Abstract
Aorto-oesophageal fistula (AEF) is a rare but life-threatening disease with an underlying infective aetiology that can cause serious complications. This study investigated the clinical outcomes of patients with AEF who received in situ cryopreserved aortic allograft replacement. From August 2000 to February 2011, 11 patients with AEF received aortic allografts; 5 for primary AEF caused by ruptured aortic aneurysm and 6 for secondary AEF that comprised 4 cases following thoracic endovascular aortic repair (TEVAR) and 2 after open graft replacement of the thoracic aorta. As for results, 2 cases of primary AEF received TEVAR and then allograft replacement, one for graft infection and the other for bleeding. Three primary AEF cases received allografts directly. Six secondary AEF cases received staged (5) or simultaneous (1) oesophagectomy and allograft replacement. There were 3 in-hospital deaths (27%), 2 because of bleeding and one because of multisystem organ failure. Four patients completed oesophageal reconstruction. There were 2 late deaths, one due to aspiration pneumonia and one of unknown cause. In conclusion, surgical results for repair of AEF using cryopreserved aortic allograft were satisfactory considering preoperative critical condition, and this type of allograft appears to be an ideal graft of choice.
Keywords: Cryopreserved aortic allograft, Aorto-oesophageal fistula, Endovascular stent
INTRODUCTION
Aorto-oesophageal fistula (AEF) is a rare but catastrophic disease with an extremely poor prognosis. Well-known causes of AEF are foreign bodies in the oesophagus, trauma, penetrating thoracic aortic aneurysm, invasive oesophagus neoplasm, and secondary causes arising from complications of oesophageal or thoracic aortic surgeries. The purpose of this report was to describe the comprehensive experience of 11 AEF cases undergoing in situ cryopreserved aortic allograft replacement of the thoracic aorta and to evaluate the preliminary efficacy of using cryopreserved aortic allografts for AEF treatment.
PATIENTS AND METHODS
From August 2000 to February 2011, 11 patients received in situ cryopreserved aortic allograft replacement for treatment of AEF (either primary or secondary). All of the aortic allografts were provided by the University of Tokyo Tissue Bank (UTTB). There were 9 male and 2 female patients from 3 hospitals, and average age at allograft surgery was 65 years. Diagnosis of AEF was made either by oesophageal endoscopy or computed tomography (CT) of the chest or by a combination of the two procedures. A series of surgeries including aortic repair, omentopexy and oesophagectomy were performed with subsequent reconstruction by teams from the cardiovascular surgery unit and the gastroenterological surgery unit by multiple-staged operations. Patients who were discharged from the hospitals were followed up at the outpatient clinic routinely, except for one patient who was lost to regular follow-up.
The data on each patient's surgical information and the perioperative clinical course was obtained by a chart review and follow-up information on recipient's general status was obtained from the referring hospital.
RESULTS
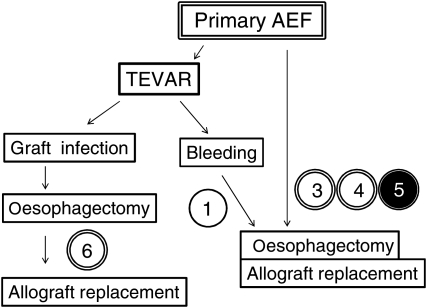
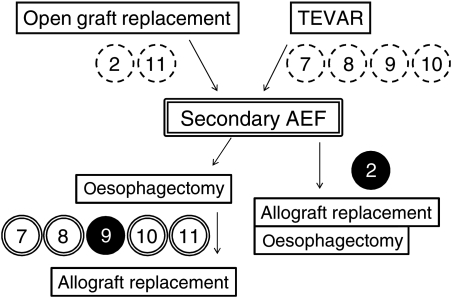
A total of 11 patients underwent in situ aortic allograft replacement for treatment of AEF. Primary AEF that was due to ruptured thoracic aortic aneurysm was diagnosed in five patients and six patients had secondary AEF. Secondary AEF occurred in two patients after open graft replacement of the thoracic aorta and in four patients after thoracic endovascular aortic repair (TEVAR). Summary of clinical courses of primary and secondary AEFs are described in Figs 1 and 2. For primary AEF cases, two cases received TEVAR, one of whom got graft infection and required a staged operation of oesophagectomy and allograft replacement, and the other presented type 1 endoleak which could not be controlled with additional stent insertion and oesophageal bleeding, and therefore received a subsequent allograft replacement and oesophagectomy. Three cases of primary AEF directly underwent oesophagectomy and allograft replacement. For secondary AEF cases, five patients underwent staged operations of oesophagectomy and allograft replacement, and one patient underwent simultaneous oesophagectomy and allograft replacement. In the short term, there were 3 (27%) in-hospital deaths including 2 patients (18%) who died within 30 days. The causes of death were fatal bleeding in two patients and multisystem organ failure (MOF) in one patient. Eight patients received concomitant omentopexy at the time of allograft replacement and seven patients survived the perioperative period. Four patients completed presternal oesophageal reconstruction within few months after the allograft replacement. There were two late deaths, one due to aspiration pneumonia and one of unknown cause. A summary of recipients' profiles and outcomes are described in Table 1. Cases are presented in chronological order according to the date of surgery. The detailed clinical course of each patient is described below as well as in Table 2. The following are additional information on cases 1, 2, 4, 5, 7 and 9–11.
Figure 1:
Clinical summary of primary AEF cases (n = 5). Double open circles represent patients who survived the perioperative period receiving omentopexy and double closed circles represent in-hospital death cases after receiving omentopexy. The single open circle represents patients who survived the perioperative period without omentopexy. The number in the circle refers to the case number.
Figure 2:
Clinical summary of secondary AEF cases (n = 6). Double open circles represent patients who survived from the perioperative period receiving omentopexy, and single closed circle presents in-hospital death cases without omentopexy. The number in the circle refers to the case number.
Table 1:
Patients' profiles and outcomes
| Number of cases | 11 |
| Female gender | 2 (18%) |
| Age at operation (year, mean ± SD) | 65 ± 13 |
| Follow-up period (days) | 9–2477 (mean 597) |
| Initial diagnosis | |
| Primary AEF | 5 |
| Secondary AEF (cases after TEVAR) | 6 (4) |
| Allograft indication | |
| Primary AEF | 3 |
| Secondary AEF | 6 |
| Uncontrolled bleeding after TEVAR | 1 |
| Stent-graft infection | 1 |
| Oesophageal management | |
| Reconstruction completed | 4 |
| Waiting for reconstruction | 2 |
| Reconstruction not achieved | 5 |
| Omentopexy | 8 (73%) |
| 30-day mortality | 2 (18%) |
| In-hospital mortality | 3 (27%) |
| Overall cumulative mortality | 5 (45%) |
| Causes of death | |
| MOF | 1 |
| Bleeding | 2 |
| Aspiration pneumonia | 1 |
| Unknown reason | 1 |
TEVAR: thoracic endovascular aortic repair.
Table 2:
Detailed description of each case
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 59 | 53 | 70 | 67 | 55 | 42 | 71 | 83 | 78 | 52 | 80 |
| Gender | Male | Male | Male | Female | Female | Male | Male | Male | Male | Male | Male |
| Primary/secondary AEF | Primary | Secondary | Primary | Primary | Primary | Primary | Secondary | Secondary | Secondary | Secondary | secondary |
| Previous procedures to thoracic aorta | TEVAR | Open graft replacement | No | No | No | TEVAR | TEVAR | TEVAR | TEVAR | TEVAR | Open graft replacement |
| Allograft indication | Uncontrolled bleeding | AEF by p-ANEU at graft anastomosis | Ruptured ANEU | Ruptured ANEU | Ruptured ANEU | Stent-graft infection | AEF at stent-graft, graft infection | AEF at stent-graft, graft infection | AEF at stent-graft, graft infection | AEF at stent-graft, graft infection | AEF by p-ANEU at graft anastomosis |
| Interval previous procedures/allograft Sx (day) | 1 | 118 | 153 | 77 | 3024 | 35 | 4 | 7 | |||
| Prior/concomitant oesophagectomy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Omental flap | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Oesophageal reconstruction | No | No | No | Yes | No | Yes | Yes | Yes | No | To be completed | To be completed |
| Infectious agent | Not detected | Serratia marcescens | MRSA | Not known | MRSA, Candida | Not identified | Not identified | Strp. anginosus | MRSA, Burkholderia cepacia | Strp. intermedius | MRSA |
| Postoperative complications | Cerebral infarction | Mediastinitis | Disuse syndrome | MRSA sepsis | |||||||
| In-hospital mortality | No | Yes | No | No | Yes | No | No | No | Yes | No | No |
| Overall outcome | Dead | Dead | Dead | Alive | Dead | Alive | Alive | Alive | Dead | Alive | Alive |
| Follow-up (day) | 934 | 19 | 799 | 2477 | 9 | 1615 | 326 | 257 | 38 | 56 | 40 |
| Cause of death | Aspiration pneumonea | Bleeding | Not known | Bleeding | MOF |
AEF: Aorto-oesophageal fistula; ANEU: aneurysm; p-ANEU: pseudoaneurysm; TEVAR: thoracic endovascular aortic repair; Sx: Surgery; MRSA: Methicilline-resistant Staphylococcus aureus; MOF: Multisystem organ failure.
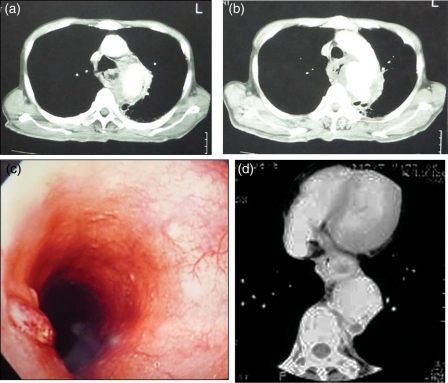
Patient 1 was a 60-year old man, who presented with haematemesis, was diagnosed with traumatic aortic pseudoaneurysm by CT of the chest (Fig. 3a and b) and underwent TEVAR. During the procedure, the oesophagus was injured and the patient experienced a massive haematemesis. Additional stent grafting was not enough to stop the oesophageal bleeding and in situ allograft replacement was performed the next day. He had experienced cerebral infarction postoperatively, and had residual left haemiplegia. He died 2.5 years after the surgery due to aspiration pneumonia.
Figure 3:
Preoperative diagnostic images. (a,b) Preoperative chest computed tomography with enhancement (case 1), showing leakage of contrast material to the mediastinum. (c) Mucosal erosion with bleeding by endoscopy (case 2). (d) An oedematous oesophageal wall next to an enlarged thoracic aortic aneurysm with abnormal air in the mediastinum (case 4).
Patient 2 was a 53-year old man who underwent open graft replacement of the distal aortic arch for an aortic aneurysm, which was complicated by graft infection shortly after the surgery. Initially, the infection was stabilized with antibiotics, however, he returned to the hospital due to a massive haematemesis 4 months later. Endoscopic examination of the oesophagus revealed a mucosal defect with active bleeding at the mid-oesophagus level (Fig. 3c), and the chest CT revealed a pseudoaneurysm at the proximal point of anastomosis of the graft. He underwent an allograft replacement and oesophagectomy simultaneously. He presented with sudden bleeding from the chest drain 11 days after the surgery and died of bleeding from the homograft anastomosis 19 days after the operation.
Patient 4 was a 67-year old woman with back pain and tarry stool who was transferred to our hospital for surgical treatment of primary AEF, which was diagnosed by oesophageal endoscopy and chest CT (Fig. 3d) at the previous hospital. She underwent allograft replacement and oesophagectomy. Postoperative course was uneventful, and presternal oesophagus reconstruction was completed 3 months later (Fig. 4).
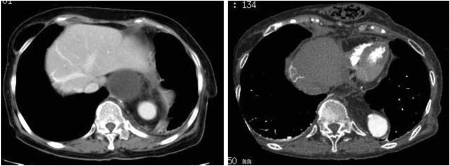
Figure 4:
Follow-up CT images from case 4. Image on the left was taken 1 week after the surgery. The omental flap can be seen clearly around the allograft. Image on the right was taken 6.5 years after the surgery, and reconstructed digestive system can be seen in presternal position.
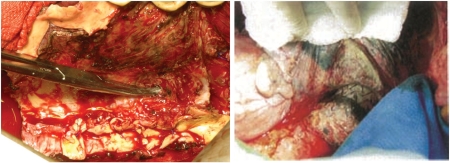
Patient 5 was a 55-year old woman with longstanding haematemesis and back pain who was referred to a nearby hospital where she was diagnosed with DeBakey type IIIb aortic dissection and AEF. She presented with haemodynamic shock, was immediately intubated and transferred to our hospital for surgery. She underwent simultaneous allograft replacement and oesophagectomy (Fig. 5, on the right). She had methicillin-resistant Staphylococcus aureus (MRSA) septisemia on admission, which was disseminated to the mediastinum, pleural cavities and the abdominal cavity. She died of graft rupture causing massive bleeding due to an uncontrolled pre-existing MRSA infection.
Figure 5:
Intraoperative views. Image on the left shows the whole view of the oesophagus after removal of the aorta (case 5). The fistula can be seen at the end of the forceps. The image on the right shows a stent graft seen through the native aortic wall next to the blue towel (case 7).
Patient 7 was a 70-year old man with previous TEVAR for a ruptured aortic aneurysm who came back to the hospital because of haematemesis. He was diagnosed with secondary AEF, stent-graft infection and mediastinitis with abscess formation in the mediastinum. He underwent staged surgery with oesophagectomy and allograft replacement, and the intraoperative findings showed complete necrosis of the aortic wall by infection and exposure of Gore®TAG® (Fig. 5, on the left). The postoperative course was stable.
Patient 9, a 78-year old man, underwent TEVAR for primary AEF (ruptured thoracic aortic aneurysm), and came back to the hospital for secondary AEF with complete exposure of the stent graft to the inner surface of oesophagus. He was diagnosed with secondary AEF with malnutrition and underwent staged surgery of oesophagectomy and allograft replacement. During the surgery, he had cardiac arrest and was resuscitated by direct cardiac massage, and the surgery was completed. However, he developed MOF, and died 38 days after the surgery.
Patient 10 was a 52-year old man who had undergone a total aortic arch replacement for acute type A dissection and endovascular stent insertion for dilation of the residual aortic dissection of the descending aorta 2 years earlier. He started to experience haematemesis 4 months prior to hospitalization, and was diagnosed with secondary AEF close to the distal end of stent graft and mediastinitis by chest CT and endoscopy. Emergency additional stent grafting, oesophagectomy and irrigation of the mediastinum were performed, and he underwent in situ allograft replacement later. He is now awaiting oesophageal reconstruction.
Patient 11, an 80-year old man, underwent graft replacement of the descending thoracic aorta for a ruptured aortic aneurysm 4 months prior to the admission. He came back to the hospital because of haematemesis and mediastinitis. Additional stent graft was inserted, and an oesophagectomy was performed followed by in situ allograft replacement.
DISCUSSION
AEF is a catastrophic disease with a poor prognosis and is a rare cause of massive bleeding from the upper gastrointestinal tract. The most frequent causes of AEF in literature have been described as thoracic aortic aneurysms, foreign bodies and oesophageal malignancies.
The incidence of secondary AEF after surgery is relatively low (4.8%). [1] However, recent reports have revealed that secondary AEF or aortobronchial fistulas, especially those following endovascular stent-grafting manoeuvre, are becoming a significant complication with very poor outcome (30-day mortality 33–100%) [2–4].
The standard treatment of choice for AEF is graft replacement of the torn aorta and oesophagectomy with subsequent reconstruction, which are usually completed in multiple-stage surgeries. Options for graft are Dacron graft [5, 6] or cryopreserved aortic allograft [7, 8]. To prevent postoperative infection (e.g. refractory mediastinitis), rifampicin-soaked grafts [9] and omental flaps to cover the replaced grafts have been described as effective surgical options. Clinical studies [10] and studies in animal models [11] have shown that cryopreserved allografts are resistant against infection; therefore, better clinical results are expected compared with regular fabric grafts. Use of omental tissue is another option for successful surgical treatment of AEF possibly preventing infective complications because of its high resistance against infection due to high vascularity and neovascularization potential [12, 13].
The TEVAR has emerged as a simple and less invasive technique than open surgical repair to treat aortic disease in very high-risk cases, and was initially reported to be useful in the salvage of critical AEF cases. [14, 15] However, follow-up results after TEVAR have shown a certain incidence of secondary AEF with adverse outcome. Stent graft sometimes causes graft infection due to the contact between the aorta and digestive tract or trauma to the neighbouring tissue by mechanical stress resulting in secondary AEF. The cause is the same as in graft replacement of the aorta using fabric grafts. A better alternative strategy to treat both primary and secondary AEF is to use aortic allografts.
Here, we reported 11 AEF cases undergoing series of surgeries with cryopreserved aortic allografts. The overall observed efficacy and outcome of allograft cases have not changed in recent years. Rather, treatment strategy for AEF and the role of the allografts seems to be changing. It can also be concluded that allograft may be able to improve the outcome of secondary AEF in combination with TEVAR. In the early days of the procedure, allograft was indicated in primary AEF for direct open allograft replacement, and this was the time when the allograft was the primary option for infective aortic disease. The technique of TEVAR emerged in early 2000 as the salvage treatment of AEF and produced ideal early clinical results. In recent years, late clinical results after TEVAR have become available and we are now aware that stent graft failure due to secondary AEF is a serious complication with very high mortality. The aortic allograft came back as an alternative graft to TEVAR in the treatment of secondary AEF. The success rate of aortic allograft for secondary AEF in our practice is satisfactory, and this may be due to the allograft's resistance against infection, as well as the use of omentopexy. The best option for treatment for such a high-risk cohort may change from time to time. However, it is important to have a good strategy for treatment of this critical condition, and to have back-up options available, whether the procedures are old or new.
In conclusion, treatment of AEF (whether primary or secondary) is still a clinical challenge, and meticulous planning of the treatment strategy is required to obtain the optimal outcome. It is noteworthy that in such a high-risk cohort the combination of initial salvage by TEVAR with consideration of allograft use for late complication after TEVAR was effective in our experience.
Conflict of interest: none declared.
Appendix. Conference discussion
Dr C. Mestres (Barcelona, Spain): Dr. Saito and colleagues present a series of 11 patients with aorto-oesophageal fistula. This is a catastrophic entity that needs aggressive treatment and is associated with high mortality as these patients are usually in extremely poor condition. Allograft thoracic aortic replacement with combined oesophagectomy was performed and a number of concomitant procedures, too. This is, of course, a challenging surgery, and not everybody is prepared to deal with this type of patient. Other than their expected 27% in-hospital mortality, staged oesophageal reconstruction was performed and 2 survivors are awaiting gastrointestinal repair.
Interestingly to me, six patients had infection after TEVAR and two after previous open-graft repair. It is not surprising that stent grafts are becoming infected, as infection may appear at any time after implantation; this has been a well-known fact in aortic surgery for decades. What happened is that the medical community erroneously thought that TEVAR would never become infected. Data shown herein are very clear in this regard. In this series, omentoplasty was performed in 73% of the cases, and two patients died from uncontrollable haemorrhage as the allograft likely became infected. In four cases, MRSA was isolated on culture, and this pathogen clearly suggests a hospital-related contamination.
This series confirms that aortic infection with aorto-oesophageal fistula is a highly serious disease. On the other hand, survivors had excellent microbiological control during the follow-up, confirming previous impressions about allografts.
I have several questions for you. Although you mentioned, and we could see on one of the slides with a colonic subcutaneous tunnel, which type of oesophageal reconstruction was performed and why was omental flap not always used in these patients? Did you always use the subcutaneous route to avoid getting into the chest once again for oesophageal reconstruction?
Dr Saito: I'm sorry. Can you -
Dr Mestres: Which type of oesophageal reconstruction did you perform in all cases? How did you reconstruct the oesophagus, the gastrointestinal tract?
Dr Saito: As I have shown in the slides, most of the cases received the interposition with the colon anterior to the sternum. Initially we create an oesophagostomy and gastrostomy, and then we are not going into the chest but we are going to reconstruct the upper digestive tract just at a superficial level.
Dr Mestres: But you should indicate that in your manuscript.
Dr Saito: I understand.
Dr Mestres: Because you only refer to oesophageal reconstruction, which is a very bad term. Second, two patients with primary aorto-oesophageal fistula had MRSA infection. Did they have a prior medical procedure other than surgery? How do you suppose that these people got MRSA infection?
Dr Saito: One of the cases which we lost because of MRSA infection was Case 5. She was already infected at the time of admission to our hospital, and she presented with septicaemia with the MRSA. So there was no way to save this patient from infection even though we used the homograft along with the omental flap.
Dr Mestres: And, finally, at the time of explanting the stent graft, did you notice any problem or were these stent grafts very easily removed?
Dr Saito: To be honest, the tissue itself is really weak, so apparently it is not too hard to take the stent graft out.
Dr Mestres: It is very easy to take them out because they just float into the aorta. So I think, finally, that this experience has to be taken into account as a warning of the possibility of stent graft infection after TEVAR which is going to become a negative part of our surgical future.
Dr A. Rajaii-Khorasani (Mashhad, Iran): I have a question regarding the aetiology. When we had this complication following open operation, it was usually due to a suture that was passing from the anastomosis to the neighbouring oesophagus. Obviously we do have primary aorto-oesophageal fistula, and that is usually an aneurysm that leaks or something happened. So my question is, did you look at the stent grafts and see if they had endoleak, because in the endoleak there is a continuing pressure, and that may be part of the aetiology.
Dr Saito: I am sorry to say that I did not specifically look into that point, so I may have to go back to the cases and check in detail regarding this aspect.
References
- 1.Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91:279–87. doi: 10.1016/0002-9343(91)90129-l. doi:10.1016/0002-9343(91)90129-L. [DOI] [PubMed] [Google Scholar]
- 2.Isasti G, Gomez-Doblas JJ, Olalla E. Aortoesophageal fistula: an uncommon complication after stent-graft repair of an aortic thoracic aneurysm. Interact CardioVasc Thorac Surg. 2009;9:683–4. doi: 10.1510/icvts.2009.207910. doi:10.1510/icvts.2009.207910. [DOI] [PubMed] [Google Scholar]
- 3.Chiesa R, Melissano G, Marone EM, Marrocco-Trischitta MM, Kahlberg A. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: a national survey. Eur J Vasc Endovasc Surg. 2010;39:273–9. doi: 10.1016/j.ejvs.2009.12.007. doi:10.1016/j.ejvs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Eggebrecht H, Mehta H, Dechene A, Tsagakis K, Kuhl H, Huptas S, et al. Aortoesophageal fistula after thoracic aortic stent-graft placement: a rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv. 2009;2:570–6. doi: 10.1016/j.jcin.2009.03.010. doi:10.1016/j.jcin.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Reardon MJ, Brewer RJ, LeMaire SA, Baldwin JC, Safi HJ. Surgical management of primary aortoesophageal fistula secondary to thoracic aneurysm. Ann Thorac Surg. 2000;69:967–70. doi: 10.1016/s0003-4975(99)01087-5. doi:10.1016/S0003-4975(99)01087-5. [DOI] [PubMed] [Google Scholar]
- 6.Coselli JS, Crawford ES. Primary aortoesophageal fistula from aortic aneurysm: successful surgical treatment by use of omental pedicle graft. J Vasc Surg. 1990;12:269–77. [PubMed] [Google Scholar]
- 7.Vogt PR, Pfammatter T, Schlumpf R, Genoni M, Kunzli A, Candinas D, et al. In situ repair of aortobronchial, aortoesophageal, and aortoenteric fistulae with cryopreserved aortic homografts. J Vasc Surg. 1997;26:11–7. doi: 10.1016/s0741-5214(97)70140-x. doi:10.1016/S0741-5214(97)70140-X. [DOI] [PubMed] [Google Scholar]
- 8.Pirard L, Creemers E, Van Damme H, Laurent S, Honore P, Limet R. In situ aortic allograft insertion to repair a primary aortoesophageal fistula due to thoracic aortic aneurysm. J Vasc Surg. 2005;42:1213–7. doi: 10.1016/j.jvs.2005.07.040. doi:10.1016/j.jvs.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Nishino T, Peng YF, Saga T. Successful one-stage operation of aortoesophageal fistula from thoracic aneurysm using a rifampicin-soaked synthetic graft. Interact CardioVasc Thorac Surg. 2008;7:322–4. doi: 10.1510/icvts.2007.164699. doi:10.1510/icvts.2007.164699. [DOI] [PubMed] [Google Scholar]
- 10.Bisdas T, Bredt M, Pichlmaier M, Aper T, Wilhelmi M, Bisdas S, et al. Eight-year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010;52:323–30. doi: 10.1016/j.jvs.2010.02.277. doi:10.1016/j.jvs.2010.02.277. [DOI] [PubMed] [Google Scholar]
- 11.Saito A, Motomura N, Kakimi K, Narui K, Noguchi N, Sasatsu M, et al. Vascular allografts are resistant to methicillin-resistant Staphylococcus aureus through indoleamine 2,3-dioxygenase in a murine model. J Thorac Cardiovasc Surg. 2008;136:159–67. doi: 10.1016/j.jtcvs.2008.01.006. doi:10.1016/j.jtcvs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Kitayama J, Morota T, Kaisaki S, Nakayama H, Ishigami H, Yamashita H, et al. Complete coverage of in situ aortograft by total omental pedicle flap as the most reliable treatment of aortoesophageal fistula. Am J Surg. 2006;192:130–4. doi: 10.1016/j.amjsurg.2005.09.011. doi:10.1016/j.amjsurg.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto S, Saiki Y, Oda K, Nitta Y, Akasaka JE, Miyazak S, et al. Successful management of esophagoparaprosthetic fistula after aortic surgery. Ann Thorac Surg. 2008;85:1449–51. doi: 10.1016/j.athoracsur.2007.10.068. doi:10.1016/j.athoracsur.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 14.Kato N, Tadanori H, Tanaka K, Yasuda F, Iwata M, Kawarada Y, et al. Aortoesophageal fistula-relief of massive hematemesis with an endovascular stent-graft. Eur J Radiol. 2000;34:63–6. doi: 10.1016/s0720-048x(99)00107-2. doi:10.1016/S0720-048X(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 15.Jonker FH, Heijmen R, Trimarchi S, Verhagen HJ, Moll FL, Muhs BE. Acute management of aortobronchial and aortoesophageal fistulas using thoracic endovascular aortic repair. J Vasc Surg. 2009;50:999–1004. doi: 10.1016/j.jvs.2009.04.043. doi:10.1016/j.jvs.2009.04.043. [DOI] [PubMed] [Google Scholar]