Abstract
Few data address the role of human mesenchymal stromal cells (MSCs) in the management of chronic ischaemic heart failure. We assessed their effect in immune-deficient animals. MSCs were cultured from bone marrow of human volunteers. Non-obese diabetes severe combined immunodeficiency (NOD/SCID) gamma null mice were randomly assigned to intramyocardial injection of human MSCs or phosphate-buffered saline 4 weeks after induction of acute myocardial infarction (MI). Echocardiography was performed 4 weeks after MI and 1 and 4 weeks after injection. Donor cell chimerism was assessed by DNA for human Alu sequences 2 and 4 weeks after injection. Histological assessment and quantification of neovascularization were determined 4 weeks after treatment. Donor MSCs at frequencies of 0.006 and 0.001% were present 2 and 4 weeks after cell injection, respectively. The infarcted ventricular wall was significantly thicker in the cohort receiving MSCs compared with control mice. There was no difference in fractional shortening, left ventricular dimensions or scar area between the groups. Small vessel density was also similar between the groups. Human MSCs increased the thickness of the infarcted ventricular wall without improving cardiac function in this chronic ischaemic heart failure model. Further studies are required to assess the benefit of MSCs in this setting.
Keywords: Cell transplantation, Myocardial infarction, Myocardial remodelling, Stem cells
INTRODUCTION
Much attention has focused on mesenchymal stromal cells (MSCs) in the management of the post-acute myocardial infarction period. We previously showed that the benefit of MSCs in this setting may be related to a switch of monocyte/macrophages infiltrating the ischaemic myocardium to an anti-inflammatory phenotype [1]. While most clinical protocols involve the immediate acute post-myocardial infarction (MI) period, several are underway to investigate the role of MSCs in chronic ischaemic heart failure (ClinicalTrials.gov ID: NCT01076920; NCT00260338) [2].
In this study, we test the hypothesis that MSCs would not improve cardiac function in a chronic ischaemia model in which inflammation is a minor component. We employed a xenogeneic immune-deficient mouse model to test the effect of human MSCs introduced by intramyocardial injection into chronic ischaemic myocardium.
MATERIALS AND METHODS
Cell preparation
Bone marrow aspirates were collected from healthy adult volunteers after informed consent according to an approved local institutional review board protocol. Ficol gradient was performed and mononuclear cells plated using MSC medium, consisting of Dulbecco's modified Eagle's medium-low glucose (DMEM-LG) supplemented with 10% foetal bovine serum (FBS) and 1% antibiotic–antimycotic solution and re-plated (passaged). Cells were passaged 3–4 times before injection and immunophenotypic analysis was conducted to determine conformity with minimal established criteria for MSCs [3]. To reflect clinical protocols as closely as possible, the cells were not labelled with fluorophores or by other means [4].
Mesenchymal stromal cell characterization
Immunophenotyping
Cells were stained using the following conjugated anti-human antibodies: CD90-fluorescein isothiocyanate (Serotec), CD44-allophycocyanin, CD105-phycoerythrin (PE) (all eBioscience), CD11b-PE, CD34-PE, CD45-PE and CD73-PE (all BD Biosciences).
Cell differentiation assays
Chondrogenic assays were performed as described previously [5]. Differentiation medium consisted of high glucose DMEM, 1% FBS, antibiotics, 50 µg/ml ascorbate 2-phosphate, ITS+ (GIBCO, #41400), 100 nM dexamethasone, 10 ng/ml transforming growth factor-β1 (Humanzyme, #HZ-1011). On day 21, wells were fixed with 0.1% glutaraldehyde and stained with Safranin-O for the detection of anionic proteoglycans.
For osteogenic differentiation, media used included the following: 100 nM dexamethasone (Sigma), 50 µg/ml ascorbate 2-phosphate and 10 mM glycerol phosphate (Sigma). On day 21, cells were fixed using cold ethanol 70% and stained with Alizarin Red S (40 mM) at pH 4.1.
Adipogenic differentiation was performed using DMEM supplemented with 10% FBS, 1 µmol/l dexamethasone, 5 µg/ml insulin, 0.5 mmol/l isobutylmethylxanthine and 60 µmol/l indomethacin for 21 days. Lipid droplets were seen under the microscope after staining with Oil Red O.
Generation of chronic ischaemic heart failure
The Institutional Animal Care and Use Committee of the Ontario Cancer Institute approved the animal protocol for this study.
We used female NOD-SCID IL2R gamma null mice aged 10–12 weeks (n = 20). Generation of chronic ischaemic heart failure was performed as previously described in rats [5]. Left thoracotomy was performed and anterior descending coronary artery was ligated 1–3 mm from tip of the left auricle.
Cell injection
Four weeks after the induction of acute MI, mice were randomized to injection of MSCs (n = 10) or PBS (n = 10) by a blinded operator. The heart was approached through a midline sternotomy. The infarcted area was localized and PBS or MSCs (5 × 105 cells) were injected in 50 μl at the borderline between infarcted and non-infarcted myocardium. The injected dose was based on previous reports [6].
Echocardiography
Transthoracic echocardiographic imaging was performed by a blinded observer using a Vevo Ultrasound System (40 MHz transducer) at baseline (4 weeks after MI), 1 week and 4 weeks after cell or medium injection. Certain haemodynamic parameters, such as left ventricular end-systolic diameter (LVESD) and left ventricular end-diastolic diameter (LVEDD), septum thickness, posterior wall thickness (PW), heart rate and fractional shortening (FS) were acquired.
Real-time polymerase chain reaction assays for human Alu sequences
At 2 and 4 weeks after cell or PBS injection, mice were sacrificed (n = 3 per group at 2 weeks and n = 7 per group at 4 weeks) with ketamine overdose and the heart was harvested. Myocardial tissue from MI border was excised and processed for molecular analysis.
Real-time polymerase chain reaction (PCR) was performed in an Applied Biosystems Sequence Detection System (SDS7900HT) using 10 µl that contained 5 µl of Taqman Universal PCR Master Mix (Applied Biosystems), 900 nM each of the forward and reverse primers (Alu forward: 5′-CATGGTGAAACCCCGTCTCTA-3′ and reverse: 5′-GCCTCAGCCTCCCGAGTAG-3′), 250 nM TaqMan probe (5′-FAM-ATTAGCCGGGCGTGGTGGCG-TAMRA-3′) and 100 ng target template. Reactions were incubated at 50°C for 2 min and at 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Total DNA in the samples was assessed by real-time PCR assays with primers that amplified both the human and the mouse gene for GAPDH (h/mGAPDH forward primer: 5′-CAGCGACACCCACTCCTCCACCTT-3′; h/mGAPDH reverse primer: 5′-CATGAGGTCCACCACCCTGTTGCT-3′). Samples were standardized by correction for total DNA and the results presented as human DNA content per 100 ng of total DNA [7].
Microscopic examination of the myocardium
Four weeks after cell injection (n = 7 per group), mice were anaesthetized with ketamine and injected with KCl (0.08 mg/g) in the inferior vena cava to obtain diastolic arrest. The heart was harvested and the left ventricle (LV) along with its septum dissected out. Cardiac LV/total weight ratio was calculated as an indicator of cardiac hypertrophy and lung weight/total weight ratio as an indicator of pulmonary congestion [8]. A unique transverse cross section was done 0.5–1 mm apically from the ligation site. The apical cardiac section was formalin fixed and embedded in paraffin. Three 4-μm thick slices from different levels were cut, stained with Masson's trichrome and scanned with Aperio Scanscope XT (Aperio Technologies Inc). The total myocardial and fibrotic areas as well as wall thickness were manually traced and calculated using ImageScope software as described previously [1]. Infarct size was expressed as percentage of total LV area as well as percentage of LV circumference [1]. The measurements were performed by a blinded observer on three slices from three different levels of each heart and the averages used for statistical analysis.
For vessel immunostaining, heart sections were stained for CD31 using a previously described protocol [1]. Slides were scanned with Aperio Scanscope XT (Aperio Technologies Inc.). Quantitative assessment of microvessels was performed on the border and in the centre of the MI scar at ×400 magnification. The procedure was repeated for three different sections obtained 100 μm apart and the number of microvessels was enumerated, averaged and expressed as the number of microvessels per 0.2 mm2 of tissue.
Statistical analysis
Quantitative data were expressed as mean ± SD. Statistical analysis was performed by two-way ANOVA, followed by Bonferroni test for comparisons between groups along different timepoints (FS, left ventricular dimensions, septum thickness). Student's t-test was used for comparison between the groups at the endpoint (scar area, scar thickness, septum thickness, septum area, vessels quantification and weights). A value of P < 0.05 was considered statistically significant.
RESULTS
Immunophenotype of human mesenchymal stromal cells
The immunophenotype and differentiation capacity of the MSCs used in our experiments demonstrated that the cells conformed to the established definition of mesenchymal stromal cells. The studies necessarily included a demonstration of the differentiation of MSCs into cells of the osteogenic, adipogenic and chondrogenic lineages [3].
Presence of human mesenchymal stromal cells in infarct tissue 2 and 4 weeks after cell injection
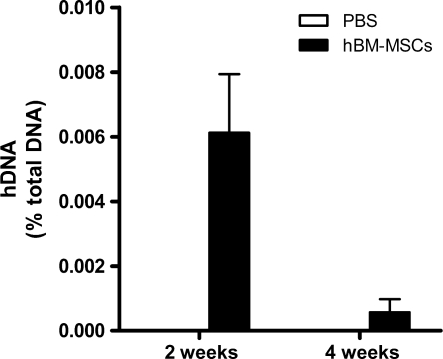
Human DNA represented 0.006 and 0.001% of total DNA at 2 and 4 weeks, respectively, after cell injection (Fig. 1).
Figure 1:
Quantification of human Alu sequences in cardiac tissue 2 and 4 weeks after cell injection. Values represent the mean ± SD, n = 3 (at 2 weeks) and 7 (at 4 weeks) per group.
Human mesenchymal stromal cells do not improve the functional outcome
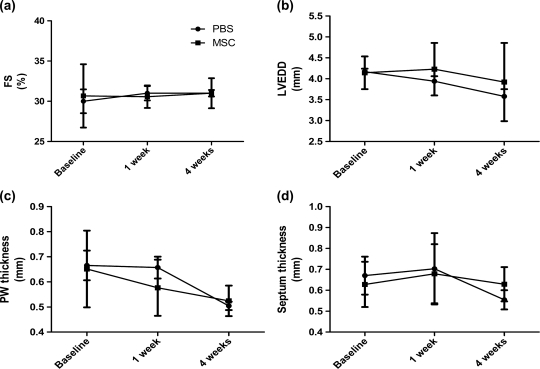
Left ventricular FS and LVEDD as well as posterior and septum thickness did not differ significantly between the groups at any of the timepoints evaluated (Fig. 2). There were no deaths in any group during the study period.
Figure 2:
Echocardiographic evaluation after cell injection. (a) Fractional shortening (FS%), (b) left ventricular end diastolic diameter (LVEDD), (c) posterior wall (PW) thickness and (d) septum thickness. Values represent mean ± SD, n = 10 (at 2 weeks) and 7 (at 4 weeks) per group.
Human mesenchymal stromal cells increase scar thickness after injection
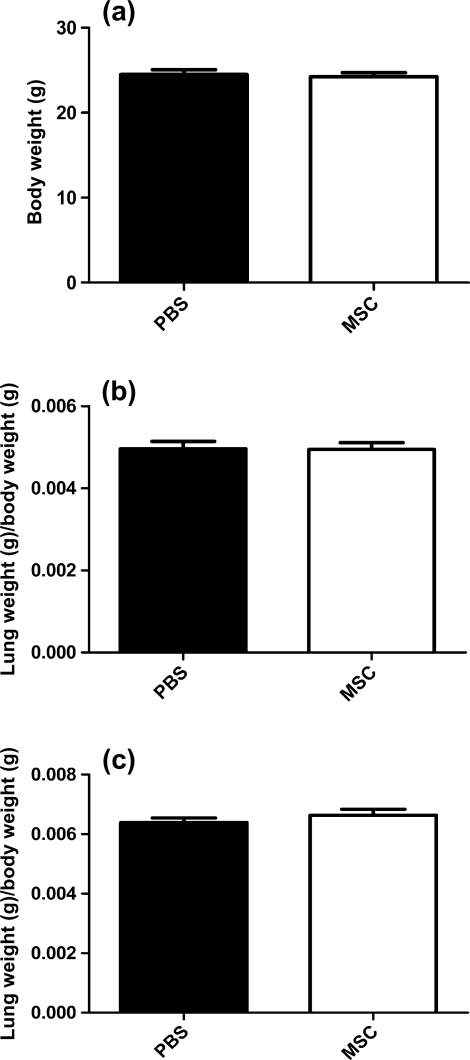
Total weight, LV and lung indexed weight 4 weeks after injection was similar in both groups.
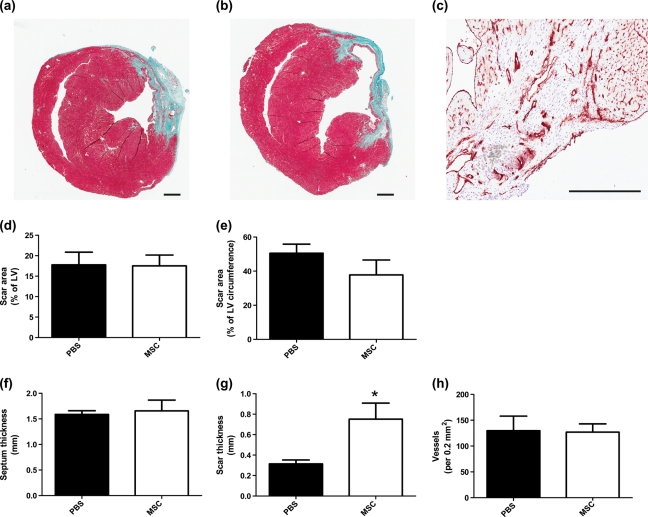
Histological assessment of the scar area and septum thickness showed no differences between groups (Fig. 3).
Figure 3:
Weight measurements 4 weeks after cell injection. (a) Total body weight. (b) Left ventricular/total body weight. (c) Lung weight/total body weight. Values represent the mean ± SD, n = 7 per group.
Scar thickness was significantly higher in MSC-treated versus control mice: 0.75 ± 0.15 and 0.3 ± 0.05 mm, respectively; P < 0.05 (Fig. 4).
Figure 4:
Histological analysis and vessel quantification of myocardial tissue 4 weeks after cell injection. Representative microphotograph of (a) MSCs and (b) PBS-injected mice. (c) Representative microphotograph of CD31-positive staining. (d) The scar area expressed as % of left ventricular area. (e) The scar area expressed as % of left ventricular circumference, (f) septum thickness, (g) scar thickness, (h) vessel quantification per 0.2 mm2 of myocardial tissue. Values represent the mean ± SD, n = 7 per group. *P < 0.05. Scale bar: 100 μm.
Vessel quantification showed no difference between MSC and PBS groups (Fig. 4).
COMMENTS
Mesenchymal stromal cells are an attractive and safe candidate cell population for cardiac regeneration. Pre-clinical and clinical studies show promise in improving cardiac function after acute MI [1, 2]. Studies implicate MSC differentiation, paracrine, immunomodulatory and anti-inflammatory effects as mechanisms mediating cardiac regeneration [1, 9, 10]. While results with pre-clinical animal models after acute MI are numerous, encouraging and form the basis for ongoing clinical trials (ClinicalTrials.gov ID: NCT01076920; NCT00260338), pre-clinical data showing a benefit of human MSCs in ischaemic heart failure are lacking.
The role of autologous or allogeneic MSCs in large animal models of chronic myocardial ischemia has been investigated [11, 12]. Schuleri et al. [11] reported an increase in infarct thickness with low (2 × 107) and high (2 × 108) doses of autologous MSCs but only the high-dose group had an improvement in ejection fraction. Quevedo et al. [12] showed improved infarct thickness, FS, endothelial differentiation and enhanced angiogenesis after the injection of 2 × 108 allogeneic MSCs. In contrast, a chronic ischaemic heart failure model in dogs failed to show improved heart function after autologous MSCs [13]. However, there are no reports of pre-clinical animal models assessing human MSCs in ameliorating chronic ischaemic cardiac failure.
A major difference between our model and previously described studies is the use of human bone marrow-derived MSCs in an immune-deficient animal model. Its main caveat is the absence of a chronic inflammatory response after MI. Here, we show for the first time the effect and safety of human MSCs in a pre-clinical chronic ischaemic heart failure model. MSC-treated mice had hearts with increased infarct wall thickness but no functional improvement or increased vascularization of the infarct zone, despite detectable human cells at the infarct site.
The mechanisms underlying increased infarct wall thickness remain uncertain. It is unlikely that the injected human cells themselves could account for the observation in view of the very low amount of human DNA detected at 4 weeks. Increased wall thickness and unchanged scar area may be explained by hypoxia-induced hypertrophy of the remaining viable cardiomyocytes [14]. The ability of MSCs to modulate matrix metalloprotease activity may also help to explain our observation [15].
The divergent results of the various studies may be due to a variety of factors, including the animal models employed and to MSC-related variables such as species, culture methodology, cell donor age, mode of cell administration and cell dose. Nevertheless, outcomes may be dose dependent, and while the cell dose we used has been conventionally accepted, higher doses may show different results [6].
Our studies showed that human MSCs did not increase cardiac function or angiogenesis in chronic ischaemic myocardium. Modulation of inflammatory factors may explain a beneficial role in other models [1]. One of the strengths of our model is that we were able to assess the direct non-immunomodulatory effect of human MSCs on chronic tissue injury. Our findings suggest that further investigation is warranted to elicit mechanisms of action other than anti-inflammatory responses.
Our study with human MSCs was necessarily constrained by the need for an immune-deficient animal model. Such a model necessarily lacks the inflammatory responses due to B, T and NK cells that may arise in chronic ischaemic heart failure, hence the full impact of human MSCs must remain unknown in this setting. Nonetheless, this pre-clinical model has been validated for other human–mouse cell interactions (for example, human haematopoietic stem cell-mouse microenvironment dynamics) and enables an in vivo assessment of the direct effects of human MSCs on cardiac regeneration largely independent of changes in the inflammatory milieu.
Given the inherent limitations of pre-clinical animal models testing human MSCs, we believe that a careful, early phase, cell dose escalation clinical study with comprehensive haemodynamic monitoring may be the next most reasonable step before embarking on advanced clinical trials in chronic myocardial ischaemia. The early phase trial could include an extensive investigation of immunological and cytokine perturbations due to human MSCs and is likely to provide valuable information to guide the most appropriate design of a randomized prospective trial for this challenging condition.
Funding
This work was supported by the Orsino Cell Therapy Translational Research Laboratory, Princess Margaret Hospital.
Conflict of interest: none declared.
REFERENCES
- 1.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–310. doi: 10.1007/s00395-011-0221-9. doi:10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 2.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Khan M, Mohsin S, Khan SN, Riazuddin S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med. doi: 10.1111/j.1582-4934.2009.00998.x. doi:10.1111/j.1582-4934.2009.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–61. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- 6.van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–52. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5:7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
- 8.Marketou M, Kintsurashvili E, Papanicolaou KN, Lucero HA, Gavras I, Gavras H. Cardioprotective effects of a selective B(2) receptor agonist of Bradykinin post-acute myocardial infarct. Am J Hypertens. 2010;23:562–8. doi: 10.1038/ajh.2010.20. [DOI] [PubMed] [Google Scholar]
- 9.Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–92. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–9. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 11.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–32. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, et al. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H1092–104. doi: 10.1152/ajpheart.01009.2005. [DOI] [PubMed] [Google Scholar]
- 14.Xu RX, Chen X, Chen JH, Han Y, Han BM. Mesenchymal stem cells promote cardiomyocyte hypertrophy in vitro through hypoxia-induced paracrine mechanisms. Clin Exp Pharmacol Physiol. 2009;36:176–80. doi: 10.1111/j.1440-1681.2008.05041.x. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, et al. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–25. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]