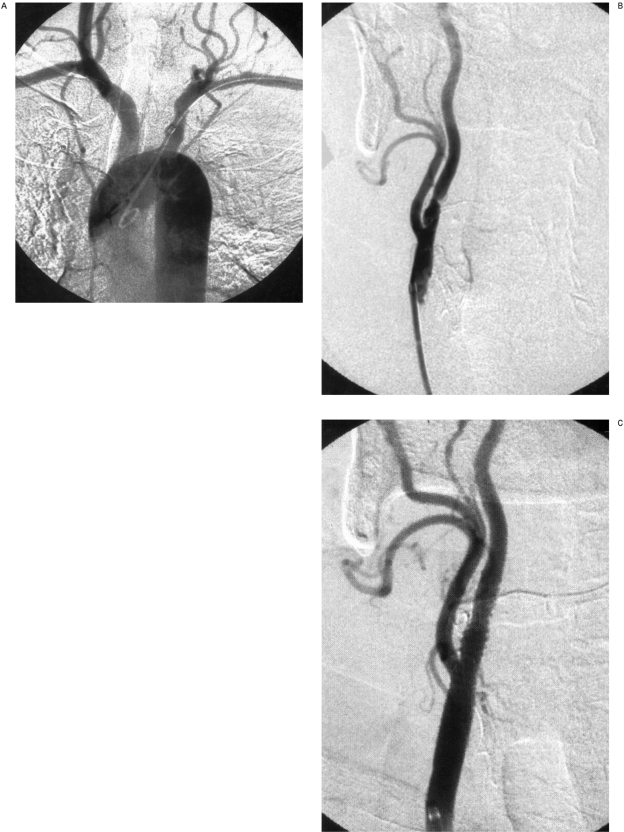
Figure 2.
A) Leriche syndrome patient with obstructed left common carotid artery and restenosis of the right internal carotid artery. Guide catheter was advanced through the left brachial artery into the right common carotid artery, B) Guide catheter tip in the right common carotid artery. Critical stenosis of the internal carotid artery, C) Angioplasty with stent traversing the common carotid artery bifurcation.
One patient whose left carotid artery developed a 95% restenosis nine months after CEA developed a hemorrhagic after PTAS and died two days later. A CT scan of the brain prior to the endovascular procedure did not reveal ischemic changes. PTAS was technically successful and the patient did well until six hours later when he developed a worsening headache with a rise in arterial blood pressure to 220/120 mm Hg. Pharmacological treatment resulted in a reduction in the arterial blood pressure to normal, but the patient clinically deteriorated. A second CT scan showed an acute intracerebral hemorrhage to cerebral trunk, presumably as a result of hyperperfusion syndrome (this is considered risk of all carotid revascularization procedures and would not be a direct technical complications from the angioplasty and stenting). The patient died 48 hours after the onset of neurological symptoms.
No neurological deficits were observed in the remaining 15 patients directly after the procedure. Ultrasonographic examinations performed before discharge in all patients revealed optimal stent expansion and normal blood flow Doppler parameters in the CCA and ICA. In 14 patients, neither neurological symptoms nor significant cervical ICA restenosis on carotid ultrasound were observed during the follow-up period. One patient reported a TIA six months after the procedure. The ultrasonographic examination performed during the second visit revealed an 80% stenosis of the CCA just proximal to the implanted stent. He was offered another angioplasty procedure but he refused. In a second patient, an asymptomatic 60% stenosis was found in the CCA just proximal to the implanted stent after 24 months. This patients is being followed, and the stenosis is stable and exhibits no neurological symptoms.
Using multivariate linear regression analysis we found the two models that can be used to predict the development of recurrent carotid stenosis after PTAS. In the first model, the degree of carotid stenosis before PTAS (Stenosis before, in percentage) and the elapsed time after PTAS (Time, elapsed, in months) taken together were related to the likelihood of developing restenosis after PTAS (Outcome, presence of narrowing -1, no - narrowing 2).
Outcome = 0.024 (Time elapsed)
− 0.014 (Stenosis before) + 1.964
F=3.396, P=0.068, R2=0.361
In the second model, three independent variables were included: the severity of the stenosis before PTAS (Stenosis before, in percentage), the elapsed time after PTAS (Time, elapsed, in months) and the presence of clinical symptoms before PTAS (Symptoms).
Outcome = 0.026 (Time elapsed)
− 0.026 (Stenosis before)
− 0.299 (Sumptoms) + 3.053
F=3.183, P=0.067, R2=0.465
The models present a good fit as expressed with square R, though no one independent variable alone had a significant effect on the outcome. The other variables such as age, sex, access approach, type of stent, and location of the stent in relation to the carotid bifurcation had no effect on the outcome, and only decreased the F and R values of our models.
Discussion
The cumulative incidence of recurrent carotid stenosis in our patient population after CEA is 9.3% during an average 12 month follow-up period. This finding is consistent with published reports on the incidence of restenosis, which risk is the highest in the first year after CEA13,14. The incidence found in our study is close to the average (6%) of several previously published values, which vary from 6% to 37% 2,3,9,10. It means that quality of our surgical CEA procedures are comparable to the quality of procedures performed in other well established institutions.
We encourage all of our patients to have the follow-up ultrasound examinations at our institution, however; a substantial percentage (36%) did not show up. These patients presumably were followed-up at their local medical facilities. We therefore do not know the overall incidence of carotid restenosis in our entire consecutive group of operated patients. We believe this rate is likely to be lower than the 9.3% restenosis rate in the studied population because no patient, who was not monitored by us, was referred back to our institution with new symptoms or a recurrent stenosis. These patients would have returned to our hospital because it serves as the only tertiary care referral center performing carotid surgery for the entire region.
Sonographic follow-up of patients after CEA is a non-invasive and accurate method to detect re-narrowing of the carotid artery. Carotid restenosis usually occurs gradually and most often during the first two years following surgery3. The primary cause of restenosis during this time interval is considered to be myointimal hyperplasia, an arterial wall cellular reaction to surgical manipulation. Recurrent stenosis caused by progressive atherosclerosis usually occurs later. Organized thrombi and cicatricial lesions in the initial and terminal sections of the endarterectomy site are other major causes of restenosis10. A ≥ 60% restenosis with recurrent ischemic symptoms and an asymptomatic ≥ 80% restenosis are considered indications for a repeat CEA7,15.
The risk of significant complications during and following re-operation is related in part to the etiology of the restenosis. Thus, preoperative characterization of the recurrent carotid stenosis may influence the therapeutic approach16.
For example, arterial myointimal hyperplasia combined with dense perivascular fibrosis significantly prolongs the surgical procedure, thereby increasing the risk of cerebral embolism, cranial nerve damage and postoperative wound complications. In some cases, vascular damage occurring during a repeat CEA may necessitate removal of an arterial segment, replacing it with a synthetic or saphenous vein graft 17. The overall risk of serious complications after repeat CEA has been reported to increase to 10.9%, compared to the 5% risk associated with a primary CEA9.
Our results indicate that an endovascular approach (PTAS) to treating carotid restenosis following CEA is safe and effective. The post-CEA PTAS complication rates in several previous studies 8,9,10,17 and in our study group are lower than that those reported after repeat CEA. Although cerebral protection methods were not utilized, no immediate complications were observed that suggested cerebral emboli occurrences.
The fibrotic lesions in carotid restenosis due to myointimal hyperplasia may be less friable and less likely to release emboli during PTAS than complex atherosclerotic plaques. Although patients with post-CEA restenosis can be treated surgically or with PTAS, our results and other similar studies suggest that the endovascular approach is associated with a lower incidence of complications. Larger randomized, prospective multicenter trials are needed to confirm these observations. Nevertheless, our data combined with data from similar studies can contribute to a meta-analysis supporting the most safe and effective approach to the treatment of carotid restenosis following CEA.
The incidence of restenosis after standard CEA may depend on the surgical technique and the experience of the vascular surgeon. Our university hospital is the only referral center performing CEA for a densely populated metropolitan area of about 3.5 million people. Our vascular surgeons are therefore well experienced. The incidence of post-CEA restenosis in this study is close to the average reported in literature.
It may be important to predict the probability of carotid restenosis after CEA and to identify the crucial contributing factors. In this study, multivariate linear regression analysis yields models that indicate that degree of initial ICA narrowing, the presence of symptoms and the time elapsed after CEA are the most powerful predictors of restenosis. Although the number of patients is low from statistical point of view the data account for the entire population of patients who underwent CEA and completed the follow-up period. Although our models have a rather low predictive accuracy, they may be useful in the decision making process.
We used specific sonographic criteria to quantify the severity of carotid artery stenosis. This can be regarded as a limitation of our study, since the specific criteria used and operator expertise may influence the accuracy of the sonographic results. We previously rigorously validated our institutional sonographic criteria based on a large number of patients who underwent CEA with angiographic and surgical correlation. Furthermore, our sonographers are very well trained and experienced in carotid ultrasonographic studies. Therefore, we believe that our ultrasound quantification of carotid artery stenosis was fairly accurate.
Conclusions
There is a substantial incidence of symptomatic or high-grade carotid restenosis following CEA. These patients can be identified reliably on regularly scheduled ultrasonographic follow-up examinations. Our institutional experience demonstrates that endovascular treatment (PTAS) of a recurrent stenosis after CEA is a safe and effective alternative to repeat carotid surgery.
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Zierler RE, Bandyk DF, Thiele BL. Carotid artery stenosis following endarterectomy. Arch Surg. 1982;117:1408–1415. doi: 10.1001/archsurg.1982.01380350016003. [DOI] [PubMed] [Google Scholar]
- 3.Mattos MA, Van-Bemmelen PS, Barkmier LD. Routine surveillance after carotid endarterectomy: does it affect clinical management? J Vasc Surg. 1993;17:819–830. [PubMed] [Google Scholar]
- 4.McDonnell CO, Legge D, et al. Carotid artery angioplasty for restenosis following endarterectomy. Eur J Vasc Endovasc Surg. 2004;27:163–166. doi: 10.1016/j.ejvs.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Moore WS, Kempczinski RF, et al. Recurrent carotid stenosis: results of the asymptomatic carotid atherosclerosis study. Stroke. 1998;29:2018–2025. doi: 10.1161/01.str.29.10.2018. [DOI] [PubMed] [Google Scholar]
- 6.Meyer FB, Piepgras DG, et al. Recurrent carotid stenosis. In: Meyer FB, editor. Sundt’s occlusive cerebrovascular disease. Philadelphia, Pa: WB Saunders Co; 1994. pp. 310–321. [Google Scholar]
- 7.O’Hara PJ, Hertzer NR, et al. Reoperation for recurrent carotid stenosis: early results and late outcome in 199 patients. J Vasc Surg. 2001;34:5–12. doi: 10.1067/mva.2001.115601. [DOI] [PubMed] [Google Scholar]
- 8.Ross CB, Naslud TC, Ranval TJ. Carotid stent-assisted angioplasty: the newest addition to the surgeons’ armamentarium in the management of carotid occlusive disease. Am Surg. 2002;68:967–975. [PubMed] [Google Scholar]
- 9.Yadav JS, Roubin GS, et al. Angioplasty and stenting for restenosis after carotid endarterectomy. Stroke. 1996;27:2075–2079. doi: 10.1161/01.str.27.11.2075. [DOI] [PubMed] [Google Scholar]
- 10.Vitek JJ, Roubin GS, et al. Carotid angioplasty with stenting in post-carotid endarterectomy restenosis. J Invas Cardiol. 2001;13:123–125. [PubMed] [Google Scholar]
- 11.Veith FJ, Amor M, et al. Current status of carotid bifurcation angioplasty and stenting based on a consensus of opinion leaders. J Vasc Surg. 2001;33(2Sup):S111–S116. doi: 10.1067/mva.2001.111665. [DOI] [PubMed] [Google Scholar]
- 12.Forteza AM, Krejza J, et al. Ultrasound imaging of cerebrovascular disease. In: Babikian VL, Wechsler L, Higashida RT, editors. Imaging Cerebrovascular Disease. Philadelphia, Pa: Butterworth-Heinemann; 2003. pp. 3–35. [Google Scholar]
- 13.Lattimer CR, Burnand KG. Recurrent carotid stenosis after carotid endarterectomy. Br J Surg. 1997;84:1206–1219. [PubMed] [Google Scholar]
- 14.Sterpetti AV, Schultz RD, et al. Natural history of recurrent carotid artery disease. Surg Gynecol Obstet. 1989;168:217–223. [PubMed] [Google Scholar]
- 15.Sander EACM, Hoeneveld H, Eikelboom BC. Residual lesions and early recurrent stenosis after carotid endarterectomy: a send follow-up study with duplex-scanning and intravenous digital subtraction angiography. J Vasc Surg. 1987;5:731–737. doi: 10.1067/mva.1987.avs0050731. [DOI] [PubMed] [Google Scholar]
- 16.Beebe HG. Scientific evidence demonstrating the safety of carotid angioplasty and stenting: do we have enough to draw conclusions yet? J Vasc Surg. 1998;27:788–790. doi: 10.1016/s0741-5214(98)70252-6. [DOI] [PubMed] [Google Scholar]
- 17.Rockman CB, Bajakian D, et al. Impact of carotid artery angioplasty and stenting on management of recurrent carotid artery stenosis. Ann Vasc Surg. 2004;18:151–157. doi: 10.1007/s10016-004-0004-y. [DOI] [PubMed] [Google Scholar]