Summary
Dissecting aneurysms involving the basilar artery (BA) are lesions with significant morbidity and mortality. Their management is controversial and often difficult. There is no generally approved strategy.
Two cases of huge dissections involving the BA presented with subarachnoid hemorrhage in one case and mass effect in both cases. The dissection of case 1 involved the upper two thirds of the BA distal to the anterior inferior cerebellar arteries (AICA). Another dissection of case 2 involved the bilateral vertebral arteries (VA) distal to bilateral PICA and extended to upper third of the BA. After making a basket with coils inside the pseudoaneursym, proximal dissection was totally occluded in case 1. Dissection on the bilateral VA distal to the bilateral PICA and proximal BA was occluded in case 2 with a small residual dissection on the left VA. Case 1 had an excellent recovery with a durable image and clinical result. But recanalization and regrowth occurred in case 2, which might have originated from the residual dissection on the left VA, induced acute mass effect and sudden coma six weeks after the initial treatment. The residual and regrown dissection had to be occluded in a second intervention. The patient died two days later.
BA occlusion is safe and efficient for dissections involving the BA as in our case and the literature. Proximal occlusion might be enough for huge and long lesions like ours. It seems that completely dense packing of proximal dissection is the key point to prevent recanalization.
Key words: basilar artery, dissection, endovascular intervention, recanalization
Abbreviations
- basilar artery (BA);
- vertebral artery (VA)
- anterior inferior cerebellar arteries (AICA)
- computed tomography (CT)
- subarachnoid hemorrhage (SAH)
- posterior cerebral arteries (PCA)
- superior cerebellar arteries (SCA)
- posterior communicating arteries (PCoA)
- dissecting aneurysm (DA)
Introduction
Dissecting aneurysms involving the basilar artery (BA), which might be either BA dissection or dissection extending from the vertebral artery (VA), have been reported much less frequently than their counterpart from the vertebral artery (VA)3-5,39. The clinical manifestations of dissections involving the BA are more varied than those for VA dissection, including SAH, ischemia, and brainstem compression3-5,13,16,21,25,37-39. Recent observations indicate that patients presenting with BA dissection aneurysms have a high rate of morbidity and mortality 13,16,21,25. Management of BA dissection aneurysms is controversial and challenging because the structure is complex and its natural history, treatment, and outcome of the treatment are not as defined as for intracranial ordinary berry aneurysms. Furthermore, only a few reports in the literature 9,17,19 specifically discussed large or huge dissections involving the BA, and strategies to relieve the problem of the mass effect of large or huge dissections as well as the risk of re-bleeding are still undefined. In this report, there is one case of huge BA dissection aneurysm and one case of vertebrobasilar dissection aneurysm.
The latter dissection involved bilateral VAs, conjunction, and two thirds of the BA trunk. Because the main portion of the vertebrobasilar dissection aneurysm was located on the proximal BA inducing symptoms and making the treatment paradox like the huge BA dissection aneurysm, we therefore named the two dissections huge dissection aneurysms involving the BA.
We present two cases with severe mass effect and rebleeding. After reviewing the literature on the various strategies, and careful evaluation of the clinical and imaging material, we designed an endovascular strategy to achieve the treatment.
Case Reports
Case 1
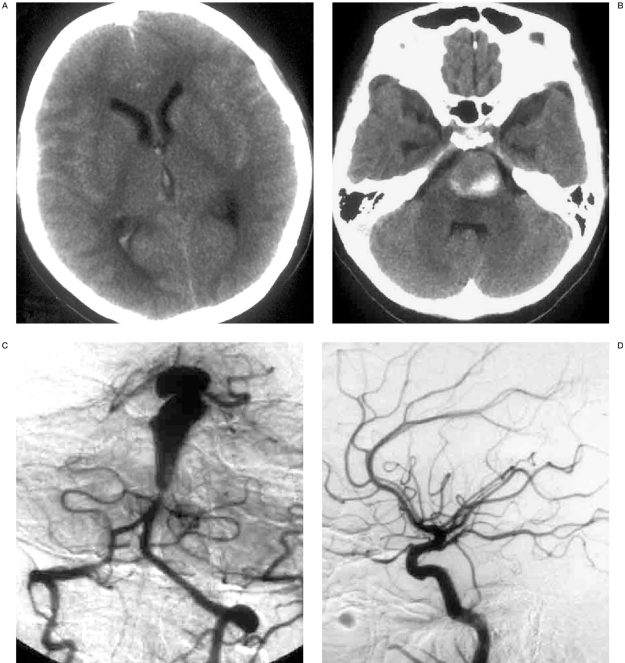
This 31-year-old woman experienced sudden violent headache, vomiting and loss of consciousness rapidly with aconuresis. Computed tomography (CT) revealed massive subarachnoid hemorrhage (SAH), slight right lateral and third intraventricle hemorrhage, and left pons was compressed by a high density mass with a diameter of 2 cm (figures 1A,B). The patient regained consciousness in 20 days. After waking up, the patient presented with dysarthria, tetraparesis, and forced laughing and crying. The patient was transferred to our institute when she was stable. Neurologic examination showed frequent laughter and crying outbursts, bilateral spasticity and weakness of limbs, bilateral ankle clonus, and right positive Babinski sign. Because of a metal implantation in her left leg prior to the onset, she could only be re-examined by CT. SAH had been absorbed; mass effect was the same as the previous one, and edema inside the pons could be seen (figure 1B).
Figure 1.
A 31-year-old woman with sudden-onset of headache, vomiting and loss of consciousness. CT revealed SAH (A) and a high density mass with a diameter of 2 cm compressing the left pons (B). Cerebral angiography showed that a huge dissection distal to the AICA (C), and bilateral PCoA were prominent (D,E). After making a basket with 3D coils (F), vertebral angiogram showed occlusion of the dissection and proximal aneurysm with patency of the bilateral AICA (G). But the upper part of the aneurysm could still be stained by bilateral PCoAs (H,I). Follow-up seven months after embolization confirmed the complete occlusion of the dissection (J), and thrombosis and vanishing of distal part of BA that had been fed by bilateral PCoAs (K,L).
Cerebral angiography was performed and a huge dissecting aneurysm distal to the bilateral anterior inferior cerebellar arteries (AICA) was noted (figure 1C). The bilateral posterior cerebral arteries (PCA) and superior cerebellar arteries (SCA) were involved in the aneurysm. Contrast medium was stagnant in the upper part of the aneurysm. Bilateral posterior communicating arteries (PCoA) were prominent (figures 1D,E).
The procedure was performed under general anesthesia after informed consent was obtained two days after the angiogram. A 5F-guiding catheter was introduced into the left cervical vertebral artery under full heparinization. Then a microcatheter was navigated into the proximal part of the dissecting aneurysm. Two large 3D coils were adjusted carefully to make a basket inside the aneurysm (figure 1F). Then more coils were detached in the dissection. Finally, the last coil was deployed extending from aneurysm to the adjacent normal BA segment just above the bilateral AICA. Vertebral angiogram showed occlusion of the DA with patency of the bilateral AICA (figure 1G). Unfortunately, the bilateral carotid artery angiogram revealed that upper part of the aneurysm could still be stained by bilateral PCoAs and the contrast medium remained stagnantly (Figures 1H,I).
The patient was monitored in the ICU after she recovered from general anesthesia. APTT was kept 1.5-2 times with heparin for three days. Heparin was changed by subcutaneous injection of low molecular heparin for ten days. Although there was no new neurological sign, the patient had violent vomiting and severe headache two days after the procedure without evidence of rebleeding or infarction on CT, which gradually resolved spontaneously in one week.
The patient was followed up for seven months after embolization. She could speak stammeringly and walk slowly by herself. Cerebral angiograms confirmed the complete occlusion of the total dissection (figure 1J). Left and right carotid angiogram showed thrombosis and vanishing of the distal part of the dissection that had been fed by bilateral PCoAs (figures 1K,L).
Case 2
This 59-year-old man had been suffering from aggressively developing ataxia, dysarthria and dysphagia for six months. He felt numbness of his left limbs two months before. His most severe problem was recurrent pneumonia because he could not cough in the recent two months. He had to be fed by stomach tube and was treated by antibiotics. The patient had a ten-year history of hypertension. And he was attacked by a large area of myocardial infarction and was treated by coronary by-pass surgery two years before. When the patient was admitted, he was weak and fragile because of long-term dystrophia and repeated infection. Coarse rales were found in his inferior lung on physical examination. CT and MR showed a high signal mass pressing on the pons (figures 2A,B).
Figure 2.
This 59-year-old man had aggressively developing dysarthria, dysphagia and recurrent pneumonia. CT and MR showed a mass pressing on the brain stem (A, B). Cerebral angiography showed a huge dissecting aneurysm distal to the bilateral PICA of the VAs and extending to the upper two thirds of the BA (C-E). The bilateral PCoA could be seen and the right PCoA was prominent (F, G). After making a basket inside the aneurysm coils were deployed inside the dissection in the BA, the junction of the bilateral VA and the segment on the right VA just distal to the PICA (H). The left VA was also occluded with coils with a small amount of residual dissection (arrows) on VA (I). The good clinical recovery was suddenly disrupted when the patient fell into coma one morning six weeks after the initial treatment. Emergent angiography showed that dissection recanalized at the posterior part (arrows) originating from a residual dissection on the left VA (J). The recanalized cavity and dissection segment on the left VA were densely packed just distal to the PICA (K). Anastomosis (arrows) from the PICA to the basilar trunk could be seen clearly (L).
Cerebral angiography was performed and a huge dissecting aneurysm (DA) distal to bilateral PICA of vertebral arteries was seen extending to the upper two thirds of the BA (figures 2C-E). Contrast medium was found stagnant in the dissection located on junction of the VAs. Bilateral PCoA could be seen and the right PCoA was prominent (figures 2F,G).
The procedure was performed under general anesthesia after informed consent was obtained seven days after the angiogram. A 5F-guiding catheter was introduced into the right VA under full heparinization. Then a microcatheter was navigated to the basilar arterial dissecting aneurysm. Two large 3D coils were adjusted carefully to make a basket inside the aneurysm and then the coils were deployed inside the aneurysm on the lower BA and the junction of the two vertebral arteries, until the dissection on the right VA just distal to the PICA was completely embolized (figure 2H). After the right VA was occluded, the guiding catheter was moved to the left VA. The left VA was also occluded with coils, but a small amount of dissection just distal to the PICA was left (figure 2I).
The patient was monitored in the ICU after he recovered from general anesthesia. APTT was kept 1.5-2 times with heparin for three days. Heparin was changed by subcutaneous injection of low molecular heparin for ten days. His ability to eat, drink and speak gradually recovered in two weeks, except for intractable mild anaemia. The patient regained the ability to cough and discharge phlegm, which led to relief of pneumonia. This optimistic situation was suddenly disrupted when the patient fell into a coma one morning six weeks after the initial treatment. Emergent angiography showed that the basilar dissecting aneurysm had recanalized at the posterior part through the left vertebral artery (figure 2J). The recanalized cavity and some of the dissecting segment on left VA were densely packed just distal to the PICA (figure 2K). Anastomosis from the PICA to the basilar trunk could be seen clearly (figure 2L). The patient awoke one night after the second intervention and regained the ability to speak and cough. But two days after the second treatment, ventricular tachycardia and then cardiac arrest suddenly occurred and the patient died in less than half an hour.
Discussion
Natural history and prognosis
Although dissecting aneurysms have been reported to represent up to 28% of the aneurysmal abnormalities involving the intracranial vertebrobasilar artery and its branches, huge BA involved dissecting aneurysms are rare3-5,9,17,19,39. Bleeding occurred in 40%-60% of these patients 5,37,39. The rerupture rate of BA dissecting aneurysms was between 19% and 74%5,13,16,21,25,37-39. The mortality rate of BA dissection aneurysms was between 17% and 79% within days to months5,13,21,39. An additional 12%-20% experienced moderate to severe disability and only 25%-33% made a good recovery5,13. Compression on the brainstem and nerve or mass effect due to huge BA dissection was much more rarely reported compared with SAH and ischemic attack. Yoshimoto et Al39 reported one dissecting BA aneurysm with compression on the brainstem, and its mass effect was found increased by MR follow-up. Boulanger et Al6 reported cranial nerve deficits because of basilar tip dissection.
Conservative treatment
Conservative treatment, including observation with serial radiological studies and anticoagulation treatment represent a rational approach for unruptured BA dissection, but conservative treatment might be life-threatening for ruptured dissections involving the BA39. It also seems that conservative treatment cannot help to relieve the symptom of mass effect. Referring to these data, our cases of huge dissections involving the BA, one with a history of hemorrhage and both with compression on the brainstem, should exclude conservative treatment. Management of dissecting aneurysms involving the BA is controversial and challenging. The two interventional options available to the patient are surgical treatment and endovascular intervention.
Neurosurgical wrapping and clipping
There are three surgical options currently available, including wrapping, proximal (Hunterian) ligation, and arterial reconstruction by direct surgical clipping. Wrapping the lesion may reduce the risk of further hemorrhage, but its efficacy is unproven. Ali et Al4 reviewed seven cases of BA dissection reported in the literature, six patients were treated by wrapping. Four of them incurred disability and died. The high failure rate of wrapping has left the technique unproved and it should not be the first choice for our cases.
Arterial reconstruction by direct surgical clipping is ideal for ordinary intracranial berry aneurysms 27,30, but it is very difficult for BA dissecting aneurysms because of the unique pathological structure of the dissection. In intracranial dissections presenting with subarachnoid hemorrhage, the plane of dissection and intramural pseudoaneurysm is between the media and adventitia10. Beyond vessel wall friability, a poorly defined neck configuration and the frequent involvement of brainstem perforators emanating from the aneurysmal segment prevent the safe use of direct clip reconstruction in the overwhelming majority of cases 4. Sano et Al28 concluded that surgical clipping was not recommended in dissections involving the BA circumferentially. Ali et Al4 reported a case of dissecting aneurysm involving the BA unilaterally, which is the first and only case managed by direct surgical clipping alone in our reviewed literature. He recommended attempting surgical clipping after passing the acute phase, allowing these lesions to mature somewhat to promote the establishment of fibrotic tissue around the lesion and, essentially, give the surgeon tissue to clip. Returning to our cases, direct surgical clipping was not approached because the dissection was huge and circumferential and it involved the main trunk of the BA artery.
Endovascular coiling with BA patency
Endovascular coiling of dissections involving the BA with BA patency was not feasible and we could only find one case embolized with coils 38. The dissection was only partially embolized, and angiogram follow-up one month later found circumscribed globular dilation of the basilar trunk 38. Although some documents reported stent-assisted coil embolization of hemorrhage VA and carotid dissection1-2,14,18,26,32,35, we could only find three cases of ruptured dissections involving the BA treated with stent-assisted coil embolization 26,35.
However re-growth was found in one case, whereas no follow-up was reported in the other two. Because of a lack of relative data on BA dissections, we have to refer to the treatment of VA dissecting aneurysms, which are most related to those involving the BA, to help to evaluate and predict the result of BA dissections. Albuquerque et Al2, Sugiu et Al32 and MacKay et Al18 reported five cases of ruptured VA dissecting aneurysms treated with stent-assisted coil embolization, all of them suffered severe recurrences. Albuquerque et Al2 warned the successors of being ready to prevent reoccurrence and fatal complications with this novel technique.
Proximal occlusion of bilateral VAs
We have found two methods of proximal occlusion for ruptured BA dissecting aneurysms by surgical or intravascular treatment. The first is occlusion of dominant unilateral or bilateral vertebral arteries. The second is occlusion of the BA trunk. Although Nakahara et Al 23 and Boulanger et Al6 successfully treated two cases of ruptured BA dissection with endovascular occlusion of the unilateral vertebral artery, Yoshimoto et Al39, Takagi et Al33 and O'Shaughnessy et Al24 reported a high tendency of redilation or re-hemorrhage after bilateral occlusion of VAs in their six cases. Enlargement of the dissection was noted along with a newly discovered 5 mm daughter sac by follow-up neuroimaging in one case of O'Shaughnessy et Al24 after bilateral vertebral artery sacrifice. The case led us to consider why dissecting aneurysms exist and grow independently of hemodynamic stress. The authors explained that orthograde basilar artery flow transmitted through cervical muscular collaterals might have restored normal intraluminal hemodynamics24. Although unilateral or bilateral VA occlusion is safer than BA occlusion because fewer perforating arteries branch from the VA, it cannot prevent re-growth or re-bleeding because of failure to induce thrombosis inside a false lumen or potential cervical muscular collaterals to the BA.
Occlusion of BA
Occlusion of BA by clipping or embolization was initially designed to treat basilar nondissecting aneurysms. The first deliberate BA occlusion was reported as successful in the treatment of a basilar bifurcation aneurysm in 196222. Some of the early reports also proved the safety of occlusion of BA7,11. Steinberg et Al31 reported the results of 201 patients treated with BA or VA occlusion for predominantly giant intracranial aneurysms. They concluded that occlusion of the lower BA was generally well tolerated with 11% irreversible ischemic neurological deficits and upper BA occlusions carried a 14% risk of irreversible ischemic complications. In these series, good or excellent outcomes were achieved in around 65-70% of cases, with severe morbidity and mortality of around 30-35%. The authors also found that the size of PCoAs was the key index for safety of BA occlusion, and the presence of one or two small posterior communicating arteries correlated significantly with an increased risk. Generous flow from the PCoA must be ascertained before considering BA occlusion revascularization. Previous reports had indicated that PCoAs > 1 mm in size on angiography or MRA were sufficient for providing collateral flow (15,29). We have found out 11 cases of BA dissection treated by BA occlusion in the literature. Five of them were ruptured, and six of them were without hemorrhage 5,12,15,20,26,36. Henkes et Al12 reported four cases of endovascular coil occlusion of the BA for the treatment of BA dissection without a history of hemorrhage. In addition to BA occlusion, the authors densely occluded aneurysms in three cases, and partially occluded the aneurysmal cavity in one case. All long-term follow-up results were excellent. Wenderoth et Al 36 reported two small BA dissections and one large BA dissection in the upper or middle part of the BA. Two of them were densely packed, one had a miniscule aneurysm remnant which led to re-growth in the follow-up. All of them were in good clinical condition. Amin-Hanjani et Al 3 clipped the BA below the level of the AICAs and kept the pseudoaneurysm from further growth. Maintenance of brainstem perfusion in this setting is dependent upon the retrograde flow supplied by the PCoAs. Kai et Al15 reported a small ruptured BA dissection at the midportion of the BA just distal to the AICA. The bilateral PCoAs were all small in size. The authors clipped the proximal BA above the level of the AICA after successfully building retrograde flow from the bypass between the left PCA and VA with the radical artery. Massimi et Al 20 reported a case of huge dissection involving the VA and lower BA. The dissection was loosely occluded with coils but the patient died from massive intracranial hemorrhage three days after embolization.
How we designed the treatment
When we were designing the treatment with parent artery occlusion, there were three technical considerations.
1) What are the key points to keep brainstem perforators and make the patient safe after BA occlusion? 2) To occlude only a proximal part of the lesion or the whole lesion? 3) Is dense packing of the whole lesion needed?
We did not worry about brainstem perforators and brainstem ischemia before occlusion of the BA. Satisfactory size of bilateral PCoAs and tolerance of simultaneous temporal balloon occlusion trial on bilateral vertebral arteries made the occlusion of the BA possible. It is highly likely that many of the brainstem perforators incorporated into the aneurysm sac are occluded by thrombus at the time of diagnosis and that the perforating artery territories involved are already supplied by collateral circulation 36. However, irrespective of the integrity of incorporated perforators, the capacity of leptomeningeal and other minor collateral pathways is remarkable, as has been noted by other investigators 8. We can also note pial anastomosis between the PICA and BA distal to the lesion in figure 2L. Even supported by the above knowledge, we still kept anticoagulation with heparin low molecular heparin for more than ten days in both of our cases. We believe that anticoagulation could help to slow down the process of thrombosis inside dissections and prevent acute infarction of the brainstem as well as other cases of occlusion of parent arteries, as emphasized by Kai et Al 15 and Chen et Al40.
Amin-Hanjani et Al5 introduced the concept of flow reversal for BA dissecting aneurysms, which allowed removal of the hemodynamic stimulus for further growth of the pseudoaneurysm. The best long-term results with endovascular treatment are achieved with obliteration of the aneurysmal inflow zone34. Dense packing of BA dissection by Henkes et Al12 and Wenderoth et Al36 proved to be image effected and clinically efficient in long-term follow-up. Amin-Hanjani et Al5 and Kai et Al15 also achieved good results from proximal occlusion of BA dissections. Besides, the dense packing might deteriorate the compression on the brainstem as reported in one case by Henkes et Al 12. There is another non-academic reason for our rejection of complete dense packing of the dissection. Compared to BA dissections treated by BA and VA occlusion in the literature, our dissections were relatively larger and involved a longer segment of the BA. Complete dense packing for our huge and long lesions with detachable coils is too expensive and not fully covered by medical insurance in our country. Therefore, we designed a loose basket woven with 3D coils inside the pseudoaneurysmal cavity and then loosely packed the distal part of the cavity with coils, and finally densely occluded the proximal dissection. Coil basket and loose packing inside the dissection with coils might help to make the retrograde flow much slower and the thrombosis inside the pseudoaneurysmal cavity easier. Follow-up in case 1 showed thrombosis in the loosely packed distal part of the dissection and there was no retrograde flow into the dissection from collateral flow.
What we have learned from our cases
There was a rapid recanalization of the dissection six weeks after the treatment in case 2, which induced dissection re-growth and acute mass effect on the brainstem. We found that the recanalization occurred from the left VA. Why the left VA and not the right VA? We found that there was a small amount of residual dissection on the left VA just distal to the PICA. Wenderoth et Al36 also described a case with proximal small residual dissection that led to re-growth and second treatment. Massimi et Al 20 reported massive hemorrhage three days after loose packing of a huge BA dissection. It seems that complete dense packing of proximal dissection is the key point. We even recommend that not only the proximal dissection but also the adjacent small part of the normal segment be packed to prevent recanalization, as was done in case 1.
The patient awoke one night after the second treatment, but he died two days later due to sudden onset of ventricular tachycardia. Although the patient was weak and fragile because of long-term dystrophia and recurrent infection, with a history of a large area of myocardial infarction and coronary by-pass, and also with postoperative intractable mild anaemia, we cannot explain clearly the reason for his sudden death.
Conclusions
Patients presenting with dissections involving the BA have a high rate of morbidity and mortality. BA occlusion is a safe and efficient treatment for BA dissections proved by our cases and the literature.
Proximal occlusion might be sufficient, especially for huge and long lesions like ours. It seems that complete dense packing of the proximal dissection is the key point to prevent recanalization.
References
- 1.Ahn JY, Chung SS, et al. Treatment of spontaneous arterial dissections with stent placement for preservation of the parent artery. Acta Neurochir (Wien) 2005;147(3):265–273. doi: 10.1007/s00701-004-0436-8. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque FC, Fiorella DJ, et al. Endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg Focus. 2005;18(2):E3. 15. [PubMed] [Google Scholar]
- 3.Alexander CB, Burger PC, Goree JA. Dissecting aneurysms of the basilar artery in 2 patients. Stroke. 1979;10:294–299. doi: 10.1161/01.str.10.3.294. [DOI] [PubMed] [Google Scholar]
- 4.Ali MJ, Bendok BR, et al. Arterial reconstruction by direct surgical clipping of a basilar artery dissection aneurysm after failed vertebral artery occlusion: technique case report and literature review. Neurosurg. 2003:1475–1481. doi: 10.1227/01.neu.0000065181.59149.36. [DOI] [PubMed] [Google Scholar]
- 5.Amin-Hanjani S, Ogilvy CS, et al. Treatment of dissecting basilar artery aneurysm by flow reversal. Acta Neurochir. 1997;139:44–51. doi: 10.1007/BF01850867. [DOI] [PubMed] [Google Scholar]
- 6.Boulanger TH, Aymand A, et al. Diagnosis and endovascular treatment of midbasilar dissecting aneurysm. J Belge Radiol. 1996;79:171–174. [PubMed] [Google Scholar]
- 7.Caplan LR. Occlusion of the vertebral or basilar artery: Follow up analysis of some patients with benign outcome. Stroke. 1979;10:277–282. doi: 10.1161/01.str.10.3.277. [DOI] [PubMed] [Google Scholar]
- 8.Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141–162. doi: 10.3171/jns.1997.87.2.0141. [DOI] [PubMed] [Google Scholar]
- 9.Drake CG. Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. J Neurosurg. 1975;43(3):255–274. doi: 10.3171/jns.1975.43.3.0255. [DOI] [PubMed] [Google Scholar]
- 10.Endo S, Nishijima M, et al. A pathological study of intracranial posterior circulation dissecting aneurysms with subarachnoid hemorrhage: Report of three autopsied cases and review of the literature. Neurosurgery. 1993;33:732–738. doi: 10.1227/00006123-199310000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Fields WS, Ratinov G, et al. Survival following basilar artery occlusion. Arch Neurol. 1966;15:463–471. doi: 10.1001/archneur.1966.00470170017002. [DOI] [PubMed] [Google Scholar]
- 12.Henkes H, Liebig T, et al. Endovascular occlusion of the basilar artery for the treatment of dissecting and dysplastic fusiform aneurysms. Der Nervenarzt. 2006;77(2):198–200. doi: 10.1007/s00115-005-1926-5. 192, 194-196. [DOI] [PubMed] [Google Scholar]
- 13.Hosoda K, Fujita S, et al. Spontaneous dissecting aneurysms of the basilar artery presenting with subarachnoid hemorrhage. Report of two cases. J Neurosurg. 1991;75:628–633. doi: 10.3171/jns.1991.75.4.0628. [DOI] [PubMed] [Google Scholar]
- 14.Joo JY, Ahn JY, et al. Treatment of intra- and extracranial arterial dissections using stents and embolization. Cardiovasc Intervent Radiol. 2005;28(5):595–602. doi: 10.1007/s00270-004-0199-x. [DOI] [PubMed] [Google Scholar]
- 15.Kai Y, Hamada J, et al. Successful treatment of a ruptured dissecting basilar artery aneurysm. Case report. J Neurosurg. 2004;100(6):1072–1075. doi: 10.3171/jns.2004.100.6.1072. [DOI] [PubMed] [Google Scholar]
- 16.Komiyama M, Yoshimura M, et al. Acute basilar artery dissection treated by emergency stenting in a 13-year-old boy. Pediatr Neurosurg. 2005;41(6):318–322. doi: 10.1159/000088734. [DOI] [PubMed] [Google Scholar]
- 17.Leibowitza R, Doa HM, et al. Parent Vessel Occlusion for Vertebrobasilar Fusiform and Dissecting Aneurysms. Am J Neuroradiol. 2003;24:902–907. [PMC free article] [PubMed] [Google Scholar]
- 18.MacKay CI, Han PP, et al. Recurrence of a vertebral artery dissecting pseudoaneurysm after successful stent-supported coil embolization: case report. Neurosurgery. 2003;53(3):754–761. doi: 10.1227/01.neu.0000080065.49651.48. [DOI] [PubMed] [Google Scholar]
- 19.Massimi L, Moret J, et al. Dissecting giant vertebro-basilar aneurysms. Childs Nerv Syst. 2003;19(4):204–210. doi: 10.1007/s00381-003-0726-0. [DOI] [PubMed] [Google Scholar]
- 20.Massimi L, Moret J, et al. Dissecting giant vertebro-basilar aneurysms. Childs Nerv Syst. 2003;19(4):204–210. doi: 10.1007/s00381-003-0726-0. [DOI] [PubMed] [Google Scholar]
- 21.Masson C, Krespy Y, et al. Magnetic resonance imaging in basilar artery dissection. Stroke. 1993;24(8):1264–1266. [PubMed] [Google Scholar]
- 22.Mount L, Taveras J. Ligation of basilar artery in treatment of an aneurysm at the basilar-artery bifurcation. J Neurosurg. 1962;19:167–170. doi: 10.3171/jns.1962.19.2.0167. [DOI] [PubMed] [Google Scholar]
- 23.Nakahara T, Satoh H, et al. Dissecting aneurysm of basilar artery presenting with recurrent subarachnoid hemorrhage. Neurosurg Rev. 1999;22:155–158. doi: 10.1007/s101430050054. [DOI] [PubMed] [Google Scholar]
- 24.O’Shaughnessy BA, Getch CC, et al. Late morphological progression of a dissecting basilar artery aneurysm after staged bilateral vertebral artery occlusion: case report. Surg Neurol. 2005;63(3):236–243. doi: 10.1016/j.surneu.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Pozzati E, Andreoli A, et al. Dissecting aneurysms of the basilar artery. Neurosurgery. 1995;36(2):254–258. doi: 10.1227/00006123-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Ramgren B, Cronqvist M, et al. Vertebrobasilar dissection with subarachnoid hemorrhage: a retrospective study of 29 patients. Neuroradiology. 2005;47(2):97–104. doi: 10.1007/s00234-005-1346-z. [DOI] [PubMed] [Google Scholar]
- 27.Redekop GJ. Microsurgical clipping or endovascular coiling for ruptured cerebral aneurysms. Stroke. 2006;37(6):1352–1353. doi: 10.1161/01.STR.0000221157.48691.8f. [DOI] [PubMed] [Google Scholar]
- 28.Sano H, Kato Y, et al. Classification and treatment of vertebral dissecting aneurysm. Surg Neurol. 1997;48(6):598–605. doi: 10.1016/s0090-3019(97)00022-0. [DOI] [PubMed] [Google Scholar]
- 29.Schomer DF, Marks MP, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 2. 1994;330(22):1565–1570. doi: 10.1056/NEJM199406023302204. [DOI] [PubMed] [Google Scholar]
- 30.Solheim O, Eloqayli H, et al. Quality of life after treatment for incidental, unruptured intracranial aneurysms. Acta Neurochir (Wien) 2006;148(8):821–830. doi: 10.1007/s00701-006-0804-7. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg GK, Drake CG, et al. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms: Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161–173. doi: 10.3171/jns.1993.79.2.0161. [DOI] [PubMed] [Google Scholar]
- 32.Sugiu K, Takahashi K, et al. Rebleeding of a vertebral artery dissecting aneurysm during stent-assisted coil embolization: a pitfall of the "stent and coil" technique. Surg Neurol. 2004;61(4):365–370. doi: 10.1016/S0090-3019(03)00515-9. [DOI] [PubMed] [Google Scholar]
- 33.Takagi M, Hirata K, et al. Unusual angiographic changes in a dissecting aneurysm of the basilar artery: case report. Neurosurgery. 1994;34(2):356–358. doi: 10.1227/00006123-199402000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Turjman F, Massoud TF, et al. Predictors of aneurysmal occlusion in the period immediately after endovascular treatment with detachable coils: a multivariate analysis. Am J Neuroradiol. 1998;19:1645–1651. [PMC free article] [PubMed] [Google Scholar]
- 35.Uhl E, Schmid-Elsaesser R, Steiger HJ. Ruptured intracranial dissecting aneurysms: management considerations with a focus on surgical and endovascular techniques to preserve arterial continuity. Acta Neurochir (Wien) 2003;145(12):1073–1083. doi: 10.1007/s00701-003-0122-2. [DOI] [PubMed] [Google Scholar]
- 36.Wenderoth JD, Khangure MS, et al. Basilar trunk occlusion during endovascular treatment of giant and fusiform aneurysms of the basilar artery. Am J Neuroradiol. 2003;24(6):1226–1229. [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaura A, Isobe K, et al. Dissecting aneurysms of the posterior inferior cerebellar artery. Neurosurgery. 1991;28:894–898. doi: 10.1097/00006123-199106000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72:183–188. doi: 10.3171/jns.1990.72.2.0183. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimoto Y, Hoya K, et al. Basilar artery dissection. J Neurosurg. 2005;102(3):476–481. doi: 10.3171/jns.2005.102.3.0476. [DOI] [PubMed] [Google Scholar]