Abstract
Plants express genes that encode enzymes that catalyse reactions to form plant secondary metabolites in specific cell types. However, the mechanisms of how plants decide their cellular metabolic fate and how cells diversify and specialise their specific secondary metabolites remains largely unknown. Additionally, whether and how an established metabolic program impacts genome-wide reprogramming of plant gene expression is unclear. We recently isolated PAP1-programmed anthocyanin-producing (red) and -free (white) cells from Arabidopsis thaliana; our previous studies have indicated that the PAP1 expression level is similar between these two different cell types. Transcriptional analysis showed that the red cells contain the TTG1-GL3/TT8-PAP1 regulatory complex, which controls anthocyanin biosynthesis; in contrast, the white cells and the wild-type cells lack this entire complex. These data indicate that different regulatory programming underlies the different metabolic states of these cells. In addition, our previous transcriptomic comparison indicated that there is a clear difference in the gene expression profiles of the red and wild-type cells, which is probably a consequence of cell-specific reprogramming. Based on these observations, in this report we discuss the potential mechanisms that underlie the programming and reprogramming of gene expression involved in anthocyanin biosynthesis.
Keywords: Anthocyanin, Arabidopsis thaliana, metabolic engineering, metabolic fate, PAP1, plant secondary metabolism
Introduction
More than two hundred thousand structurally diverse plant secondary metabolites (PSMs, plant natural products) have been reported in the plant kingdom.1,2 In general, PSMs provide diverse functions such as protection against herbivores and pathogens and attraction of pollinators and seed dispersers. Because they are biosynthesised from primary metabolites (e.g., amino acids), PSMs have been concisely categorised into nitrogen-containing secondary metabolites (alkaloids, glucosinolates, cyanogenic glycosides, amines and non-protein amino acids), terpenoids, and phenolics.3 An obvious biosynthetic property of PSMs is that their genes are expressed in a family-, plant-, tissue-, cell- or time-specific manner.2-4 Phytoalexins are also PSMs as a rapidly expressed result of plant-microbial interactions. PSMs are biosynthesised in pathways that are catalysed by enzymes encoded by genes,4,5 and these metabolic pathways are regulated by transcription factors.6 In a previous review, Vom Endt et al.6 asked the important question of what regulates the regulators. Some recent studies have provided relevant evidence and attempted to answer this question. For example, treatment of suspension-cultured Medicago truncatula cells with either jasmonic acid or yeast extract induces the expression of genes that encode for transcription factors that can reprogram secondary metabolism.7,8 However, exactly how specific metabolic fates of plant cells are determined and how specific metabolic pathways are memorised by plant cells remain unknown. The answers to these two questions will increase our understanding of PSM biosynthesis and metabolic engineering.
Anthocyanins are a group of natural pink/red/blue/purple pigments that are widely produced in the plant kingdom. Anthocyanin pigments are an ideal model for studying the complex process of natural product biosynthesis in plants.9 There are three common types of anthocyanins that are classified based on their core chromophore structure: cyanin, delphinin, and pelargonin.10 The main anthocyanin molecules that are biosynthesised in A. thaliana are cyanins derived from cyanidin. Beginning with phenylalanine, there are nine enzymes involved in this pathway that are essential for the biosynthesis of red anthocyanidin pigment in A. thaliana (Fig. 1B). Of these nine enzymes, phenylalanine amino-lyase (PAL), cinnamate-4-hydroxylase (C4H), and 4-coumaroyl: CoA-ligase (4CL) are also required for the biosynthesis of other phenylpropanoids. Chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), and flavonoid 3′-hydroxylase (F3′H) are normally referred to as the early flavonoid pathway enzymes, whereas dihydroflavonol 4-reductase (DFR) and anthocyanidin synthase (also called leucoanthocyanidin dioxygenase, LDOX) are normally referred to as the late anthocyanin pathway enzymes. The glycotransferases (GT) add sugars to anthocyanidins, which diversifies the anthocyanin structure. Flavonoid 3′5′-hydroxylase (F3′5′H) is another enzyme that is required for delphinin production. Based on current genomic information, the Arabidopsis genome does not contain a gene that codes for this enzyme, a fact supported by the main previously reported anthocyanin molecular structures.11-13 To produce anthocyanins, the expression of genes that code for these enzymes is essential. Correspondingly, mutations in these genes (e.g., tt3, a DFR mutant) cause the loss of anthocyanin expression in plant tissue.14
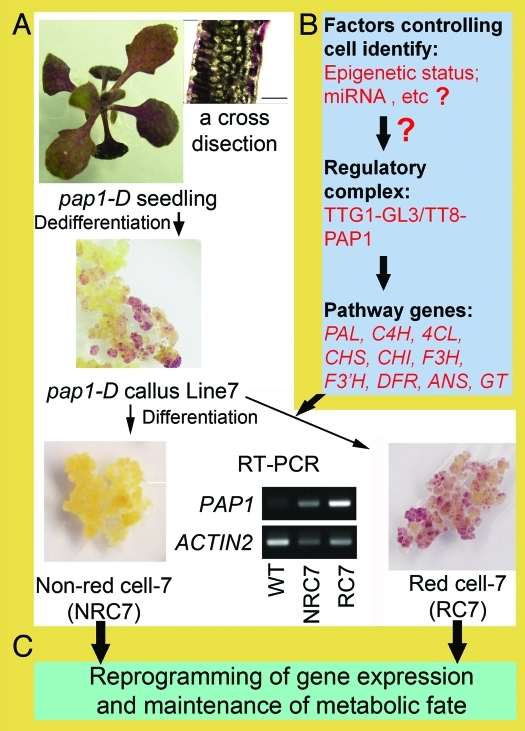
Figure 1.
A scheme showing the differentiation of red and white PAP1-programmed Arabidopsis cells and the regulation of metabolism toward anthocyanin biosynthesis in the red cells. (A) The white box illustrates the dedifferentiation of cells from a PAP1-programmed purple seedling with anthocyanin accumulation in the epidermal cells; the acquisition of calli with different accumulation patterns of anthocyanins; re-differentiation of red and white cells; and a RT-PCR image showing the expression of PAP1 in both the white and red cells. The thin arrows indicate experimental procedures that were performed to differentiate cells from leaves. (B) The light blue box presents the components that are required for the development of an anthocyanin biosynthesis metabolic fate in red cells; these include 10 pathway genes, the TTG1-GL3/TT8-PAP1 regulatory complex we recently described11 and factors that potentially control the regulatory complex. The wide black arrows indicate regulatory activation, and the question marks indicate unknowns. (C) The light green box illustrates the process of establishing and maintaining a particular metabolic fate, which involves the genome-wide reprogramming of metabolic gene expression. The thick black arrows between boxes A and C indicate that the establishment of a metabolic fate can lead to the reprogramming of gene expression. Our previous study showed that the red cells have reprogrammed gene expression.11
Although numerous regulators (Fig. 1) from Arabidopsis (e.g., PAP1, GL3 and TTG1)11 and the C gene from maize15 have been functionally characterised, how these regulators are themselves regulated remains unknown. We recently developed anthocyanin-producing Arabidopsis cells and tobacco cells that are programmed by the expression of PAP1 to study these unknown mechanisms.11,16 These two systems are ideal models for understanding how PAP1, an R2R3-MYB transcription factor, programs anthocyanin biosynthesis in cells and how PAP1 function is in turn regulated by other factors.11,16 Based on our recent work, the goal of this addendum is to provide insight into the programming and reprogramming mechanisms that determine the cellular metabolic fate of anthocyanin biosynthesis in A. thaliana.
Differentiation of PAP1-Based Programming into Anthocyanin Biosynthesis in Arabidopsis Cells
Previous studies have shown that PAP1 is a master activator of anthocyanin biosynthesis in A. thaliana. However, PAP1 regulation in vivo is restricted to specific cell types. pap1-D plants overexpress the PAP1 gene under the control of a 35S promoter with four enhancers.17 Previous studies have shown that PAP1 overexpression either activates or increases the expression of the most of genes encoding the nine enzymes and GTs described above that catalyse the formation of anthocyanins in plants.13,17,18 However, PAP1 overexpression does not lead to anthocyanin production in all plant cells. PAP1 activation of anthocyanin biosynthesis is strongly restricted to certain cell types, e.g., epidermal and hypodermal cells of leaves, as well as parenchymal cells in and around the vascular bundles of leaf veins.12 Interestingly, PAP1 overexpression does not lead to the formation of anthocyanins in trichomes, which contrasts with the results for ectopic expression of PAP1 in tobacco plants.19 Thus, these data indicate that PAP1 function and subsequent anthocyanin biosynthesis in trichomes is differentially regulated, even in similar cell types.
PAP1 regulation is restricted to specific cell types in vitro. We recently described the metabolic engineering of anthocyanin production in Arabidopsis cells from pap1-D plants that were transcriptionally programmed by overexpression of the PAP1 gene encoding MYB75.11 To obtain PAP1-programmed cells that produce anthocyanins, we screened multiple lighting conditions and a large number of media consisting of multiple combinations of phytohormones, inorganic nutrients and organic nutrients. Newly induced cells that were dedifferentiated from red leaf explants produced low levels of anthocyanins. Red cells (Fig. 1A) with high levels of anthocyanins were obtained from media containing very low levels of nitrogen source.11 We observed that although the cellular PAP1 expression level was high, the selected cells had anthocyanin accumulation patterns that ranged from low to high (Fig. 1A), which indicates that PAP1 regulation differs in various parts of the callus. Recently, we isolated white (e.g., NRC7) and red cell lines (RC7) from a single explant (Fig. 1A). We performed RT-PCR assays to determine whether PAP1 was also over expressed in the white cells. The results showed that the PAP1 expression level is very similar between the NRC7 and RC7 cell lines (Fig. 1 A). This indicates that the posttranscriptional status of PAP1must differ because although both cell types overexpress PAP1, only the red cells biosynthesise anthocyanins.
PAP1 regulation in engineered cells requires additional regulators, and the generation and characterisation of numerous knockout mutants has increased our knowledge of anthocyanin biosynthesis regulation in Arabidopsis. Previous reports have indicated that the WBM (WD-40/bHLH/MYB) complexes are likely to control anthocyanin biosynthesis.20,21 The WBM complexes in Arabidopsis seedlings are TTG1/WD-40-GL3/bHLH-PAP1/MYB75, TTG1-GL3-PAP2/MYB90, TTG1 -GL3-MYB113 and/or 114.21 In seeds, the most likely regulatory complex is the TTG1-TT8/bHLH- TT2/MYB complex located in the endothelium.21,22 In our engineered red cells that were programmed by PAP1, microarray and RT-PCR analyses indicated that PAP1, GL3, TT8 and TTG1 were all expressed; however, PAP1 expression was not detected and there were low expression levels of GL3 and TT8 in the wild-type control cells.11 We also recently analysed the expression levels of these genes in PAP1-programmed white cells (NRC7, Fig. 1A). The GL3 expression level was extremely low, and TT8 expression was not detected (Table 1, PCR image data not shown). These results indicate that low or no expression of these two genes may lead to no anthocyanin production in the white cells, which supports the hypothesis that PAP1 regulation may be dependent on GL3 and/or TT8. These experiments also show that there are different regulatory programs in these two types of metabolic cells.
Table 1. TTG1-GL3/TT8-PAP1 complex gene expression in PAP1-programmed anthocyanin-producing (RC7) cells, PAP1-programmed anthocyanin-free (NRC7) cells and wild-type (WT) cells.
| Cell type | TTG1 | GL3 | TT8 | PAP1 |
|---|---|---|---|---|
| RC7 |
+ |
+ |
+ |
+ |
| NRC7 |
+ |
d |
- |
+ |
| WT | + | - | - | - |
“+”: expression; “-”: no expression; “d”: at the limit of detection.
Metabolic Cell Fate and Gene Expression Reprogramming
Little is known about the mechanisms that determine cellular metabolic fate and plant secondary metabolism. As described above, PAP1-based programming is strongly restricted to specific cell types; however, the mechanisms underlying this observation are unclear. Here, we hypothesise that this difference in cellular metabolic fate toward anthocyanin biosynthesis programmed by a WBM complex is controlled by epigenetics. Our engineered red cells are an ideal model for studying the differentiation of metabolic fate toward anthocyanin biosynthesis.
In our experiment, we isolated red and white cells from PAP1-programmed leaves (Fig. 1A). We hypothesised that the red cells (Fig. 1A) inherited their epigenetic modifications from the epidermal and hypodermal cells of leaves or parenchymal cells in the vascular bundles of the leaf veins, which have active PAP1-based programming promoting anthocyanin production. In contrast, we hypothesised that the white cells (NRC7, Fig. 1A) originate from mesophyll cells or other cell types that do not produce anthocyanins.12 In addition, we hypothesised that although the in vitro cultured red cells morphologically differ from the in planta anthocyanin-producing cells (e.g., epidermal cells) in leaves, the red cells inherit their “metabolic context” from their somatic “mother” cells. Furthermore, we suggest that the engineered white cells (e.g., NRC7, Fig.1A) lack the metabolic context and epigenetic modifications required for anthocyanin production. The most direct evidence is the expression of TTG1, TT8 and GL3 (GLABRA3) in the red cells. TTG1 may be a genetic marker for an epidermal cellular and metabolic fate in Arabidopsis tissue. TTG1 is an essential component of the WBM complexes that regulate anthocyanin biosynthesis described above. Additionally, TTG1 is globally involved in determining the fate of epidermal cells, including pavement cells and trichomes.23-26 TTG1 recruitment may trigger the development of plant tissue into epidermis. Similarly, GL3 may be another marker gene. GL3 is specifically expressed in the epidermis of Arabidopsis tissues.26,27 In our experiments, we observed that GL3 was expressed in the red cells but not in the wild-type control cells.11 Recently, we showed that trace expression of GL3 occurs in PAP1-programmed white cells (PCR data not shown). TT8 may be yet another marker gene, although it has been reported to regulate anthocyanidin and proanthocyanidin biosynthesis in the seed coat.28,29 In our experiments, TT8 expression was detected in the red cells but not in the wild-type control or PAP1-programmed white cells.
The morphological differences between the in vitro cultured cells and epidermal cells can be explained by their EGL3 expression pattern. Previous studies have shown that EGL3 (Enhancer of GL3) plays a role in the determination of pavement cell fate,23,27 and EGL3 gene deletion results in abnormal epidermis. However, additional studies have reported that EGL3 does not play a role in anthocyanin production.30 In our engineered red and control cells, EGL3 expression was not detected.11 Therefore, although these engineered red cells do not inherit epidermal cell morphology, they contain the epigenetic modifications that promote anthocyanin biosynthesis.
One appropriate explanation for metabolic fate differentiation between similar cell types is different epigenetics. Although there are few studies describing plant epigenetics related to metabolic differentiation, knowledge regarding how a plant determines cell fate during vegetative and reproductive growth bears on the likeliness of epigenetic differences in various metabolic cell types. The Polycomb (PcG) and trithorax (trxG) groups are two protein families that are evolutionarily conserved in the fungal, animal, and plant kingdoms.31-34 These proteins have been shown to help determine plant cell development. In general, these two groups of proteins are antagonistically expressed at specific times during organismal development and at various transitions. The PcG proteins repress homeotic gene (HOX gene) expression, whereas the trxG proteins activate and maintain the expression of HOX genes, thereby promoting development and differentiation. Currently, the main PcG protein members identified in Arabidopsis include MEA (MEDEA), CLF (CRULY LEAF), SWN (SWINGER), FIE (FERTILIZATION INDEPENDENT ENDOSPERM), MSI1 (MULTICOPY SUPPRESSOR OF IRA1), FIS2 (FERTILIZATION-INDEPENDENT SEED 2), EMF2 (EMBRYONIC FLOWER 2), and VRN2 (VERNALIZATION 2). Other potential members include VRN1 (VERNALIZATION 1) and VIN3 (VERNALIZATION INSENSITIVE 3). These proteins form different types of Polycomb Repressive Complex 2s (PRC2s), including FIS PRC2-like, EMF PRC2-like, and VRN PRC2-like complexes. Several recent reviews have summarized and discussed their functions.34-37 One of the main functions of PRC2 is to catalyse the trimethylation of histone H3 at lysine 27 (H3K27me3), which leads to the reversible repression of gene expression. PRC2 also catalyses the trimethylation of histone H3 at lysine 9 (H3K9me3), which results in long-term repression of gene expression.35,38 The trxG proteins identified in Arabidopsis include ATX1 (Arabidopsis Trithorax 1), ULT1 (ULTRAPETALA 1), PKL (PRICKLE), and EFS (EARLY FLOWRING IN SHORT DAYS).35,36 ATX1 catalyses the trimethylation of histone H3 at lysine 4 (H3K4me3), which activates and maintains the expression of genes involved in flower development.36,39 In our experiments, we mined microarray data and found that in the both the red and control cells, MEA, CLF, SWN, FIE, MSI1, EMF2, VRN2 and VRN1 were expressed. In addition to the PcG members, EFS (a trxG member) was also expressed. However, FIS2 was not expressed. When we performed our microarray analysis, the ATX1 probe was not available on the Affymetrix ATH1 microarray; thus, we could not analyse ATX1 expression.
Interestingly, the MEA expression level was nearly 1.8-fold lower in the red cells compared to the wild-type control cells (false discovery rate, FDR, 0.02). In contrast to MEA, the VRN1 expression level was nearly 1.5-fold higher in the red cells than in the control cells (FDR, 0.02). MEA, which is a self-controlling protein and an essential component of the FIS PRC2 complex, has been shown to regulate seed development.40-42 Additionally, increased MEA expression has been proposed to cause callus formation (dedifferentiation) on clf swn double mutant leaves when grown on plant tissue culture medium.34 Thus, it seems likely that MEA expression was associated with the leaf callus formation observed in our experiments. However, it is unclear whether these PRC 2-like complexes control the expression or function of the TTG1-GL3/TT8-PAP1 complex. Future studies are needed to analyse these possible interactions, and such data will provide a more thorough understanding of the mechanisms involved in programming plant gene expression towards the biosynthesis of anthocyanins.
It is likely that established metabolic cells reprogram gene expression. However, there is little information regarding the reprogramming of gene expression in programmed cells towards anthocyanin biosynthesis or that of other metabolites. In general, the biosynthesis of many plant secondary metabolites is very responsive to biotic and abiotic stimulants. For example, phytoalexins are produced after transient reprogramming of gene expression in plant tissues in response to pathogens or other external signals. Evidence from a number of sources has shown that plant cells can rapidly program gene transcription profiles in response to elicitors,7 pathogen attacks,43 and nutrients such as nitrogen.44 However, whether the elicited programming can reprogram gene expression and can be inherited by progeny cells remains unclear.
In our experiment, the red cells programmed by PAP1 expression are an ideal model for studying the genome-wide reprogramming of gene expression. As previously reported, when we used 0.05 as a false discovery rate, we found that the expression profiles of nearly 6.5% of the genes in the Arabidopsis genome were altered in the red cells,11 which indicates that metabolic engineering for anthocyanins led to genome-wide reprogramming. However, the mechanisms that are responsible for this reprogramming are unknown. We hypothesise that the overexpression of PAP1 alters the epigenetic context of the red cells, and that the progeny cells inherit this alteration during long-term subculture under optimised conditions. Interestingly, anthocyanin formation in the red cells is regulated by additional factors such as plant hormones and nitrogen concentration (data not shown). These observations indicate that anthocyanin biosynthesis as programmed by PAP1 is reversible.
Conclusion
Our studies regarding PAP1-programmed anthocyanin biosynthesis and our genome-wide gene expression analysis have shown that the TTG1-GL3/TT8-PAP1 regulatory complex plays a key role in this process. The presence of this regulatory complex in Arabidopsis cells appears to promote anthocyanin production in vitro. Furthermore, genome-wide gene expression analyses of the red and wild-type cells showed that these cells have different transcriptomes, which likely mediates their different reprogrammed phenotypes. Our data provide fundamental information about the mechanisms whereby plant secondary metabolism is programmed and reprogrammed. Furthermore, these studies suggest that regulatory programming may eventually be applied to engineer medicinally and nutritionally significant metabolites.
Glossary
Abbreviations:
- PAP1
production of anthocyanin pigment 1
- TTG1
transparent testa glabra 1
- TT8
transparent testa 8
- GL3
glabra 3
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/17786
References
- 1.Springob K, Kutchan TM. Introduction to the different classes of natural products. In: Osbourn AE, Lanzotti V, eds. Plant-derived Natural Products. New York: Springer, 2009:3-50. [Google Scholar]
- 2.Wink M. Plant secondary metabolism: Diversity, function and its evolution. Nat Prod Commun. 2008;3:1205–16. [Google Scholar]
- 3.Dey PM, Harborne JB. Plant Biochemistry. San Diego London: Academic Press, 1997. [Google Scholar]
- 4.Croteau R, Kutchan T, Lewis N. Natural Products (Secondary Metabolites). In: Buchanan BB, Gruissem W, Jones RL, eds. Biochemistry & Molecular Biology of Plants 2000:1250-342. [Google Scholar]
- 5.Dixon RA. Engineering of plant natural product pathways. Curr Opin Plant Biol. 2005;8:329–36. doi: 10.1016/j.pbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Vom Endt D, Kijne JW, Memelink J. Transcription factors controlling plant secondary metabolism: what regulates the regulators? Phytochemistry. 2002;61:107–14. doi: 10.1016/S0031-9422(02)00185-1. [DOI] [PubMed] [Google Scholar]
- 7.Naoumkina MA, He XZ, Dixon RA. Elicitor-induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula. BMC Plant Biol. 2008;8:132. doi: 10.1186/1471-2229-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, Reddy MSS, Naoumkina M, Aziz N, May GD, Huhman DV, et al. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta. 2005;220:696–707. doi: 10.1007/s00425-004-1387-2. [DOI] [PubMed] [Google Scholar]
- 9.Springob K, Nakajima H, Yamazaki M, Saito K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat Prod Rep. 2003;20:288–303. doi: 10.1039/b109542k. [DOI] [PubMed] [Google Scholar]
- 10.Harborne JB, Baxter H. Anthocyanins. In: Harborne JB, Baxter H, eds. The Handbook of Natural Flavonoids John Wiley & Sons Inc, 1999:1-115. [Google Scholar]
- 11.Shi MZ, Xie DY. Engineering of red cells of Arabidopsis thaliana and comparative genome-wide gene expression analysis of red cells versus wild-type cells. Planta. 2011;233:787–805. doi: 10.1007/s00425-010-1335-2. [DOI] [PubMed] [Google Scholar]
- 12.Shi M-Z, Xie D-Y. Features of anthocyanin biosynthesis in pap1-D and wild-type Arabidopsis thaliana plants grown in different light intensity and culture media conditions. Planta. 2010;231:1385–400. doi: 10.1007/s00425-010-1142-9. [DOI] [PubMed] [Google Scholar]
- 13.Tohge T, Nishiyama Y, Hirai MY, Yano M, Nakajima J, Awazuhara M, et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005;42:218–35. doi: 10.1111/j.1365-313X.2005.02371.x. [DOI] [PubMed] [Google Scholar]
- 14.Winkel-Shirley B. Flavonoid Biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–93. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grotewold E, Drummond BJ, Bowen B, Peterson T. The myb -homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell. 1994;76:543–53. doi: 10.1016/0092-8674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L-L, Zeng H-N, Shi M-Z, Xie D-Y. Development of tobacco callus cultures over expressing Arabidopsis PAP1/MYB75 transcription factor and characterization of anthocyanin biosynthesis. Planta. 2008;229:37–51. doi: 10.1007/s00425-008-0809-y. [DOI] [PubMed] [Google Scholar]
- 17.Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12:2383–94. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowan DD, Cao M, Kui L-W, Cooney JM, Jensen DJ, Austin PT, et al. Environmental regulation of leaf colour in red 35S:PAP1 Arabidopsis thaliana. New Phytol. 2009;182:102–15. doi: 10.1111/j.1469-8137.2008.02737.x. [DOI] [PubMed] [Google Scholar]
- 19.Xie D-Y, Sharma SB, Wright E, Wang Z-Y, Dixon RA. Metabolic engineering of proanthocyanidins through co-expression of anthocyanidin reductase and the PAP1 MYB transcription factor. Plant J. 2006;45:895–907. doi: 10.1111/j.1365-313X.2006.02655.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay NA, Glover BJ. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005;10:63–70. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53:814–27. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 22.Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004;39:366–80. doi: 10.1111/j.1365-313X.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development. 2008;135:1991–9. doi: 10.1242/dev.016873. [DOI] [PubMed] [Google Scholar]
- 24.Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG1 gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–54. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- 25.Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasdan N, Blundell TL, et al. The TRANSPARENT TESTA GLABRA 1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell. 1999;11:1337–50. doi: 10.1105/tpc.11.7.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne CT, Zhang F, Lloyd A. GL3 Encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics. 2000;156:1349–62. doi: 10.1093/genetics/156.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J. The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development. 2003;130:6431–9. doi: 10.1242/dev.00880. [DOI] [PubMed] [Google Scholar]
- 28.Baudry A, Caboche M, Lepiniec L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J. 2006;46:768–79. doi: 10.1111/j.1365-313X.2006.02733.x. [DOI] [PubMed] [Google Scholar]
- 29.Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell. 2000;12:1863–78. doi: 10.1105/tpc.12.10.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feyissa DN, Løvdal T, Olsen K, Slimestad R, Lillo C. The endogenous GL3, but not EGL3, gene is necessary for anthocyanin accumulation as induced by nitrogen depletion in Arabidopsis rosette stage leaves. Planta. 2009;230:747–54. doi: 10.1007/s00425-009-0978-3. [DOI] [PubMed] [Google Scholar]
- 31.Surani A, Smith A. Differentiation and gene regulation programming, reprogramming and regeneration - Editorial overview. Curr Opin Genet Dev. 2003;13:445–7. doi: 10.1016/j.gde.2003.08.013. [DOI] [Google Scholar]
- 32.Köhler C, Grossniklaus U. Epigenetic inheritance of expression states in plant development: the role of Polycomb group proteins. Curr Opin Cell Biol. 2002;14:773–9. doi: 10.1016/S0955-0674(02)00394-0. [DOI] [PubMed] [Google Scholar]
- 33.Simon JA, Tamkun JW. Programming off and on states in chromatin: mechanisms of Polycomb and trithorax group complexes. Curr Opin Genet Dev. 2002;12:210–8. doi: 10.1016/S0959-437X(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 34.Köhler C, Villar CBR. Programming of gene expression by Polycomb group proteins. Trends Cell Biol. 2008;18:236–43. doi: 10.1016/j.tcb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Köhler C, Hennig L. Regulation of cell identity by plant Polycomb and trithorax group proteins. Curr Opin Genet Dev. 2010;20:541–7. doi: 10.1016/j.gde.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Pien S, Grossniklaus U. Polycomb group and trithorax group proteins in Arabidopsis. Biochim Biophys Acta. 2007;1769:375–82. doi: 10.1016/j.bbaexp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Guyomarc'h S, Bertrand C, Delarue M, Zhou DX. Regulation of meristem activity by chromatin remodelling. Trends Plant Sci. 2005;10:332–8. doi: 10.1016/j.tplants.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15:163–76. doi: 10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Venegas R, Pien S, Sadder M, Witmer X, Grossniklaus U, Avramova Z. ATX-1, an Arabidopsis homolog of trithorax, activates flower homeotic genes. Curr Biol. 2003;13:627–37. doi: 10.1016/S0960-9822(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 40.Jullien PE, Katz A, Oliva M, Ohad N, Berger F. Polycomb group complexes self-regulate imprinting of the polycomb group gene MEDEA in Arabidopsis. Curr Biol. 2006;16:486–92. doi: 10.1016/j.cub.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Baubec T, Scheid OM. Medea in full self-control. Trends Plant Sci. 2006;11:469–71. doi: 10.1016/j.tplants.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Arnaud P, Feil R. MEDEA takes control of its own imprinting. Cell. 2006;124:468–70. doi: 10.1016/j.cell.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 43.La Camera S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, et al. Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunol Rev. 2004;198:267–84. doi: 10.1111/j.0105-2896.2004.0129.x. [DOI] [PubMed] [Google Scholar]
- 44.Scheible W-R, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, et al. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004;136:2483–99. doi: 10.1104/pp.104.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]