Abstract
Human trisomy 21 is the most frequent live-born human aneuploidy and causes a constellation of disease phenotypes classified as Down syndrome, which include heart defects, myeloproliferative disorder, cognitive disabilities and Alzheimer-type neurodegeneration. Because these phenotypes are associated with an extra copy of a human chromosome, the genetic analysis of Down syndrome has been a major challenge. To complement human genetic approaches, mouse models have been generated and analyzed based on evolutionary conservation between the human and mouse genomes. These efforts have been greatly facilitated by Cre/loxP-mediated mouse chromosome engineering, which may result in the establishment of minimal critical genomic regions and eventually new dosage-sensitive genes associated with Down syndrome phenotypes. The success in genetic analysis of Down syndrome will further enhance our understanding of this disorder and lead to better strategies in developing effective therapeutic interventions.
Keywords: chromosome engineering, Down syndrome, genetic dissection, mouse models, trisomy 21
Human trisomy 21, the chromosomal basis of Down syndrome (DS), is a leading genetic cause of congenital heart disease, acute megakaryoblastic leukemia and developmental cognitive disabilities. It causes early-onset Alzheimer-type neurodegeneration in nearly every individual with DS. The mechanisms of this disorder are not well understood. The prevailing hypothesis suggests triplications of one or more human chromosome 21 (Hsa21) genes underlie a DS phenotype.1 To identify genomic regions that contain the critical genes associated with DS phenotypes, human genetic approaches have been used to analyze the data of individuals with segmental trisomies.2-5 To complement such an effort, the mouse has been employed as a model organism based on the evolutionary conservation between the regions on Hsa21 and three regions in the mouse genome located on chromosome 10 (Mmu10), Mmu16 and Mmu17. Ts65Dn and Ts1Cje models, the products of random mutagenesis, have served as the essential platforms in establishing the importance of mouse mutants in DS research.6,7 More recently, two new groups of mouse models have emerged. The first carries a duplication of a specific Hsa21 orthologous region on a mouse chromosome generated by Cre/loxP-mediated chromosome engineering, with the advantage that the duplications’ endpoints can be predetermined (Fig. 1). This group includes Ts1Rhr,8 Dp(16)1Yey/+,9 Ts1Yah,10 Dp(10)1Yey/+, Dp(17)1Yey/+11 and Dp(16)2Yey/+12 models (Fig. 2). The second consists of transchromosomic models, generated to carry Hsa21 or a fragment of it.13,14 Among them, Tc1 carries Hsa21 with two small deletions (Fig. 2).13 The availability of these mouse models has significantly expedited genetic analysis of the major DS-associated disease phenotypes.
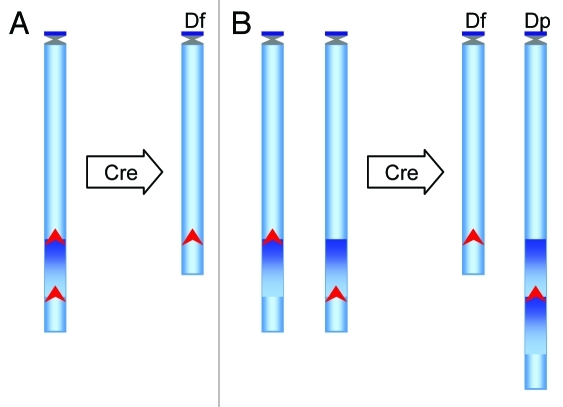
Figure 1. Schematic representation of the strategy to generate deletions and duplications in mouse ES cells using Cre/loxP-mediated chromosome engineering. To generate these rearrangements, loxP is targeted to the proximal and distal endpoints of an orthologous region of Hsa21 in the genome of mouse ES cells. Two different positive selection markers are used to isolate the ES cell clones carrying the targeted alleles. An expression vector for Cre recombinase is then transfected into the double-targeted cells to induce recombination. (A) If two targeted loxP sites are located in cis on the same chromosome homolog, the Cre/loxP-mediated recombination will lead to a deletion. (B) If two targeted loxP sites are located in trans on two homologs of the same chromosome, the recombination will lead to a duplication and a deletion. Recombination efficiency can be used to assist in identifying a cis or trans event because a cis recombination is much more efficient than a trans event. The ES cells carrying the desired chromosomal rearrangements are analyzed by various genotyping procedures, such as DNA gel blot analysis, fluorescence in situ hybridization and/or microarray-based comparative genomic hybridization. Upon confirmation, these cells are injected into wild-type mouse blastocysts to generate chimeric males, and mutant mice carrying a desired duplication or deletion can be derived by crossing the chimeras with wild-type mice. Arrow head, loxP site; Df, deletion; Dp, duplication.
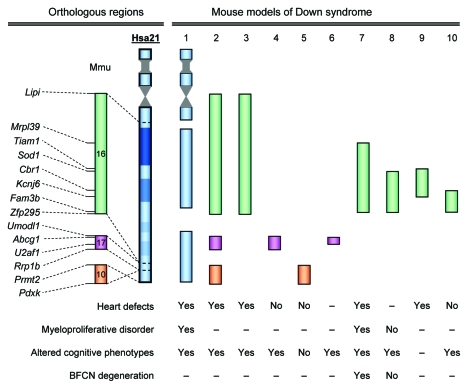
Figure 2. Genetic analysis of DS in mice. Endpoints of the trisomic regions are shown. Mouse models: 1, Tc1; 2, Dp(10)1Yey/+;Dp(16)1Yey/+;Dp(17)1Yey/+; 3, Dp(16)1Yey/+; 4, Dp(17)1Yey/+; 5, Dp(10)1Yey/+; 6, Ts1Yah; 7, Ts65Dn; 8, Ts1Cje; 9, Dp(16)2Yey/+; 10, Ts1Rhr. –, data not available.
Congenital heart defects are detected in 40–60% of children with DS.15 DS-related heart defects were observed in Ts65Dn, Tc1, Dp(16)1Yey/+ and Dp(10)1Yey/+;Dp(16)1Yey/+;Dp(17)1Yey/+ models, but not in Dp(10)1Yey/+ or Dp(17)1Yey/+ models,9,12,13,16,17 indicating the causative gene(s) for heart defects is located in the Hsa21 orthologous region on Mmu16, but not on Mmu10 or Mmu17 (Fig. 2). The detection of heart defects in Dp(16)2Yey/+ models, but not in the Dp(16)1Yey/Df(16)2Yey model, suggests that triplication of the Tiam1-Kcnj6 region is necessary and sufficient to cause heart defects, which further narrow down the critical genomic region for heart defects (Fig. 2). This conclusion is also supported by the elevated expressions of genes located within the Tiam1-Kcnj6 region in the pharyngeal arch and heart of Dp(16)2Yey/+ embryos.12 Since heart defects were not observed in the Ts1Rhr model,12,17 we can therefore conclude that the Tiam1-Cbr1 region contains a causative gene(s) (Fig. 2).
As many as 10% of children with DS develop transient myeloproliferative disorder, and about 30% of these patients develop acute megakaryoblastic leukemia (AMKL), which equates to an approximately 500-fold increased risk of developing AMKL.18 It has recently been determined that nearly all children who have DS and develop AMKL have acquired somatic mutations in exon 2 of the GATA1 gene on the X chromosome, which leads to the generation of a mutant protein, GATA1s.19 In mice, the same mutation in the mouse ortholog Gata1 alone results in hyperproliferation of the CD41+ progenitor cells of megakaryocytes in the E12.5 developmental stage, but the level of progenitor cells drops back to normal in the later developmental stage.20 Myeloproliferative disorder, including megakaryocytosis, was observed in Ts65Dn and Tc1 mice,21,22 and the addition of the Gata1 mutation to Tc1 mice did not lead to AMKL.22 On the other hand, by converting three copies of Erg to two copies, Ng et al. provided evidence that triplication of Erg is required for the myeloproliferative phenotype in Ts65Dn mice.23 An unresolved puzzle is that myeloproliferative disorder was not detected in the Ts1Cje model even though it also carried three copies of Erg24 (Fig. 2).
Human trisomy 21 is the most common genetic cause for developmental cognitive disabilities and there is no effective treatment for this clinical manifestation at present. Recent analysis of Ts1Rhr mice indicates that the triplication of this region led to decreased hippocampal long-term potentiation (LTP) and impaired cognitive behaviors.25 Within the duplicated region of the Ts1Rhr model, Dyrk1a and Kcnj6 are interesting candidates for these phenotypes.25 However, transgenic mice harboring a single copy of the BAC clone carrying the human DYRK1A gene showed increased hippocampal LTP.26 Introducing a heterozygous null allele of Kcnj6 into Ts65Dn mice has led to a decrease in the resting membrane potential of CA1 pyramidal neurons,27 but the direct impact of Kcnj6 on hippocampal LTP remains unknown. The duplications in Hsa21 orthologous regions on Mmu17 resulted in increased hippocampal LTP.10,28 Together, these data point to contributions and genetic interactions of different genes in the orthologous regions with regard to hippocampal LTP and cognitive behaviors. The compound effects of these and possibly other genes may have led to decreased hippocampal LTP and impaired cognitive behaviors in Tc1 and Dp(10)1Yey/+;Dp(16)1Yey/+;Dp(17)1Yey/+ mice.11,13 Besides the hippocampus, recent studies have also shown defects in other brain regions in Ts65Dn mice, e.g., the forebrain, which may have a significant implication for developmental cognitive disabilities.29,30 Interestingly, converting Olig1 and Olig2 back to two copies in Ts65Dn mice rescued some major neuronal phenotypes observed in the forebrain of the mutant mice.29
The brains of individuals with DS over the age of 40 show the neuropathological alterations of Alzheimer disease: amyloid plaques, neurofibrillary tangles, synapse loss and cellular atrophy,31-33 including degeneration of basal forebrain cholinergic neurons (BFCNs).34 Although the amyloid plaques and neurofibrillary tangles of Alzheimer disease have not been replicated in the current mouse models of DS,11,13,35 age-related BFCN degeneration has consistently been observed in Ts65Dn mice,36-38 which is associated with intra-axonal failure in retrograde transport of nerve-growth factor (NGF).36 In fact, NGF transport in Ts65Dn mice was decreased to 10–15% of the wild-type control.37 Crossing Ts65Dn mice to an App KO strain, NGF transport was markedly increased and the BFCN degeneration phenotype was rescued.37 This indicates that App played a conspicuous role in axonal transportation and BFCN degeneration.37 App is not triplicated in Ts1Cje mice (see Fig. 2) but axonal transportation in this mutant can only reach ~70% of the wild-type control,37 suggesting another gene(s), in addition to App, located within the Sod1-Zfp295 region also affects NGF transport.
From the aforementioned analyses of cardiovascular, hematopoietic and neurological phenotypes, it is apparent that mouse mutants play a pivotal role in DS research. At this point, as in the cases of human segmental trisomies, the number of desirable mouse mutants for genetic analysis of DS is also limited (see Fig. 2). But unlike the human cases, the number of new mouse mutants can be increased significantly by chromosome engineering in research laboratories. Therefore, the mouse can serve as an effective platform for a comprehensive genetic dissection of DS. On the other hand, because of species-specific differences, it would be interesting to replace mouse orthologous regions using human 21 genomic fragments. A BAC-based replacement of a mouse genomic segment with the equivalent human orthologous region has been reported.39 Cre/loxP-mediated recombination could also be used to transfer a megabase Hsa21 genomic fragment to a mouse chromosome40 although such a targeted transfer have not yet led to any desired mutant mice. If successful, mouse models carrying three copies of human chromosomal segments could be developed using this approach, and alternative strategy of genetic dissection of DS could be pursued by engineering and analyzing mouse mutants carrying defined segmental trisomies of Hsa21. Upon the identification of minimal critical regions, segmental trisomic mice could be compounded with null alleles of the genes located within the region in order to search for the dosage-sensitive genes associated with disease phenotypes. The ongoing international mouse gene knockout projects would provide the essential null alleles for this effort. Therefore, chromosome engineering may turn out to be one of the most critical technological innovations that propels contemporary DS research and hastens the pace of unraveling the underlying mysteries of this complex disorder, which in turn should speed up development of novel therapeutic interventions for the major clinical presentations of the disorder.
Acknowledgments
Projects in the authors’ laboratories are supported in part by grants from the Children’s Guild Foundation, the Jerome Lejeune Foundation, the Larry L. Hillblom Foundation, the Down Syndrome Research and Treatment Foundation, and the NIH (R01HL91519, R01NS55371 and R01NS66072).
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/17696
References
- 1.Epstein CJ. The consequences of chromosome imbalance. Am J Med Genet Suppl. 1990;7:31–7. doi: 10.1002/ajmg.1320370706. [DOI] [PubMed] [Google Scholar]
- 2.Korbel JO, Tirosh-Wagner T, Urban AE, Chen XN, Kasowski M, Dai L, et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc Natl Acad Sci USA. 2009;106:12031–6. doi: 10.1073/pnas.0813248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korenberg JR, Chen XN, Schipper R, Sun Z, Gonsky R, Gerwehr S, et al. Down syndrome phenotypes: the consequences of chromosomal imbalance. Proc Natl Acad Sci USA. 1994;91:4997–5001. doi: 10.1073/pnas.91.11.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyle R, Bena F, Gagos S, Gehrig C, Lopez G, Schinzel A, et al. Genotype-phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21. Eur J Hum Genet. 2009;17:454–66. doi: 10.1038/ejhg.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinet PM, Theophile D, Rahmani Z, Chettouh Z, Blouin JL, Prieur M, et al. Mapping of the Down syndrome phenotype on chromosome 21 at the molecular level. Biomed Pharmacother. 1994;48:247–52. doi: 10.1016/0753-3322(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 6.Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–84. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 7.Sago H, Carlson EJ, Smith DJ, Kilbridge J, Rubin EM, Mobley WC, et al. Ts1Cje, a partial trisomy 16 mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proc Natl Acad Sci USA. 1998;95:6256–61. doi: 10.1073/pnas.95.11.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson LE, Richtsmeier JT, Leszl J, Reeves RH. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science. 2004;306:687–90. doi: 10.1126/science.1098992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Yu T, Morishima M, Pao A, LaDuca J, Conroy J, et al. Duplication of the entire 22.9-Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities. Hum Mol Genet. 2007;16:1359–66. doi: 10.1093/hmg/ddm086. [DOI] [PubMed] [Google Scholar]
- 10.Pereira PL, Magnol L, Sahun I, Brault V, Duchon A, Prandini P, et al. A new mouse model for the trisomy of the Abcg1-U2af1 region reveals the complexity of the combinatorial genetic code of down syndrome. Hum Mol Genet. 2009;18:4756–69. doi: 10.1093/hmg/ddp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu T, Li Z, Jia Z, Clapcote SJ, Liu C, Li S, et al. A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions. Hum Mol Genet. 2010;19:2780–91. doi: 10.1093/hmg/ddq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Morishima M, Yu T, Matsui SI, Zhang L, Fu D, et al. Genetic analysis of Down syndrome-associated heart defects in mice. Hum Genet. 2011 doi: 10.1007/s00439-011-0980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S, et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science. 2005;309:2033–7. doi: 10.1126/science.1114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinohara T, Tomizuka K, Miyabara S, Takehara S, Kazuki Y, Inoue J, et al. Mice containing a human chromosome 21 model behavioral impairment and cardiac anomalies of Down's syndrome. Hum Mol Genet. 2001;10:1163–75. doi: 10.1093/hmg/10.11.1163. [DOI] [PubMed] [Google Scholar]
- 15.Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. 1998;77:431–8. doi: 10.1002/(SICI)1096-8628(19980605)77:5<431::AID-AJMG15>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Moore CS. Postnatal lethality and cardiac anomalies in the Ts65Dn Down syndrome mouse model. Mamm Genome. 2006;17:1005–12. doi: 10.1007/s00335-006-0032-8. [DOI] [PubMed] [Google Scholar]
- 17.Dunlevy L, Bennett M, Slender A, Lana-Elola E, Tybulewicz VL, Fisher EM, et al. Down syndrome-like cardiac developmental defects in embryos of the transchromosomic Tc1 mouse. Cardiovasc Res. 2010;88:287–95. doi: 10.1093/cvr/cvq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Athale UH, Razzouk BI, Raimondi SC, Tong X, Behm FG, Head DR, et al. Biology and outcome of childhood acute megakaryoblastic leukemia: a single institution's experience. Blood. 2001;97:3727–32. doi: 10.1182/blood.V97.12.3727. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32:148–52. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Godinho FJ, Klusmann JH, Garriga-Canut M, Yu C, Orkin SH. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet. 2005;37:613–9. doi: 10.1038/ng1566. [DOI] [PubMed] [Google Scholar]
- 21.Kirsammer G, Jilani S, Liu H, Davis E, Gurbuxani S, Le Beau MM, et al. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood. 2008;111:767–75. doi: 10.1182/blood-2007-04-085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alford KA, Slender A, Vanes L, Li Z, Fisher EM, Nizetic D, et al. Perturbed hematopoiesis in the Tc1 mouse model of Down syndrome. Blood. 2010;115:2928–37. doi: 10.1182/blood-2009-06-227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng AP, Hyland CD, Metcalf D, Carmichael CL, Loughran SJ, Di Rago L, et al. Trisomy of Erg is required for myeloproliferation in a mouse model of Down syndrome. Blood. 2010;115:3966–9. doi: 10.1182/blood-2009-09-242107. [DOI] [PubMed] [Google Scholar]
- 24.Carmichael CL, Majewski IJ, Alexander WS, Metcalf D, Hilton DJ, Hewitt CA, et al. Hematopoietic defects in the Ts1Cje mouse model of Down syndrome. Blood. 2009;113:1929–37. doi: 10.1182/blood-2008-06-161422. [DOI] [PubMed] [Google Scholar]
- 25.Belichenko NP, Belichenko PV, Kleschevnikov AM, Salehi A, Reeves RH, Mobley WC. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci. 2009;29:5938–48. doi: 10.1523/JNEUROSCI.1547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn KJ, Jeong HK, Choi HS, Ryoo SR, Kim YJ, Goo JS, et al. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiol Dis. 2006;22:463–72. doi: 10.1016/j.nbd.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Cramer NP, Best TK, Stoffel M, Siarey RJ, Galdzicki Z. GABAB-GIRK2-mediated signaling in Down syndrome. Adv Pharmacol. 2010;58:397–426. doi: 10.1016/S1054-3589(10)58015-3. [DOI] [PubMed] [Google Scholar]
- 28.Yu T, Liu C, Belichenko P, Clapcote SJ, Li S, Pao A, et al. Effects of individual segmental trisomies of human chromosome 21 syntenic regions on hippocampal long-term potentiation and cognitive behaviors in mice. Brain Res. 2010;1366:162–71. doi: 10.1016/j.brainres.2010.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarti L, Best TK, Cramer NP, Carney RS, Isaac JT, Galdzicki Z, et al. Olig1 and Olig2 triplication causes developmental brain defects in Down syndrome. Nat Neurosci. 2010;13:927–34. doi: 10.1038/nn.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakrabarti L, Galdzicki Z, Haydar TF. Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J Neurosci. 2007;27:11483–95. doi: 10.1523/JNEUROSCI.3406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278–82. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 32.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–6. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 33.Mann DM. The neuropathology of Alzheimer's disease: a review with pathogenetic, aetiological and therapeutic considerations. Mech Ageing Dev. 1985;31:213–55. doi: 10.1016/0047-6374(85)90092-2. [DOI] [PubMed] [Google Scholar]
- 34.Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–83. doi: 10.1016/S0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- 35.Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci USA. 1996;93:13333–8. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, et al. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci USA. 2001;98:10439–44. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Granholm AC, Sanders L, Seo H, Lin L, Ford K, Isacson O. Estrogen alters amyloid precursor protein as well as dendritic and cholinergic markers in a mouse model of Down syndrome. Hippocampus. 2003;13:905–14. doi: 10.1002/hipo.10130. [DOI] [PubMed] [Google Scholar]
- 39.Wallace HA, Marques-Kranc F, Richardson M, Luna-Crespo F, Sharpe JA, Hughes J, et al. Manipulating the mouse genome to engineer precise functional syntenic replacements with human sequence. Cell. 2007;128:197–209. doi: 10.1016/j.cell.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 40.Kazuki Y, Schulz TC, Shinohara T, Kadota M, Nishigaki R, Inoue T, et al. A new mouse model for Down syndrome. J Neural Transm Suppl. 2003;67:1–20. doi: 10.1007/978-3-7091-6721-2_1. [DOI] [PubMed] [Google Scholar]