The distribution of volume doubling times of cancers diagnosed in annual rounds of CT screening is not significantly different from that previously reported in an earlier study for all cancers (P = .51) or for non–small cell cancers only (P = .69).
Abstract
Purpose:
To empirically address the distribution of the volume doubling time (VDT) of lung cancers diagnosed in repeat annual rounds of computed tomographic (CT) screening in the International Early Lung Cancer Action Program (I-ELCAP), first and foremost with respect to rates of tumor growth but also in terms of cell types.
Materials and Methods:
All CT screenings in I-ELCAP from 1993 to 2009 were performed according to HIPAA-compliant protocols approved by the institutional review boards of the collaborating institutions. All instances of first diagnosis of primary lung cancer after a negative screening result 7–18 months earlier were identified, with symptom-prompted diagnoses included. Lesion diameter was calculated by using the measured length and width of each cancer at the time when the nodule was first identified for further work-up and at the time of the most recent prior screening, 7–18 months earlier. The length and width were measured a second time for each cancer, and the geometric mean of the two calculated diameters was used to calculate the VDT. The χ2 statistic was used to compare the VDT distributions.
Results:
The median VDT for 111 cancers was 98 days (interquartile range, 108). For 56 (50%) cancers it was less than 100 days, and for three (3%) cancers it was more than 400 days. Adenocarcinoma was the most frequent cell type (50%), followed by squamous cell carcinoma (19%), small cell carcinoma (19%), and others (12%). Lung cancers manifesting as subsolid nodules had significantly longer VDTs than those manifesting as solid nodules (P < .0001).
Conclusion:
Lung cancers diagnosed in annual repeat rounds of CT screening, as manifest by the VDT and cell-type distributions, are similar to those diagnosed in the absence of screening.
© RSNA, 2012
Introduction
Concern has been expressed that lung cancers diagnosed as a result of computed tomographic (CT) screening may not have anywhere near the same growth rates as cancers diagnosed in the absence of screening, so that screening may lead to excessive diagnostic work-up and treatment of cancers that are indolent and thus not life threatening. This concern is based mainly on the growth-rate and cell-type distributions of cancers diagnosed in the baseline rounds of CT screening (1–3). But screening inherently provides a different growth-rate distribution of diagnosed cancers in the baseline round as compared with subsequent repeat rounds of screening (4–7). The cancers diagnosed in the baseline round of screening naturally are, on average, less aggressive—that is, slower growing—than those diagnosed in the absence of screening. But cancers diagnosed in repeat rounds of screenings, in combination with those diagnosed on the prompting of symptoms between screenings, should be representative of lung cancers diagnosed in the absence of screening (4,5). Research on cancers diagnosed in repeat rounds of CT screening has not previously been reported, to our knowledge. This report will empirically address the distribution of the volume doubling time (VDT) of lung cancers diagnosed in annual repeat rounds of CT screening in the International Early Lung Cancer Action Program (I-ELCAP), first and foremost with respect to rates of tumor growth but also in terms of cell types.
Materials and Methods
This report draws from the database of the I-ELCAP, in which CT screening for lung cancer was performed according to a common protocol (8). In this program, consent had been obtained from all participants according to Health Insurance Portability and Accountability Act–compliant protocols, with approval by the institutional review boards of the collaborating institutions. From the I-ELCAP database for 1993–2009, we identified all instances of first primary lung cancer diagnosed after a negative result of the prior screening examination 7–18 months earlier, regardless of whether the pursuit of the diagnosis was prompted by a positive result of the initial CT examination in a repeat round of CT screening or by symptoms prior to the next scheduled repeat screening examination. To identify all those diagnosed cases prompted by symptoms prior to the next scheduled repeat screening, all participants who did not return for their scheduled appointment after 12 months were approached, and, if they were not available, family members and the referring physician were contacted to determine whether lung cancer had been diagnosed in the interim.
The CT images for all patients diagnosed with lung cancer were re-reviewed on a radiology workstation by a thoracic radiologist (C.I.H., with more than 20 years of experience) together with a research fellow (A.F., with 10 years of experience interpreting CT scans); both investigators were blinded to the cell types and stages of the cancers. The nodule consistency was defined by the same reviewers as solid if the nodule obscured the entire lung parenchyma within it and subsolid if it did not (9). The length and width of the cancerous nodule were measured at two points in time: T1, when the nodule was first identified for further work-up, and T0, when the most recent prior scanning had been performed, 7–18 months earlier. For both T1 and T0, the length and width measurements were made on the magnified image that represented the largest cross-sectional area of the cancerous nodule. The diameter of the cancerous nodule was calculated as the average of length and width. These measurements were repeated another time (C.I.H., A.F.), with the second time at least 2 months after the first time. For eight of the patients, at least one of the screening CT studies was performed at an institution other than an I-ELCAP institution and was not available for review, so the documented findings on nodule consistency, reported length, and width of the nodule in the I-ELCAP data management system were used in the calculation of the VDT measurement.
Given the diameters at T0 and T1 of each cancerous nodule, the corresponding VDT was calculated on the premise of exponential growth (10–15) according to the following equation: VDT = [(T1 − T0) · log 2]/[log (V1/V0), where V1 and V0 are volume at T1 and T0, and volume equals π/(6 · D2), where D is diameter.
The diagnosis of lung cancer, including cell type and extent of disease, was established as part of clinical care at each participating site. Beyond this, the diagnoses were confirmed by a five-member panel of experts in pulmonary disease (16,17), who reviewed the submitted histologic slides. The clinical stage of the cancer was classified on the basis of clinical examination and imaging tests as defined by the 6th edition of the TNM staging classification manual (8,18). The presence or absence of lymph node and distant metastases (N and M status) was examined on the basis of the most recent CT study prior to diagnosis, as well as on the basis of findings at positron emission tomography (PET), if performed. The status was classified as N0M0 if the short axis (width) of all mediastinal lymph nodes at CT was less than 10 mm, no hilar lymph nodes or distant metastases were identified, and PET scanning, if performed, showed no abnormal uptake except than in the cancer itself.
Intraobserver variability of the repeated diameter measures was assessed by using the paired t test separately for time T0 (excluding nodules not visible) and for time T1 and was found not to be significantly different (P = .24 and P = .15, respectively). The geometric mean of the two calculated VDT values for each case was used for this report. If the nodule was not visible on the CT study at T0, the diameter was presumed, conservatively, to have been 2.0 mm, and we performed an additional analysis by calculating the VDT by using an initial diameter at T0 of 1.0 mm, which is still within the resolution of CT imaging. Comparison of VDT distributions (eg, for solid and subsolid nodules) was performed by using the χ2 test statistic. Both median and mean values are provided for the VDT, as for potentially skewed distributions, the median value is more representative than the mean value. P < .05 was considered to indicate a significant difference.
Results
A total of 111 cases of lung cancer were diagnosed, 110 (99%) at screening (screen diagnoses) and one (1%) prompted by symptoms (interim diagnosis). Of the 111 cases, 88 (79%) were clinical stage I. The median VDT for all 111 cancers was 98 days (mean, 136 days). The value was less than 100 days for 56 (50%) of the cancers and more than 400 days for three (3%) cancers (Table 1). For the 90 non–small cell cancers, the median VDT was 121 days (mean, 154).
Table 1.
Distribution of Lung Cancer Cases Diagnosed in Annual Rounds of CT Screening according to VDT and Nodule Consistency
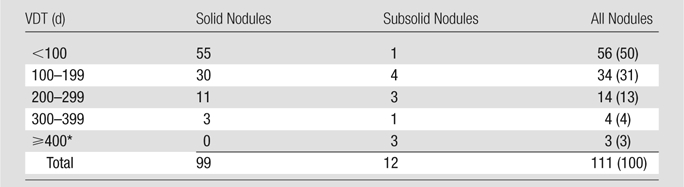
Note.—Data are numbers of nodules, with percentages in parentheses. Comparison of the two distributions showed a significant difference (P < .0001, χ2 statistic).
Nodules with a VDT of more than 400 days had VDTs of 418, 531, and 884 days.
The cancer was not visible at the prior CT examination in 28 of the 111 cases. With use of 1.0 instead of 2.0 mm as the diameter for the threshold value of visibility at T0 for these 28 cancers, the median VDT for all 111 cancers was faster, as expected, at 90 days (mean, 132 days).
Adenocarcinoma was the most frequent cell type (50%), followed by squamous cell carcinoma (19%), small cell carcinoma (19%), and cancers of other cell types (13%) (Table 2). The distribution of the VDTs according to cell type on an ordinal scale from the fastest growing to the slowest growing was as follows: small cell cancers (median, 43 days), large cell/neuroendocrine cancers (median, 82 days), squamous cell cancers (median, 88 days), adenocarcinomas manifesting as solid nodules (median, 140 days), and adenocarcinomas manifesting as subsolid nodules (median, 251 days).
Table 2.
Distribution according to Cell Type of Lung Cancers Diagnosed in Annual Rounds of CT Screening
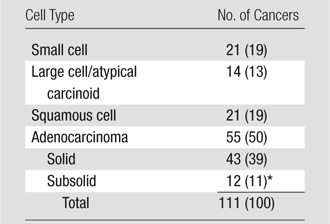
Note.—Data in parentheses are percentages.
Three of these 12 cancers were bronchioloalveolar subtypes of adenocarcinoma.
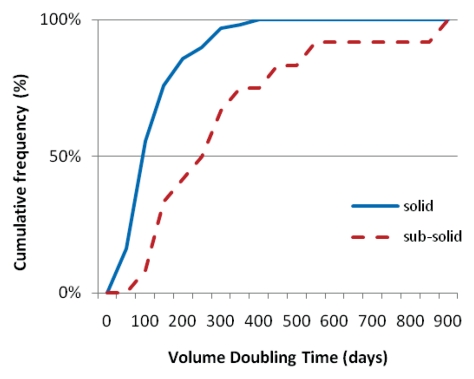
Cancers manifested most frequently as solid nodules (99 [89%] of 111) and were of all cell types. The 12 subsolid nodules were all adenocarcinomas (Table 2). All of the 28 cancers that were not identifiable even with retrospective review of the CT study manifested as solid nodules and were of all cell types. The Figure provides the cumulative distribution of the VDTs, separately for the cancers manifesting as solid and those manifesting as subsolid nodules. The distribution of the VDTs for cancers manifesting as solid nodules and that for cancers manifesting as subsolid nodules was significantly different (P < .0001).
Graph shows cumulative frequency distribution of the calculated VDT, separately for cancers manifesting as solid (n = 99) and those manifesting as subsolid (n = 12) nodules.
Discussion
VDTs of lung cancers diagnosed in “clinical practice,” in the absence of screening, have been reported to range from 20 to 360 days (19,20), while those for benign pulmonary nodules typically are less than 30 days or more than 480 days (21). From a systematic review of the literature, focusing on non–small cell carcinomas, Detterbeck and Gibson (20) recently reported a mean VDT of approximately 135 days for such cancers diagnosed in “routine medical care.” Their median value was 121 days, with values of less than 100 days for 44% of cancers and values of more than 400 days for 4% (Table 3). The distribution of VDTs of cancers diagnosed in annual rounds of CT screening in our study is not significantly different from that reported by Detterbeck and Gibson (20) for all cancers (P = .51) or for non–small cell cancers only (P = .69). The VDT distribution of non–small cell cancers in our report showed that 41% of the values were less than 100 days and 3% were more than 400 days (Table 3).
Table 3.
Distribution of Non–Small Cell Lung Cancers Diagnosed in Annual Rounds of CT Screening in I-ELCAP (n = 90) and in the Absence of Screening (n = 318) by Means of VDT
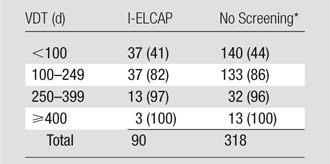
Note.—Data are numbers of cancers, with cumulative percentages in parentheses. Comparison of the two distributions showed no significant difference (P = .69, χ2 statistic).
In the study by Detterbeck and Gibson described in reference 20.
When we used 1.0 mm instead of 2.0 mm as the threshold of visibility for the cancers not identifiable on retrospective review, the median VDT for all 111 cancers in our study was even faster, at 90 days (mean, 132 days) instead of 98 days (mean, 136 days). Other reports on VDTs of CT screening–diagnosed cancers did not differentiate between cancers found in the baseline and annual repeat rounds of screening and thus are not comparable to those found in the absence of screening (15).
In lung cancers diagnosed in the absence of screening, the frequencies of small cell carcinoma and adenocarcinoma have been reported to be approximately 20% and 50%, respectively (22), nearly identical to the values of 19% and 50% we found in repeat rounds of CT screening (Table 2). This distribution of cell type is, as expected, quite different from that observed in the baseline round of screening, where the proportion of adenocarcinoma is higher (7).
Relevant to the VDTs reported here, it is of note that adenocarcinomas manifesting as subsolid nodules do not only progress by increasing in size but also by internal growth that is manifested as increasing CT attenuation and/or the development of solid components without an increase in external diameter. Therefore, our measured values for the VDTs of these cancers may well be quite conservative.
Limitations of the approach to calculating the VDT in this study include the assumption that the nodule was a sphere whose diameter was the average of the length and width measured on the CT scan. Although this assumption may not fully reflect the complexity of the nodule, particularly the complexity of subsolid nodules, this approach has been used widely in reporting VDTs of nodules in the absence of screening (10–15). For a subsolid nodule, the internal growth was not considered in calculating the VDT, and thus the actual VDT may be faster than the calculated VDT. When the nodule was not visible on the prior study even on retrospective review, the assumed nodule diameter was 2 mm, given the current visibility threshold of CT imaging. This value provides a conservative estimate of the VDT, as the use of a smaller nodule diameter results in a faster VDT, as illustrated by the sensitivity analysis using a diameter of 1 mm.
The comparability of the VDTs for lung cancers diagnosed in annual rounds of CT screening with those of cancers diagnosed in clinical practice tends to refute the two assertions that have been made regarding growth rates of the lung cancers diagnosed at CT screening. One is that the screening tends to identify cancers that are so slowly growing that they would not become life threatening—that is, that the screening results in major overdiagnosis (1,2). The second contention is that the life-threatening cancers grow so fast that they cannot be detected at screening while still curable (3). The main basis for these assertions has been the interpretation of prior chest radiography screening trial results in which no mortality benefit was demonstrated but an excess of early stage lung cancers was identified in the screening arm. These ideas have led to viewing screen-detected lung cancer as potentially representing a disease entity of its own, one that is not a precursor of advanced-stage disease (23). In the I-ELCAP experience, however, lung cancers diagnosed in annual repeat rounds of CT screening are, by direct measures of their growth rates, quite similar to those diagnosed in the absence of screening. Our results thus confirm that lung cancers detected in annual repeat rounds of CT screening have a natural course similar to those detected in the absence of screening.
Advances in Knowledge.
• The volume doubling times (VDTs) of lung cancers detected in annual rounds of CT screening were not significantly different from those detected in the absence of screening (P = .69).
• Lung cancers manifesting as subsolid nodules had significantly longer VDTs than those manifesting as solid nodules (P < .0001).
Implication for Patient Care.
• Because the distribution of VDTs of lung cancers diagnosed in annual screening rounds is significantly different for cancers manifesting as solid nodules than for those manifesting as subsolid nodules, work-up and treatment may become more tailored according to nodule consistency.
Disclosures of Potential Conflicts of Interest: C.I.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a named inventor on, and entitled to receive money and royalties from, a number of patents and patent applications relating to the evaluation of diseases of the chest, including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are nonexclusively licensed to General Electric. As an inventor of these patents, C.I.H. is entitled to a share of any compensation that CRF may receive from its commercialization of these patents. As of April 2009, C.I.H. has renounced all financial benefits related to these patents. Other relationships: none to disclose. D.F.Y. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution has grants or grants pending with AstraZeneca; is a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest, including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are nonexclusively licensed to General Electric. As an inventor of these patents, D.F.Y. is entitled to a share of any compensation, in the form of money or royalties, that CRF may receive from its commercialization of these patents. R.Y. No potential conflicts of interest to disclose. A.P.R. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest, including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are nonexclusively licensed to General Electric. As an inventor of these patents, A.P.R. is entitled to a share of any compensation, in the form of money or royalties, that CRF may receive from its commercialization of these patents. Other relationships: none to disclose. A.F. No potential conflicts of interest to disclose. D.X. No potential conflicts of interest to disclose. J.P.S. No potential conflicts of interest to disclose. D.M.L. No potential conflicts of interest to disclose. M.W.P. No potential conflicts of interest to disclose. O.S.M. No potential conflicts of interest to disclose.
Acknowledgments
We gratefully acknowledge the essential work and dedication of the I-ELCAP investigators in performing the screening research: Lijuan Zhang and David S. Mendelson at Mount Sinai School of Medicine, New York, NY; Dorothy I. McCauley and Mildred Chen at Weill Cornell Medical College, New York, NY; A. Biancardi at Cornell University, Ithaca, NY; Albert Miller at CBNS, City University of New York at Queens College, Queens, NY; Shusuke Sone and Takaomi Hanaoka at Azumi General Hospital, Nagano, Japan; Heidi Roberts and Demetris Patsios at University of Toronto, Princess Margaret Hospital, Toronto, Ontario, Canada; Javier Zulueta, Luis Montuenga, and Gorka Bastarrika at Clinica Universitaria de Navarra, Pamplona, Spain; Thomas Bauer at Christiana Care, Helen F. Graham Cancer Center, Newark, Del; Salvatore Giunta at National Cancer Institute Regina Elena, Rome, Italy; Karl Klingler at LungenZentrum Hirslanden, Zurich, Switzerland; Ralph Aye at Swedish Medical Center, Seattle, Wash; John H. M. Austin and Gregory D. N. Pearson at Columbia University Medical Center, New York, NY; Dorith Shaham at Hadassah Medical Organization, Jerusalem, Israel; Enzer Cole at St Agnes Cancer Center, Baltimore, Md; Georgeann McGuinness at New York Univeristy Medical Center, New York, NY; Cheryl Aylesworth at Holy Cross Hospital Cancer Institute, Silver Spring, MD; Matthew Rifkin at State University of New York at Stony Brook, Stony Brook, NY; Samuel Kopel at Maimonides Medical Center, Brooklyn, NY; Donald Klippenstein at Roswell Park Cancer Institute, Buffalo, NY; Peter Loud and Alan Litwin at State University of New York, Buffalo, NY; Leslie J. Kohman and Ernest M. Scalzetti at Upstate Medical Center, Syracuse, NY; Barry Sheppard at Dorothy E. Schneider Cancer Center, Mills-Peninsula Health Services, San Mateo, Calif; M. Kristin Thorsen at ProHealth Care Regional Cancer Center, Waukesha and Oconomowoc Memorial Hospitals, Oconomowoc, Wis; Arfa Khan and Rakesh Shah at North Shore-Long Island Jewish Health System, New Hyde Park, NY; Richard Thurer at Jackson Memorial Hospital, University of Miami, Miami, Fla; Davood Vafai at Eisenhower Lucy Curci Cancer Center, Rancho Mirage, Calif; Xueguo Liu at the 5th Affiliated Hospital of Sun Yat-Sen University, Zhuhai, China; Shahriyour Andaz at South Nassau Communities Hospital, Long Island, NY; Jose Cervera Deval at Fundacion Instituto Valenciano de Oncologia, Valencia, Spain; Michael V. Smith at Georgia Institute for Lung Cancer Research, Atlanta, Ga; Patrick Meyers at Nebraska Methodist Hospital, Omaha, Neb; Diana Y. Yeh at Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; Dan Luedke at St Joseph Health Center, St Charles, Mo; Michelle S. Ginsberg at Memorial Sloan-Kettering Cancer Center, New York, NY; Terence A. S. Matalon at New York Medical College, Valhalla, NY; Shari-Lynn Odzer at Mount Sinai Comprehensive Cancer Center, Miami Beach, Fla; William Mayfield at Wellstar Health System, Marietta, Ga; Fred Grannis and Arnold Rotter at City of Hope National Medical Center, Duarte, Calif; Daniel Ray at Evanston Northwestern Healthcare Medical Group, Evanston, Ill; David Olson at Aurora St Luke’s Medical Center, Milwaukee, Wis; Mary Salvatore at Staten Island University Hospital, Staten Island, NY; Peter H. Wiernik at Our Lady of Mercy Medical Center, Bronx, NY; Robert Korst at the Valley Hospital Cancer Center, Paramus, NJ; David Mullen at Greenwich Hospital, Greenwich, Conn; Louis DeCunzo at Glens Falls Hospital, Glens Falls, NY; Harvey Pass and Carmen Endress at Karmanos Cancer Institute, Detroit, Mich; Michael Kalafer at Sharp Memorial Hospital, San Diego, Calif; Michaela Straznicka at John Muir Cancer Institute, Concord, Calif; Melissa Lim at Comprehensive Cancer Center, Sequoia Hospital, Redwood City, Calif; Gary Cecchi at Alta Bates Summit Medical Center, Berkeley, Calif; Albert Koch at Bend Memorial Hospital, Bend, Ore; Paul Scheinberg at St Joseph’s Hospital, Atlanta, Ga; and Edson Cheung at Baylor University Medical Center, Dallas, Tex.
The I-ELCAP pooled database has been supported in part by National Institutes of Health grants R01-CA-63393 and R01-CA-78905; Department of Energy grant DE-FG02-96SF21260; the City of New York, Department of Health and Mental Hygiene; New York State Office of Science, Technology and Academic Research (NYSTAR); American Cancer Society; Israel Cancer Association; the Starr Foundation; the New York Community Trust; the Rogers Family Fund; the Foundation for Lung Cancer: Early Detection, Prevention, and Treatment (primary source was an unrestricted gift in 2000–2003 from the Vector Group, the parent company of Liggett Tobacco); Dorothy R. Cohen Foundation; Research Foundation of Clinic Hirslanden; Yad-Hanadiv Foundation; Jacob and Malka Goldfarb Charitable Foundation; Auen/Berger Foundation; Princess Margaret Foundation; Berger Foundation; Mills Peninsula Hospital Foundation, Tenet Healthcare Foundation; Ernest E. Stempel Foundation; Academy for Medical Development and Collaboration; Columbia University Medical Center, Empire Blue Cross and Blue Shield; Eastman-Kodak; GE Healthcare; Weill Cornell Medical College; Cornell University; New York Presbyterian Hospital; Clinic Hirslanden; Swedish Hospital; Christiana Care Helen F. Graham Cancer Center; Holy Cross Hospital; Eisenhower Hospital; Jackson Memorial Hospital Health System; and Evanston Northwestern Healthcare.
Received February 10, 2011; revision requested March 30; revision received December 6; accepted December 13; final version accepted January 13, 2012.
Current address: Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Quebec, Canada.
Funding: This research was supported by the National Institutes of Health (grants R01-CA-63393 and R01-CA-78905).
Abbreviations:
- I-ELCAP
- International Early Lung Cancer Action Program
- VDT
- volume doubling time
References
- 1.Black WC. Computed tomography screening for lung cancer: review of screening principles and update on current status. Cancer 2007;110(11):2370–2384 [DOI] [PubMed] [Google Scholar]
- 2.Bach PB. Overdiagnosis in lung cancer: different perspectives, definitions, implications. Thorax 2008;63(4):298–300 [DOI] [PubMed] [Google Scholar]
- 3.Bach PB, Silvestri GA, Hanger M, Jett JR; American College of Chest Physicians. Screening for lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132(3 Suppl):69S–77S [DOI] [PubMed] [Google Scholar]
- 4.Day NE, Walter SD, Collette B. Statistical models of disease natural history: their use in the evaluation of screening programmes. In: Prorok PC, Miller AB, eds. Screening for cancer. I. General on evaluation of screening for cancer and screening for lung, bladder, and oral cancer. UICC Technical Report Series No 78. Geneva, Switzerland: Union for International Cancer Control, 1984; 55–70 [Google Scholar]
- 5.Morrison A. Screening in chronic disease. New York, NY: Oxford University Press, 1992 [Google Scholar]
- 6.Duffy SW, Tabar L, Fagerberg G, et al. Breast screening, prognostic factors and survival: results from the Swedish two county study. Br J Cancer 1991;64(6):1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter D, Vazquez M, Flieder DB, et al. Comparison of pathologic findings of baseline and annual repeat cancers diagnosed on CT screening. Lung Cancer 2007;56(2):193–199 [DOI] [PubMed] [Google Scholar]
- 8.International Early Lung Cancer Action Program : enrollment and screening protocol. http://www.ielcap.org/professionals/docs/ielcap.pdf. Published July 1, 2011. Accessed February 21, 2012
- 9.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178(5):1053–1057 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M. A biomathematical approach to clinical tumor growth. Cancer 1961;14:1272–1294 [DOI] [PubMed] [Google Scholar]
- 11.Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217(1):251–256 [DOI] [PubMed] [Google Scholar]
- 12.Kostis WJ, Reeves AP, Yankelevitz DF, Henschke CI. Three-dimensional segmentation and growth-rate estimation of small pulmonary nodules in helical CT images. IEEE Trans Med Imaging 2003;22(10):1259–1274 [DOI] [PubMed] [Google Scholar]
- 13.Kostis WJ, Yankelevitz DF, Reeves AP, Fluture SC, Henschke CI. Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology 2004;231(2):446–452 [DOI] [PubMed] [Google Scholar]
- 14.Reeves AP, Chan AB, Yankelevitz DF, Henschke CI, Kressler B, Kostis WJ. On measuring the change in size of pulmonary nodules. IEEE Trans Med Imaging 2006;25(4):435–450 [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73(876):1252–1259 [DOI] [PubMed] [Google Scholar]
- 16.Vazquez M, Flieder D, Travis W, et al. Early lung cancer action project pathology protocol. Lung Cancer 2003;39(2):231–232 [DOI] [PubMed] [Google Scholar]
- 17.Vazquez M, Flieder D, Travis W, et al. International Early Lung Cancer Action Program: pathology protocol. http://www.ielcap.org/professionals/docs/pathology_protocol.pdf. Published March 1, 2011. Accessed February 21, 2012 [DOI] [PubMed]
- 18.Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th ed. New York, NY: Wiley-Liss, 2002 [Google Scholar]
- 19.Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD; American College of Chest Physicians. The solitary pulmonary nodule. Chest 2003;123(1 Suppl):89S–96S [DOI] [PubMed] [Google Scholar]
- 20.Detterbeck FC, Gibson CJ. Turning gray: the natural history of lung cancer over time. J Thorac Oncol 2008;3(7):781–792 [DOI] [PubMed] [Google Scholar]
- 21.Gould MK, Fletcher J, Iannettoni MD, et al. ; American College of Chest Physicians. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132(3 Suppl):108S–130S [DOI] [PubMed] [Google Scholar]
- 22.Ginsberg MS, Grewal RK, Heelan RT. Lung cancer. Radiol Clin North Am 2007;45(1):21–43 [DOI] [PubMed] [Google Scholar]
- 23.Bach PB. Is our natural-history model of lung cancer wrong? Lancet Oncol 2008;9(7):693–697 [DOI] [PubMed] [Google Scholar]