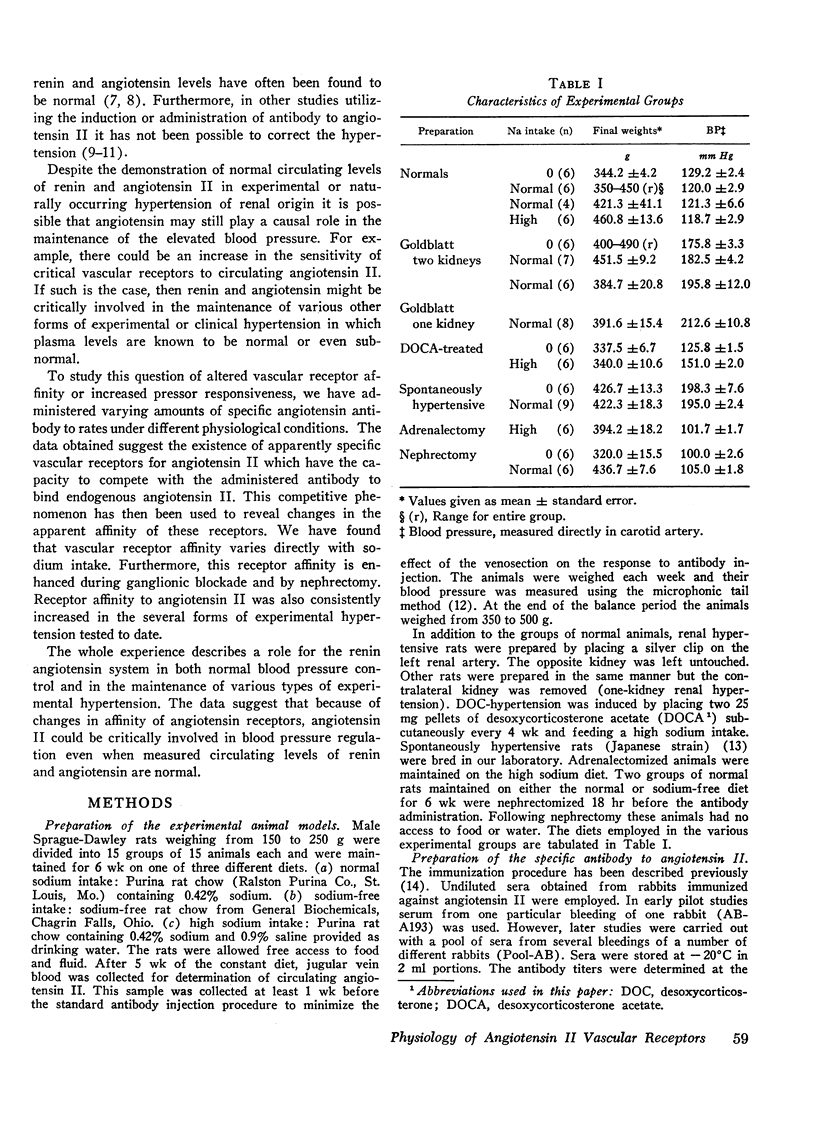
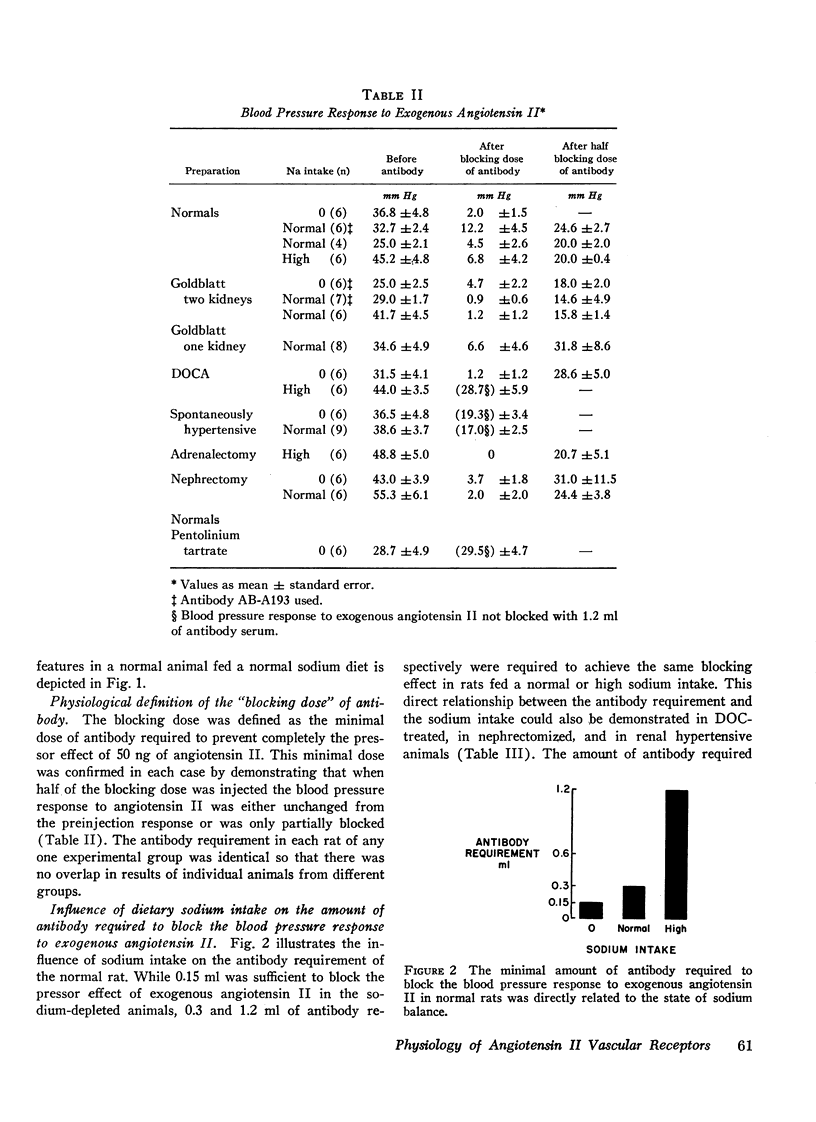
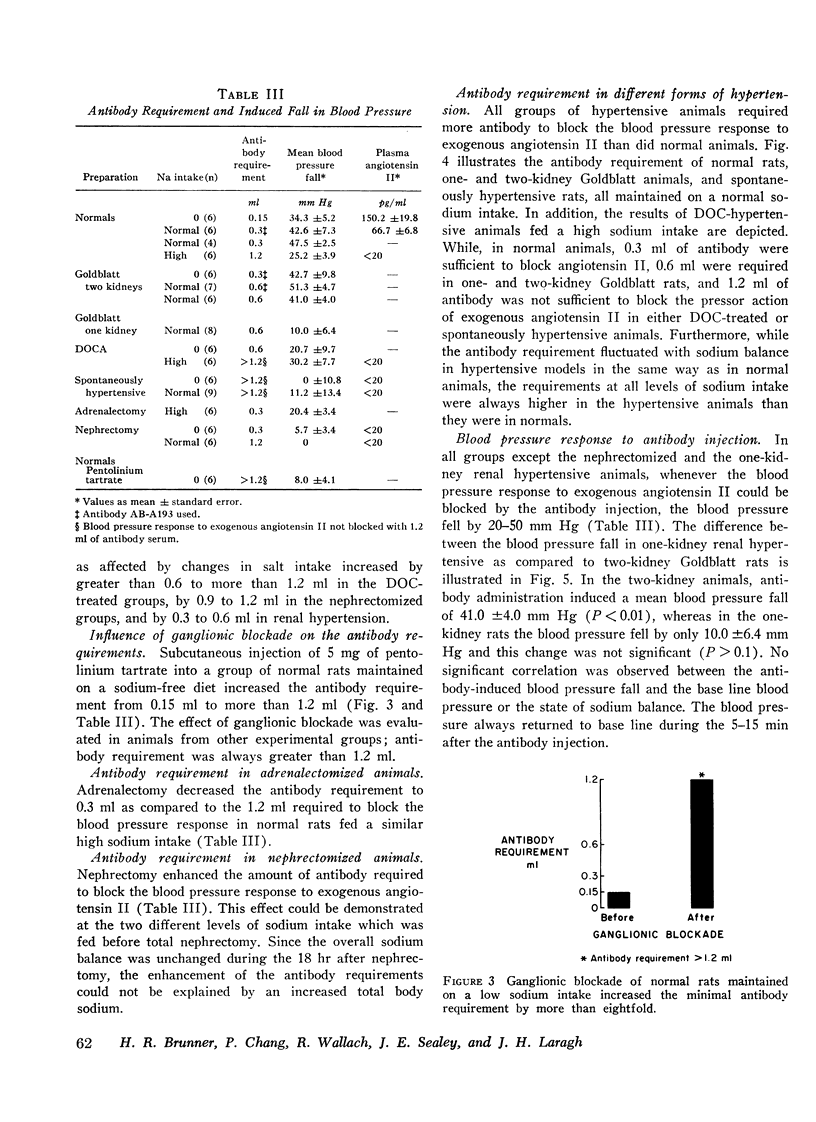
Abstract
During intravenous administration of varying doses of angiotensin II antibody to anesthetized rats, apparently specific vascular receptors were characterized. These receptors compete with administered antibody to bind circulating angiotensin. This competitive phenomenon was used to evaluate the affinity of these receptors for angiotensin. Apparent vascular receptor affinity was defined by the amount of antibody required to block the blood pressure response to exogenous angiotensin. It was found that this receptor affinity varies directly with sodium intake so that the amount of antibody required to block was eightfold greater in normal animals on a high sodium intake, as compared with those on a low sodium intake. Sodium dependence of receptors was also demonstrated in nephrectomized animals, in desoxycorticosterone (DOC)-treated rats, and in chronic renal hypertension. Thus the observed changes in receptor affinity were usually inversely related to measured endogenous angiotensin II levels. Ganglionic blockade increased antibody requirement eightfold. All of these changes were consistent, with no overlap observed in response of individual animals from different groups. These results may explain the variation in pressor activity of angiotensin associated with changes in salt balance and ganglionic blockade.
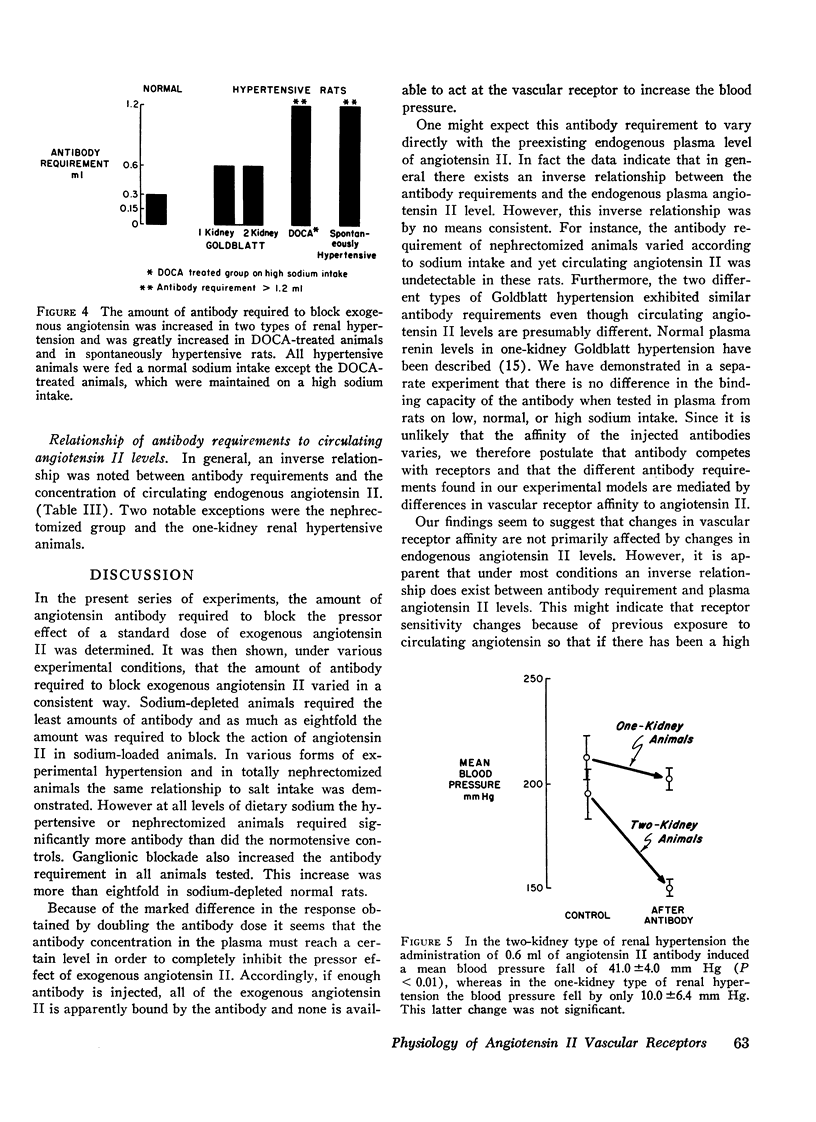
In general, when sufficient antibody was injected to block the effect of exogenous angiotensin a blood pressure lowering effect was also observed. Two exceptions were the nephrectomized and the one-kidney renal hypertensive animals, in both of which antibody administration had no effect on blood pressure.
Additional results suggest that changes in receptor affinity are involved in the pathogenesis of various types of experimental hypertensions because the amount of antibody required to block angiotensin was enhanced in renal (twofold), DOC (fourfold), and genetic (fourfold) hypertension. Accordingly, changes in the affinity of these receptors could be critically involved in normal blood pressure control and in various forms of experimental and clinical hypertension, even when circulating angiotensin II levels are normal.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES R. P., BORKOWSKI A. J., SICINSKI A. M., LARAGH J. H. PROLONGED INFUSIONS OF ANGIOTENSIN II AND NOREPINEPHRINE AND BLOOD PRESSURE, ELECTROLYTE BALANCE, AND ALDOSTERONE AND CORTISOL SECRETION IN NORMAL MAN AND IN CIRRHOSIS WITH ASCITES. J Clin Invest. 1965 Jul;44:1171–1186. doi: 10.1172/JCI105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN J. J., DAVIES D. L., LEVER A. F., ROBERTSON J. I. VARIATIONS IN PLASMA RENIN CONCENTRATION IN SEVERAL PHYSIOLOGICAL AND PATHOLOGICAL STATES. Can Med Assoc J. 1964 Jan 25;90:201–206. [PMC free article] [PubMed] [Google Scholar]
- Bailie M. D., Rector F. C., Jr, Seldin D. W. Angiotensin II in arterial and renal venous plasma and renal lymph in the dog. J Clin Invest. 1971 Jan;50(1):119–126. doi: 10.1172/JCI106465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christlieb A. R., Biber T. U., Hickler R. B. Studies on the role of angiotensin in experimental renovascular hypertension: an immunologic approach. J Clin Invest. 1969 Aug;48(8):1506–1518. doi: 10.1172/JCI106117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creditor M. C., Loschky U. K. Plasma renin activity in hypertension. Am J Med. 1967 Sep;43(3):371–382. doi: 10.1016/0002-9343(67)90193-3. [DOI] [PubMed] [Google Scholar]
- Davis W. W., Burwell L. R., Bartter F. C. Inhibition of the effects of angiotensin II on adrenal steroid production by dietary sodium. Proc Natl Acad Sci U S A. 1969 Jul;63(3):718–723. doi: 10.1073/pnas.63.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara A., Grollman A. Pressor activity of renal venous effluent following constriction of renal artery in dogs. Am J Physiol. 1968 Jan;214(1):1–5. doi: 10.1152/ajplegacy.1968.214.1.1. [DOI] [PubMed] [Google Scholar]
- Eide I., Aars H. Renal hypertension in rabbits immunized with angiotensin. II. Scand J Clin Lab Invest. 1970 Mar;25(2):119–127. doi: 10.3109/00365517009049193. [DOI] [PubMed] [Google Scholar]
- Gocke D. J., Gerten J., Sherwood L. M., Laragh J. H. Physiological and pathological variations of plasma angiotensin II in man. Correlation with renin activity and sodium balance. Circ Res. 1969 May;24(5 Suppl):131–148. [PubMed] [Google Scholar]
- Gordon R. D., Küchel O., Liddle G. W., Island D. P. Role of the sympathetic nervous system in regulating renin and aldosterone production in man. J Clin Invest. 1967 Apr;46(4):599–605. doi: 10.1172/JCI105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedwall P. R. Effect of rabbit antibodies against angiotensin-II on the pressor response to angiotensin-II and renal hypertension in the rat. Br J Pharmacol. 1968 Nov;34(3):623–629. doi: 10.1111/j.1476-5381.1968.tb08491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON C. I., JOSE A. D. REDUCED VASCULAR RESPONSE TO ANGIOTENSIN II IN SECONDARY HYPERALDOSTERONISM. J Clin Invest. 1963 Sep;42:1411–1420. doi: 10.1172/JCI104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C. I., Hutchinson J. S., Mendelsohn F. A. Biological significance of renin angiotensin immunization. Circ Res. 1970 Oct;27(Suppl):215+–215+. [PubMed] [Google Scholar]
- KAPLAN N. M., SILAH J. G. THE EFFECT OF ANGIOTENSIN II ON THE BLOOD PRESSURE IN HUMANS WITH HYPERTENSIVE DISEASE. J Clin Invest. 1964 Apr;43:659–669. doi: 10.1172/JCI104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUCHEL O., HORKY K., PAZOUREK M., GREGOROVA I. PRESSOR HYPOREACTIVITY TO ANGIOTENSIN ADDISON'S DISEASE. Lancet. 1964 Dec 19;2(7373):1316–1317. doi: 10.1016/s0140-6736(64)91107-9. [DOI] [PubMed] [Google Scholar]
- Khairallah P. A., Page I. H., Turker K. R. Potentiation of vascular myotropic responses by metanephrine and other noncatecholamines. Circ Res. 1966 Sep;19(3):538–543. doi: 10.1161/01.res.19.3.538. [DOI] [PubMed] [Google Scholar]
- LARAGH J. H., ANGERS M., KELLY W. G., LIEBERMAN S. Hypotensive agents and pressor substances. The effect of epinephrine, norepinephrine, angiotensin II, and others on the secretory rate of aldosterone in man. JAMA. 1960 Sep 17;174:234–240. doi: 10.1001/jama.1960.03030030014003. [DOI] [PubMed] [Google Scholar]
- LARAGH J. H. Hormones and the pathogenesis of congestive heart failure: vasopressin, aldosterone, and angiotensin II. Further evidence for renal-adrenal interaction from studies in hypertension and in cirrhosis. Circulation. 1962 Jun;25:1015–1023. doi: 10.1161/01.cir.25.6.1015. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Goodfriend T. L. Angiotensin receptors. Am J Physiol. 1970 May;218(5):1319–1328. doi: 10.1152/ajplegacy.1970.218.5.1319. [DOI] [PubMed] [Google Scholar]
- Macdonald G. J., Louis W. J., Renzini V., Boyd G. W., Peart W. S. Renal-clip hypertension in rabbits immunized against angiotensin II. Circ Res. 1970 Aug;27(2):197–211. doi: 10.1161/01.res.27.2.197. [DOI] [PubMed] [Google Scholar]
- Meyer P., Worcel M. Role of angiotensin in the salt-hypertension of rats. Pflugers Arch. 1970;317(4):327–335. doi: 10.1007/BF00586581. [DOI] [PubMed] [Google Scholar]
- Miksche L. W., Miksche U., Gross F. Effect of sodium restriction on renal hypertension and on renin activity in the rat. Circ Res. 1970 Dec;27(6):973–984. doi: 10.1161/01.res.27.6.973. [DOI] [PubMed] [Google Scholar]
- OSTROVSKY D., GORNALL A. G. EFFECTS OF ALDOSTERONE AND OTHER ADRENAL HORMONES ON THE BLOOD PRESSURE RESPONSES TO RENIN AND ANGIOTENSIN. Can Med Assoc J. 1964 Jan 25;90:180–184. [PMC free article] [PubMed] [Google Scholar]
- Okamoto K. Spontaneous hypertension in rats. Int Rev Exp Pathol. 1969;7:227–270. [PubMed] [Google Scholar]
- Reid W. D., Laragh J. H. Sodium and potassium intake, blood pressure, and pressor response to angiotensin. Proc Soc Exp Biol Med. 1965 Oct;120(1):26–29. doi: 10.3181/00379727-120-30434. [DOI] [PubMed] [Google Scholar]
- TOBIAN L., Jr, BINION J. Artery wall electrolytes in renal and DCA hypertension. J Clin Invest. 1954 Oct;33(10):1407–1414. doi: 10.1172/JCI103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander A. J. Control of renin release. Physiol Rev. 1967 Jul;47(3):359–382. doi: 10.1152/physrev.1967.47.3.359. [DOI] [PubMed] [Google Scholar]
- WAKERLIN G. E. Antibodies to renin as proof of the pathogenesis of sustained renal hypertension. Circulation. 1958 Apr;17(4 Pt 2):653–657. doi: 10.1161/01.cir.17.4.653. [DOI] [PubMed] [Google Scholar]
- Weiser R. A., Johnson A. G., Hoobler S. W. The effect of antirenin on the blood pressure of the rat with exprimental renal hypertension. Lab Invest. 1969 Apr;20(4):326–331. [PubMed] [Google Scholar]


