Abstract
Recently, a novel observation was made in which nonischemic trauma at a site remote from the heart produced by a transverse abdominal incision resulted in a marked reduction of infarct size (IS) in the mouse heart via activation of sensory nerve fibers in the skin and subsequent activation of bradykinin 2 receptors (BK2R). This phenomenon was termed remote preconditioning of trauma (RPCT). Since RPCT may have potential clinical implications we attempted to confirm these findings in a large animal model, the dog. The epoxyeicosatrienoic acids (EETs) have also recently been shown to be antinociceptive and have been shown to mimic ischemic preconditioning (IPC) and postconditioning (POC) in dogs, therefore, we tested the role of the EETs in RPCT. Anesthetized adult mongrel dogs of either sex were subjected to 60 min of left anterior descending (LAD) coronary artery occlusion followed by 3 h of reperfusion. In all groups except the controls (no slit), a transverse slit (9 cm) was applied to the abdominal wall of the dog being careful to only slit the skin. Subsequently, 15 min after the slit the heart was subjected to the ischemia/reperfusion protocol. In the control dogs, the IS as a percent of the area at risk (AAR) was 22.5 ± 2.4%, whereas in the dogs subjected to the slit alone the IS/AAR was reduced to 9.2 ± 1.2% (*P<0.01). The BR2R blocker, HOE 140 (50 ug/kg, iv) given 10 min prior to the slit, completely abolished the protective effects of RCPT as did pretreatment with 14,15-EEZE, a putative EET receptor blocker or pretreatment with the selective EET synthesis inhibitor, MSPPOH. These results suggest that BK and the EETs share cardioprotective properties in a large animal model of RPCT.
Introduction
Since the phenomenon of ischemic preconditioning (IPC) was first described in 1986 [1], there have been a plethora of studies in which the mechanisms responsible for the protective effects of IPC have been investigated and many types of receptors and signal transduction pathways responsible for its protective effects identified [2]. In the ensuing time, a number of phenomena similar to classical IPC have also been identified and characterized including the second window of IPC (SWOP), postconditioning (POC), remote preconditioning (RIPC) at a distance and remote ischemic perconditioning [3,4,5,6]. Unfortunately, in spite of a great deal of effort to find a clinically useful preconditioning mimetic, no drug candidate or device has been found that is commonly used in patients presenting with an acute myocardial infarction and has been shown to consistently reduce infarct size.
More recently a novel form of preconditioning has been described by Ren et al.[7] and Jones et al.[8] in the mouse heart subjected to an ischemia/reperfusion insult. This form of preconditioning was shown to involve skin nociception and activation of cardiac sensory and sympathetic nerves with bradykinin serving as a chemical mediator. This protective effect was also mediated by activation of PKCε and suppression of PKCδ with the subsequent activation of the ATP-sensitive K channel (KATP). This phenomenon was termed remote preconditioning of trauma (RPCT). Interestingly, RPCT was mimicked by topical application with capsaicin which suggests that this noninvasive treatment would be easily adapted to clinical use. RPCT was also shown to be effective when administered just prior to reperfusion which suggests that this treatment could be given during an ongoing infarction. Since this may have clinical applications, we decided to test whether RPCT also occurs in a larger animal model such as the dog and determine the role of bradykinin in this species. We also have shown that the epoxyeicosatrienoic acids (EETs) are involved in classical IPC and POC in dogs, thus, we decided to test if they play a role in RPCT by using a selective EET receptor blocker and an EET synthesis inhibitor.
Methods
All experiments conducted in these studies were performed in accordance with the Position of the American Heart association on Research and Animal Use and approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. The Medical College of Wisconsin is accredited by the American Association of Laboratory Animal Care.
Materials
N-methylsulfonyl-6-(propargyloxyphenyl)hexanamide (MSPPOH) and 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) were synthesized in the laboratory of Dr. J. R. Falck. HOE 140 was purchased from Tocris Bioscience. All other chemicals were of the highest analytic or purity grades. Distilled, deionized water was used in all experiments.
General preparation of dogs
The protocol used has been thoroughly described in detail in previous publications from our laboratory [9]. Briefly, adult mongrel dogs of either sex, weighing 15–25 kg, were fasted overnight, anesthetized with the combination of barbital sodium (200 mg/kg) and pentobarbital sodium (15 mg/kg), and ventilated with room air supplemented with 100% oxygen. Body temperature was carefully controlled at 38 ± 1°C with a heating pad. Atelectasis was prevented by maintaining an end-expiratory pressure of 5–7 cm with a trap. Arterial blood pH, PCO2, and PO2 were monitored at selected intervals by an AVL blood-gas analyzer and maintained within normal physiological limits (pH = 7.35–7.45, PCO2 30–40 Torr, and PO2 85–130 Torr) by adjusting the respiratory rate and the oxygen flow rate or by adding 1.5% sodium bicarbonate intravenously if necessary. An electromagnetic flowmeter (Statham 2202) was used to measure left anterior descending (LAD) coronary artery blood flow. A mechanical occluder was placed distal to the flow probe and the occluder, such that there were no branches between the flow probe and occluder. A double-tipped Millar (model 770) catheter was placed into the carotid artery and left ventricle (LV) to measure aortic and LV pressures and to determine LV change in pressure over time (positive and negative). The left atrium was cannulated via the appendage for radioactive microsphere injections which were administered at 30 min of occlusion and at the end of 3 h of reperfusion.
Experimental protocol
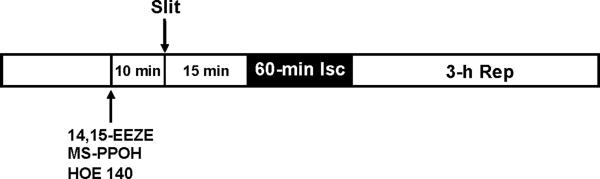
Dogs were sequentially assigned to 6 groups for different treatments (Fig. 1). In all groups, eight dogs were included for statistical analysis. The control group was subjected to 60 min of LAD occlusion and 3 h of reperfusion. At 15 min before ischemia, a 9 cm transverse skin incision was made via the abdominal midline with a scalpel and was termed the RPCT stimulus. In three subsequent series of dogs, the putative EET receptor antagonist,14,15-EEZE (0.128 mg/kg), the EET synthesis inhibitor, MSPPOH(1.29 mg/kg), were administered intracoronary 10 min before the RPTC stimulus to determine whether EETs were mediators of RPCT. Similarly, the BR2R blocker, HOE 140, was administered intravenously (50 ug/kg) 10 min before the RPCT stimulus to confirm a role for bradykinin in dogs subjected to RPCT. In an additional 3 control series of experiments, 14,15-EEZE, MS-PPOH and HOE 140, were administered 25 min before LAD occlusion in the absence of the RPCT stimulus to determine whether these compounds had any effect on IS/AAR alone in the absence of the RPTC stimulus. In all groups, hemodynamic measurements, blood-gas analyses, and regional myocardial blood flow (radioactive microspheres) measurements were performed at baseline and at 30 min into the 60-min occlusion period. Following reperfusion, hemodynamics and blood gases were measured every hour, and regional myocardial blood flow was determined at the end of 3 h of reperfusion. At the end of the experiment, the hearts were electrically fibrillated, removed, and prepared for IS determination and regional myocardial blood flow calculations the following day.
Figure 1.
Experimental protocol for remote preconditioning with trauma in dogs.
IS determination
IS was determined as previously described by our laboratory [10]. Briefly, at the end of the 3-h reperfusion period, the LAD was reoccluded and cannulated distal to the occlusion site. To determine the anatomic area at risk (AAR) and the nonischemic area, 5 ml of Patent Blue dye and 5 ml of saline were injected at equal pressure into the LAD and left atrium, respectively. The heart was then immediately fibrillated and removed. The LV was dissected and sliced into serial transverse sections 6–7 mm in width. The nonstained ischemic area and the blue-stained normal area were separated and incubated in a solution containing 1% 2,3,5-triphenyltetrazolium chloride (Sigma) in 0.1 mol/l phosphate buffer, pH 7.4, at 37°C for 15 min. After incubation overnight in 10% formaldehyde, the noninfarcted and infarcted tissues within the AAR were separated and determined gravimetrically. IS was expressed as a percentage of the AAR (IS/AAR).
Regional myocardial blood flow
Regional myocardial blood flow, expressed as milliliters per minute per gram of tissue (ml/min/g), was measured by the radioactive microsphere technique, as previously reported [10]. Microspheres were administered 30-min into the 60-min occlusion period and at the end of 3 h of reperfusion. Transmural blood flow was calculated as the weighted average of the subepicardium, midmyocardium, and subendocardium of each region.
Statistical analysis
All values are expressed as means ± SE (N = 8/group). Differences between groups in hemodynamics and blood gases were compared by using a two-way ANOVA. Differences between groups in tissue blood flows, AAR, and IS/AAR were compared by a one-way ANOVA followed by Tukey's post hoc test. Differences between groups were considered significant if P < 0.05. Linear regression analysis was performed to determine the correlation between transmural collateral blood flow in the ischemic area and myocardial IS (IS/AAR). Analysis of covariance, with collateral blood flow as the covariate, was used to determine whether differences in this relationship were observed among the treatment groups analyzed.
Results
Exclusion Criteria
Dogs were excluded if 1) heartworms were found after the dogs were euthanized; 2) transmural collateral blood flow was >0.20 ml/min /g, 3) heart rate was >180 beats/min at the beginning of the experiment, or 4) more than three consecutive attempts were needed to convert ventricular fibrillation with low-energy DC pulses applied directly to the heart.
Hemodynamics
There were no differences in hemodynamics (see Table 1) or blood gases (data not shown) between the control and treated dogs at any time throughout the protocol. This suggests that changes in infarct size were not the result of improved hemodynamics in the dogs subjected to the slit prior to ischemia or to the dogs treated with pharmacological antagonists to bradykinin or the EETs.
Table 1.
HEMODYNAMIC VALUES
| Heart Rate (beats/min) | CON | 30-min OCC | 3-H REP |
|---|---|---|---|
| Control | 155±5 | 152±4 | 154±4 |
| RPCT | 153±6 | 156±5 | 157±4 |
| HOE 140 | 154±6 | 157±4 | 158±5 |
| HOE + RPCT | 158±6 | 156±5 | 162±5 |
| 14,15-EEZE | 155±4 | 152±4 | 152±4 |
| 14,15-EEZE + RPCT | 152±3 | 153±4 | 152±4 |
| MS-PPOH | 155±6 | 156±5 | 154±5 |
| MS-PPOH + RPCT | 153±4 | 153±2 | 152±2 |
| Mean Arterial Pressure(mmHg) | CON | 30-min OCC | 3-H REP |
|---|---|---|---|
| Control | 112±5 | 118±3 | 113±4 |
| RPCT | 99±6 | 102±5 | 104±2 |
| HOE 140 | 97±7 | 92±6 | 98±5 |
| HOE + RPCT | 96±6 | 97±8 | 101±7 |
| 14,14-EEZE | 108±5 | 98±8 | 99±6 |
| 14,15-EEZE + RPCT | 112±8 | 104±4 | 112±4 |
| MS-PPOH | 122±5 | 110±7 | 111±3 |
| MS-PPOH + RPCT | 117±2 | 121±8 | 122±4 |
All values are the mean ± SEM.( N=8/group). There were no significant differences between groups by ANOVA followed by the Newman-Kuels posthoc test.
Abbreviations: CON = Control
OCC = Occlusion
REP = Reperfusion
Infarct Size Data
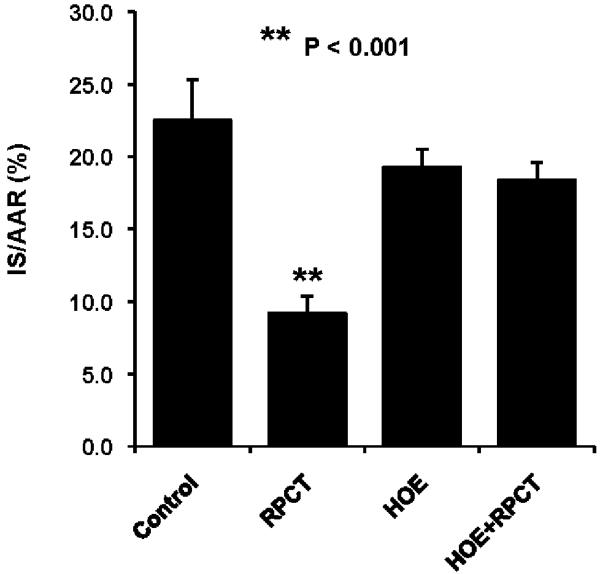
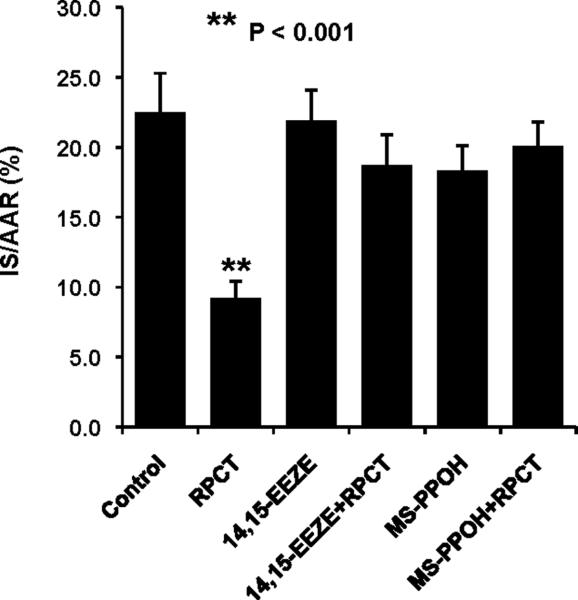
In an initial series of experiments, we decided to determine whether RPCT could produce a reduction in myocardial infarct size in the dog heart similar to what had been previously been shown in the mouse heart (7,8). We found that RPCT produced a marked reduction in IS/AAR from 22.5± 2.5% to 9.2 ± 1.2 % (P<0.001, Fig 2). Similar to what had been shown in the mouse heart (7,8 ) the bradykinin receptor (BK2R) antagonist, HOE 140, completely blocked the protective effect of RPCT (Fig 2) at a dose which had no effect by itself. Similarly, 14,15-EEZE, an EET receptor antagonist [12] and MS-PPOH, an EET synthesis inhibitor [13 ], completely abolished the effect of RPCT to reduce infarct size (Fig 3). In an earlier study from our laboratory, we showed that these same doses of 14,15-EEZE and MS-PPOH blocked the effect of ischemic preconditioning [11].
Figure 2.
Remote preconditioning with trauma (RPCT) results in a significant reduction in myocardial infarct size expressed as a percent of the area at risk (IS/AAR) in the canine heart. This effect was abolished by pretreatment with the selective bradykinin 2 receptor (BK2R) antagonist, HOE 140 (50 ug/kg, iv). All values are the mean ± SEM of 8 animals/group. **P<0.001 vs the Control group.
Figure 3.
The effect of RPCT to reduce IS/AAR is abolished by the epoxyeicosatrienoic acid (EET) receptor antagonist 14,15-EEZE and by the EET synthesis inhibitor, MSPPOH. All values are the mean ± SEM of 7/8 animals/group. **P<0.001 vs the Control group.
Regional Myocardial Blood Flow
Transmural myocardial blood flow in the nonischemic left circumflex perfusion bed and the ischemic LAD bed were measured at 30 min of occlusion and at 3 h of reperfusion . There were no differences in nonischemic transmural blood flow among groups or transmural collateral blood flow in the ischemic bed at 30 minutes of occlusion (Table 2). Most importantly, there were no differences in the ischemic-reperfused area (area at risk, AAR) during coronary occlusion, which suggested that all groups were subjected to similar degrees of ischemia (Data not shown). There were also no differences in AAR or AAR/LV among groups. Since there were no differences in AAR, coronary collateral blood flow (Table 2), and hemodynamics (Table 1), the three major determinants of IS/AAR, it appears that RPCT is exerting its cardioprotective effects through mechanisms in which bradykinin and the EETs play a supporting role. Finally, in Figure 4 we demonstrate the relationship between IS/AAR and transmural coronary collateral blood flow measured at 30 min into the ischemic period. In the four groups analyzed, there was a significant (P < 0.04 to 0.0003) inverse relationship between these two parameters, as shown by linear regression analysis. In the RPCT group there was a marked parallel shift downward (Panel B) compared with the control group (Panel A), which clearly indicates that at any given collateral blood flow one would predict a smaller IS/AAR in the RPCT group. Similar results were obtained with bradykinin as with the RPCT group (Data not shown). Interestingly, pretreatment with 14,15-EEZE (Panel C) or MS-PPOH (Panel D), the 2 EET blockers, shifted these two lines nearly back to that of the control group. These data further indicate that the changes observed in IS/AAR are occurring independent of changes in transmural coronary collateral blood flow.
Table 2.
Blood flow values (ml/min/g)
| GROUP | NONISCHEMIC REGION TRANS | ISCHEMIC REGION TRANS | ||
|---|---|---|---|---|
| 30 MIN OCC | 3 H REP | 30 MIN OCC | 3 H REP | |
| Control | 1.38 ± 0.15 | 1.46 ± 0.13 | 0.10 ± 0.02 | 1.03 ± 0.12 |
| HOE 140 | 1.62 ± 0.26 | 1.58 ± 0.16 | 0.09 ± 0.03 | 0.82 ± 0.05 |
| HOE 140 + RPCT | 1.14 ± 0.10 | 1.27 ± 0.16 | 0.10 ± 0.04 | 0.58 ± 0.10 |
| 14,15-EEZE | 1.71 ± 0.10 | 1.46 ± 0.17 | 0.10 ± 0.02 | 1.03 ± 0.18 |
| 14,15-EEZE + RPCT | 1.18 ± 0.13 | 1.67 ± 0.14 | 0.07 ± 0.02 | 0.81 ± 0.12 |
| MS-PPOH | 1.22 ± 1.13 | 1.13 ± 0.05 | 0.08 ± 0.01 | 0.69 ± 0.07 |
| MS-PPOH + RPCT | 1.33 ± 0.16 | 1.19 ± 0.09 | 0.08 ± 0.01 | 0.72 ± 0.07 |
All values are the mean ± SEM (N = −8).
Abbreviations: TRANS = Transmural
OCC = Occlusion
REP = Reperfusion
Figure 4.
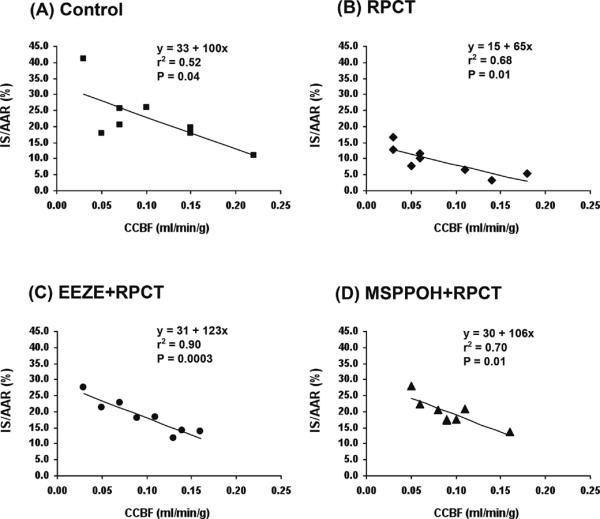
The relationship between IS/AAR and transmural coronary collateral blood flow measured at 30 min into the ischemic period. In the four groups analyzed, there was a significant (P < 0.04 to 0.0003) inverse relationship between these two parameters, as shown by linear regression analysis. In the RPCT group there was a marked parallel shift downward ( Panel B) compared with the control group (Panel A), which clearly indicates that at any given collateral blood flow one would predict a smaller IS/AAR in the RPCT group. Similar results were obtained with bradykinin as with the RPCT group [Data not shown]. Interestingly, pretreatment with 14,15-EEZE (Panel C) or MS-PPOH (Panel D), the 2 EET blockers, shifted these two lines nearly back to that of the control group.
Discussion
The results of the present study suggest that the canine heart is preconditioned by an abdominal surgical incision (RPCT) similar to the results of a previous study performed in the murine heart [8]. Furthermore, bradykinin is a central mediator of this protective effect via activation of the bradykinin 2 receptor (BK2R) since the effect of RPCT was blocked in our study by the BK2R selective antagonist HOE140. These data suggest that this same sensory neural pathway, which is very efficacious in the mouse at reducing infarct size, is also present in a large animal model such as the dog (59% reduction in IS/AAR) and may be expected to translate to humans as well. Since this effect was mimicked by capsaicin in the mouse [8] which is effective when administered topically, suggests that application of capsaicin could be safely used in humans with ischemic heart disease.
In addition to confirming the phenomenon of RPCT in a large animal model and the importance of bradykinin in mediating the cardioprotective effect of RPCT, we also have found another piece to the puzzle of RPCT, that being neurogenic activation of the CYP-450 epoxygenase pathway since the cardioprotective effect of RPCT was blocked by administration of the putative selective EET antagonist, 14,15-EEZE [10] and the EET synthesis inhibitor, MSPPOH [11]. Since the EETs have previously been shown to mediate both ischemic preconditioning (IPC) and postconditioning (POC) in the canine heart [11], the finding that the EETs are involved in RPCT in the dog suggests that similar to IPC and POC, the CYP epoxygenase pathway has a broad spectrum of cardioprotective effects in a variety of animal models with several different endpoints, most importantly a reduction in infarct size which has been observed in all species thus far tested. The EETs have also been recently shown to preserve mitochondrial function [12] in the ischemic heart, attenuate the remodeling process post myocardial infarction [13], preserve endothelial function [14] and exert potent antinflammatory effects in various organs such as the kidney [15]. This plethora of beneficial effects suggest that a more stable, orally available EET analogue or sEH inhibitor, the enzyme which metabolizes the EETs to the less biologically active DiHETEs [16] or a combination of the two may have considerable therapeutic promise in the treatment of patients with ischemic heart disease. However, more studies are needed to investigate the mechanisms and sites of actions of the EETs , particularly, in reducing infarct size and preventing remodeling following a myocardial infarction, two desirable clinical scenarios.
Limitations of this study
Although the current study suggests that similar pathways mediate RPTC in mice and dogs the previous study by Jones et al. [8] has several additional steps in the signaling pathway which were not touched on by our study. These authors demonstrated a key component of the neurogenic pathway occurred via activation and depression of PKCε and PKCδ, respectively, following activation of sensory and sympathetic nerve fibers. Finally, a role for activation of myocardial ATP-sensitive potassium channels was demonstrated for RPCT. Both glibenclamide, a nonselective KATP channel antagonist, and 5-HD, a mitochondrial selective KATP antagonist, abrogated the protection afforded by RPCT. Many of these factors have already been shown to mediate IPC, POC as well as EET-induced cardioprotection in different animal models so it is likely that similar results would be found in the dog if these signaling pathways were investigated in more detail. Nevertheless, this fascinating phenomenon deserves further study because of its potential clinical use and relative safety as compared to other invasive and noninvasive methods being touted for producing IPC and POC.
Acknowledgements
This work was supported by funds from the National Institutes Grant # HL-74314.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. The late phase of preconditioning. Circ Res. 2000:972–983. doi: 10.1161/01.res.87.11.972. 2000. [DOI] [PubMed] [Google Scholar]
- 3.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress proteins 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 4.Vinten-Johansen J, Yellon DM, Opie LH. Postconditioning: a simple clinically applicable procedure to improve revascularization in acute myocardial infarction. Circulation. 2005;112:2085–88. doi: 10.1161/CIRCULATIONAHA.105.569798. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–46. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt MR, Smerup M, Konstantinov IE, Shimisu M, Li J, Cheung M, White PA, Kristiansen SB, Sorenson K, Dzavik V, Redington AN, Kharbanda RK. Intermittant peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic preconditioning. Am J Physiol Circ Physiol. 2007;292:H1883–H90.7. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ren X, Wang Y, Jones WK. TNF-a is required for late phase ischemic preconditioning but not for remote preconditioning of trauma. J Surg Res. 2004;121:120–29. doi: 10.1016/j.jss.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120(Suppl 1):S1–S9. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nithipatikom K, Gross ER, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, Gross GJ. Inhibition of cytochrome -hydroxylase: A novel endogenous cardioprotective pathway. Circ Res. 2004;95:e65–e67. doi: 10.1161/01.RES.0000146277.62128.6f. [DOI] [PubMed] [Google Scholar]
- 10.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol. 2008;294:H2838–H44. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross GJ, Gauthier KM, Moore J, Campbell WB, Falck JR, Nithipaikom K. Evidence for a role of epoxyeicosatrienoic acids in mediating ischemic preconditioning and postconditioning in dog. Am J Physiol Heart Circ Physiol. 2009;297:H47–H52. doi: 10.1152/ajpheart.01084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol. 2009;46:867–75. doi: 10.1016/j.yjmcc.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Xu D, Li N, He Y, Timofeyev V, Lu HJ, Tsai HJ, Kim IH, Tuteja D, Mateo RKP, Singpuri A, Davis BB, Low R, Hammock BD. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. PNAS. 2006;103:18733–38. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–H31. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 15.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F03. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]