Summary
Although cure of cerebral arteriovenous malformations (AVMs) is the ideal goal it is often only possible with smaller sized lesions. This is certainly true if surgery or radiotherapy is used as the treatment strategy. Endovascular treatment, however, allows the possibility of partial treatment directed at specific areas of AVM architecture. With this in mind we retrospectively reviewed our cohort of AVMs to establish which morphological areas we had identified as targets and how the treatment of the AVM influenced the clinical picture.
Over a 36-month period 42 AVMs were treated. In 22 of the patients who presented with a hemorrhage an intranidal aneurysm was identified as a target and treated in nine patients.
Other patients presented with headaches (2), neurological deficit (4) and seizures (16) with two patients having their AVM picked up coincidentally. Targets identified included high flow fistulas (12), decreasing venous flow (17) and cure of the AVM (8).
Results of targeted treatment showed a lower rehemorrhage rate than anticipated by the natural history. Patients cured did best and targeted embolization improved seizures in nine patients, neurological deficit in four patients and headache in 20 patients.
Until safer methods exist to cure large AVMs the use of targeted embolization is acceptable as it protects patients against rehemorrhage and translates into an improvement in their clinical picture.
Key words: arteriovenous malformation, target embolization, intranidal aneurysm, delayed venous drainage, venous fistula, cure
Introduction
The natural history of arteriovenous malformations (AVM) is considered fairly benign with an annual hemorrhage rate of 4%. AVMs may present with other manifestations which may be an indication for treatment. Post mortem have shown the incidence of AVMs in the general population to be between 0.06 and 0.11%7-9 and the incidence of symptomatic hemorrhage to be between 1.24 per 100 000 and 0.51 per 100 0003,2.
The ideal treatment of AVMs, cure, must be achieved with a low morbidity and mortality. In an attempt to beat the morbidity and mortality of the natural history various treatment methods have been utilized and these methods have subtle variations, which in an individual case may favor one treatment method over another. In common these treatments are all able to treat lower Spetzler Grades well and higher grades are either not suitable (radiotherapy) or with an unacceptable complication rate (surgery) 10. Endovascular treatment of a high grade AVM may change the natural history of the AVM even if the AVM is partially treated9.
We sought to assess whether we could identify targets within an AVM and we wondered whether, even though in some cases we were only partially treating the AVM, this would change the natural history and clinical picture. With this in mind we reviewed our cohort of AVMs treated between 2004 and 2005 to assess if we could identify features which we believe were responsible for the conversion of an occult to a clinically evident AVM and what the impact of treatment was on these patients.
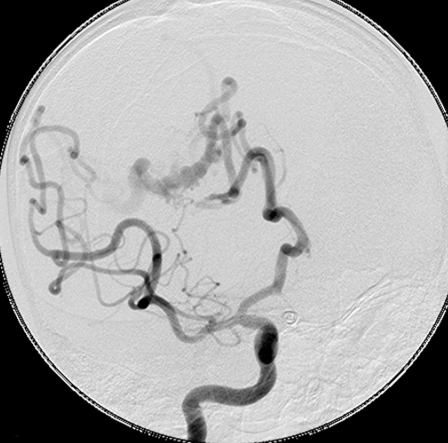
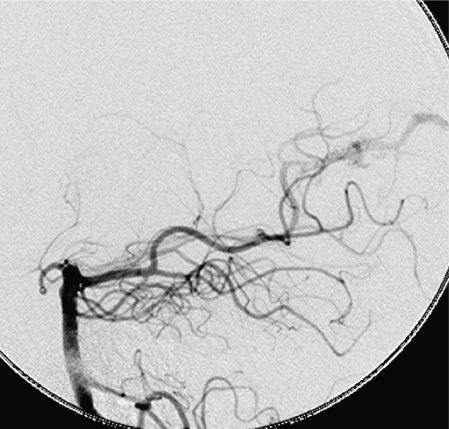
Figure 1.
A high flow fistula of the anterior cerebral artery. Note the passage of artery into vein with no intervening nidus.
Methods
Over a 36-month period from 2004 we treated 46 patients with AVMs. Retrospectively we looked at how these patients presented. We looked at how we had treated these patients and whether we had been able to identify a target prior to our embolization. This was done looking at the post procedure notes or the angiogram. We looked at whether we had been able to successfully treat the target we had identified. The results were then correlated with the patient's clinical picture to assess whether the endovascular success translated into clinical success. We also looked at whether specific targets were more beneficial to treat than others.
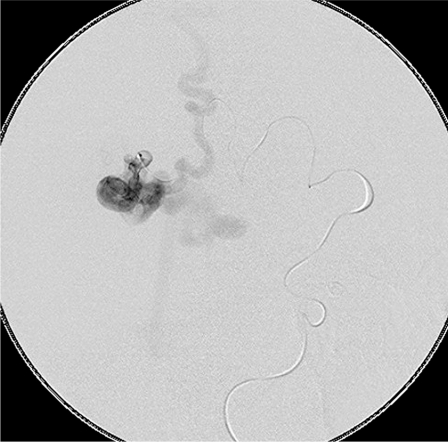
Figure 2.
A microcatheter in the fistula showing lack of intervening nidus.
Patients were assessed clinically as well as using a questionnaire where they were asked about seizure activity, headaches as well as general well being. Both the GOS and the Modified Rankin Score were used. Lastly in patients who had had more than one different mode of treatment we asked the patient about their experiences and which treatments were more easily tolerated.
Results
Over a 36-month period 46 patients were treated. The size in centimeters and Spetzler-Martin Grades are summarized in Tables 1 and 2. Treatment comprised of 63 embolizations, surgery on three patients and radiosurgery in two patients. Of the patients operated on all had embolization prior to surgery and two of the patients who were treated with radiosurgery required embolization - one before and one after radiosurgery. Twenty-two patients presented with a hemorrhage, 16 patients presented with seizures and another six patients who presented with other symptoms also had seizures. Two patients had AVMs picked up coincidentally. Two patients presented with headaches but 24 of the patients complained of headaches as a problem without it being the presenting feature. Four patients presented with a neurological deficit and five patients on examination were found to have a neurological deficit although it was not the reason for this presentation.
Table 1.
Size of AVMs treated/number of patients.
| 0-3 cm | 24 |
| 3.6 cm | 14 |
| > 6 cm | 8 |
Table 2.
Spetzler-Martin Grading
| I | 5 |
| II | 16 |
| III | 10 |
| IV | 9 |
| V | 6 |
In the evaluation of targets identified for endovascular treatment in nine patients an intranidal aneurysm was found. In 12 patients a high flow fistula was identified. In 17 patients the target was venous flow reduction as it was felt the drainage of the normal brain was not normal. In eight patients the target was AVM cure.
On follow-up we had 858 months patient follow-up, which translates to 71.5 patient years.
The Modified Rankin scores as well as the Glasgow Outcome Scores are summarized in Tables 3 and 4.
Table 3.
Modified Rankin Score.
| 0 | 25 |
| 1 | 6 |
| 2 | 5 |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| 6 | 0 |
Table 4.
Glasgow Outcome Score.
| 5 | 33 |
| 4 | 3 |
| 3 | 0 |
| 2 | 0 |
| 1 | 0 |
When a fistula was targeted, on follow-up, five patients claimed they generally felt better, in two patients seizures were better and one patient had no further seizures. Five of the patients claimed their headaches were better and one claimed it was worse.
In the patients in whom the target was cure all of the patients claimed to feel generally better. Three of the patients experienced no further seizures. Six claimed their headaches were better and three of the patients had improvement of their neurological function. In patients in whom the target was decreasing venous flow, ten of the patients claimed to feel generally better. Four of the patients had improvement in their seizure activity and one patient had no further seizures. Eight of the patients claimed their headaches were better and one patient had improvement in their neurological function.
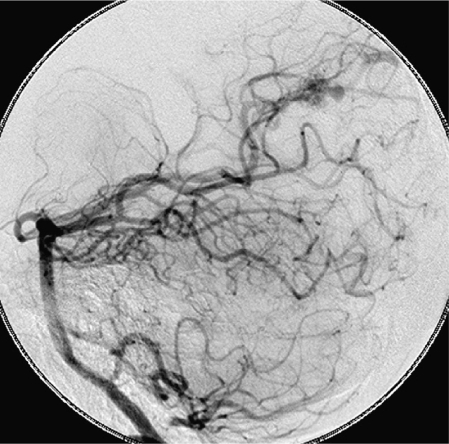
Figure 3.
An intranidal aneurysm on the posterior cerebral artery in a patient who presented with a hemorrhage.
In summary for the patients in whom fistulas were targeted seven of the 12 showed some form of improvement, of the patients whose target was cure seven out of eight showed some form of improvement and when the target was decreasing venous flow ten out of 14 showed some form of improvement. The results are summarized in Table 5.
Table 5.
| TARGET | SIGN/SYMPTOM | overall | seizures | headache | neurological deficit |
|---|---|---|---|---|---|
| fistula | 5 better | 2 improved 1 no further seizures |
5 improved 1 worse |
||
| decreased venous flow |
10 better | 4 improved 1 no further seizures |
8 improved | 1 improved | |
| cure | 8 better | 3 no further seizures |
6 improved | 3 improved | |
Figure 4.
Microcatheter placement to target only the intranidal aneurysm. Although it was tempting to try to cure this AVM, concern for the calcarine artery prompted targeting of the intranidal aneurysm only.
In nine patients the target treated was an intranidal aneurysm and in each case it was felt the aneurysm was successfully embolized. We had one patient who rebled but the intraventricular hemorrhage caused no morbidity.
There were two other complications in our cohort. One was thrombo-embolic secondary to an exchange and the other was reflux of NBCA into the labyrinthine artery, which resulted in deafness of the ear. In two patients the catheters were not removed with no complications.
Patients who had undergone more than one treatment modality claimed that endovascular treatment was the least distressing.
Discussion
When there is more than one modality that may be utilized to treat a condition the implication is that no one method has a clear benefit over another. When the condition has a benign natural history whatever mode is utilized must have a complication morbidity and mortality which is less than the natural history i.e. doing nothing. This is especially true for AVMs. Minor variations and subtleties aside, surgery, radiotherapy and endovascular embolization are all comparable and effective ways of treating small AVMs. Unfortunately when it comes to larger more complex AVMs this is no longer the case when trying to cure them. Radiotherapy can only treat AVMs up to a certain volume. Surgical morbidity and mortality is unacceptably high in large AVMs - much higher than the natural history of the AVM. Endovascular embolization may be unsuitable because of the number of embolizations required to cure the AVM. Sometimes more than one treatment method is used but this then exposes the patient to the cumulative complications. Spetzler Martin Grade 1, 10 ml volume or a lesion of less than 3 cm can be almost equally well treated by surgery, radiosurgery or endovascularly respectively. Conversely large AVMs are equally difficult to cure using any three of the modalities. However because of the ability to target certain areas of an AVM endovascular treatment potentially offers an advantage over the other two methods.
Classifications of AVMs have mirrored the treatments used. Initially because surgery was the first treatment available various classifications for a surgical perspective were published 5 but the most commonly now used is still that proposed by Spetzler and Martin10. However it is clear that a surgical classification does not fit when a non-surgical treatment is used. This is the motivation behind Valavanis and Yasargil's descriptive classification which is based on the topographical features of the AVM11 for endovascular treatment and was also acknowledged by Pollack and Flickinger who noted that grading systems for AVMs did not reliably predict outcomes in patients treated with radiosurgery8. Therefore depending on which modality of treatment is being used both an appropriate grading system and appropriate outcomes must be anticipated. Where partial targeted embolization reduces the rehemorrhage rate partial AVM resection increases the hemorrhage rate to double that of the natural history3,4.
Of our 46 patients we were only able to cure eight patients. This figure is in keeping with other studies where cure varies between 10% and 40% 6. All of these patients had AVMs smaller than 3 cm. It is clear that these patients fare better than patients who fail to have their AVMs cured.
In patients who presented with a hemorrhage we were only able to identify an intranidal aneurysm in nine patients. These were targeted as we agree with Valavanis and Yasargil that particularly nidal aneurysms if targeted in untreatable AVMs reduce the risk of hemorrhage11. There is therefore a group of patients who present with a hemorrhage where there is no intranidal aneurysm as a clear target. Although our follow-up is still short we have had less rehemorrhage than would be normally expected with the natural history of an AVM. For 71.5 patient years of follow-up one would expect three hemorrhages if a hemorrhage rate of 4% annually is accepted. We had one. Soderman et Al describe intranidal fistula as a "weak spot" for hemorrhage9. This is a target we chose in those patients in whom an intranidal aneurysm was not seen or could be targeted. These findings are in keeping with those of Crawford et Al who showed that partial targeted embolization with NBCA reduced the long term risk of hemorrhage by 24-78% when intranidal aneurysms or fistulae were targeted3.
Intranidal fistulas not only reduced the rehemorrhage rate but also improved the seizure activity and headaches in patients. Surprisingly when the target was decreasing venous flow more than half the patients felt better and had fewer seizures and/or headaches. One patient who presented with continuous athetoid movements of his arm was able for the first time in years to have an undisturbed full night's sleep following the treatment decreasing venous flow in a basal ganglial AVM. Patients we were able to cure did very well.
Deciding on the target when treating an AVM that cannot be cured may be straightforward. An intranidal aneurysm in a patient who has bled can be seen as an objective straightforward target. When it comes to what constitutes an intranidal fistula objectivity is more difficult. A fistula implies a high flow hole into the venous side and may be suspected radiologically when large caliber vessels with no intervening nidus are seen and confirmed hydrodynamically when a microcatheter is placed in the artery and the contrast is seen to rapidly wash away.
When the target is decreasing venous flow from the AVM it is even more difficult to be objective. The assessment of decreasing venous flow is a dynamic one that is made by comparing venous drainage of the surrounding brain before and after the embolization. If the venous drainage of the normal brain appears not to be delayed or less delayed when compared prior to embolization, then it can be assumed that there has been a reduction in blood flow through the AVM. By reducing the volume of blood flowing through the AVM venous congestion of the surrounding brain is reduced and this translates clinically into an improved picture.
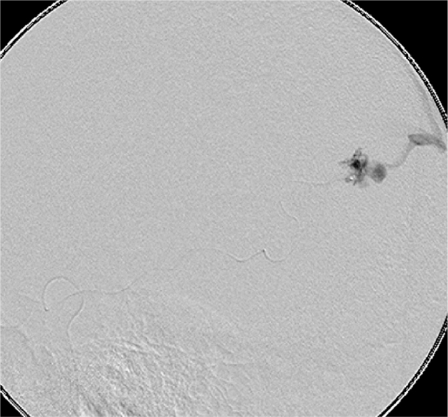
Figure 5.
Image shows successful treatment of the intranidal aneurysm only.
The improvement seen in patients when a fistula is treated may also be on this basis.
Of the five patients who had endovascular treatment as well as either surgery or radiosurgery four of the patients said given the option they would choose endovascular embolization for treatment of their AVM. Both of the radiosurgically treated patients preferred embolization to radiosurgery.
In conclusion the decision to treat an AVM must ensure that the complication of the treatment is safer than the natural history. Large AVMs have a high treatment complication rate if cure is the objective. In smaller AVMs where cure is possible this should be safely attempted as these patients do best. In larger AVMs targeted embolization is safe and is beneficial to the patient.
These benefits are translated into clinical improvement as well. We identified four targets namely aneurysms, venous fistula, high venous flow impairing normal brain venous drainage and cure of the AVM. Some targets can be more objectively assessed than others but perhaps as we understand AVMs better this subjectivity will be reduced. Until we have a safer way of curing large AVMs partial targeted embolization is an acceptable treatment.
References
- 1.Alexander MJ, Tolbert ME. Targeting Cerebral Arteriovenous Malformations for Minimally Invasive Therapy. Neurosurgery. 2006;59:S178–S183. doi: 10.1227/01.NEU.0000238530.44912.01. [DOI] [PubMed] [Google Scholar]
- 2.Al Shahi R, Bhattacharya JJ, et al. Prospective population based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS) Stroke. 2003;34:1163–1169. doi: 10.1161/01.STR.0000069018.90456.C9. [DOI] [PubMed] [Google Scholar]
- 3.Crawford P, West C, et al. Arteriovenous malformations of the brain; natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1–10. doi: 10.1136/jnnp.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han PP, Ponce FA, Speltzer RF. Intention to treat analysis of Spetzler - Martin grade IV and V arteriovenous malformations: Natural history and treament paradigm. J Neurosurg. 2003;98:3–7. doi: 10.3171/jns.2003.98.1.0003. [DOI] [PubMed] [Google Scholar]
- 5.Luessenhopp AJ, Gennarelli TA. Anatomical grading of supratentorial arterial venous malformations for determining operatability. Neurosurgery. 1977;1:30–35. doi: 10.1227/00006123-197707000-00007. [DOI] [PubMed] [Google Scholar]
- 6.McCormick WF, Rosenfield DB. Massive brain hemorrhage review of 144 cases and examination of their causes. Stroke. 1973;4:946–954. doi: 10.1161/01.str.4.6.946. [DOI] [PubMed] [Google Scholar]
- 7.Ondra S, Troupp H, et al. The natural history of symptomatic arteriovenous malformations of the brain; a 24 year follow up assessment. J Neurosurg. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 8.Pollock BE, Flickinger JC. A proposed radio-surgery based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79–85. doi: 10.3171/jns.2002.96.1.0079. [DOI] [PubMed] [Google Scholar]
- 9.Soderman M, Andersson T, et al. Management of patients with brain arteriovenous malformations. EJR. 2003;46:195–205. doi: 10.1016/s0720-048x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 10.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformation. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 11.Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131–214. doi: 10.1007/978-3-7091-6504-1_4. [DOI] [PubMed] [Google Scholar]