Abstract
Aggressive intravenous thrombolysis of pulmonary emboli after major thoracic surgery has rarely been reported and is controversial because of an assumed risk of fatal bleeding. We report a 62-year old female who underwent left upper lobectomy. Her postoperative course was complicated with symptomatic pulmonary embolism and on postoperative day 5 she was successfully treated with intravenous thrombolysis using alteplase (Actilyse®) without signs of bleeding. She was discharged from the hospital 12 days postoperatively.
Keywords: Pulmonary embolism, Lung cancer, Thrombolysis
INTRODUCTION
Acute pulmonary embolism (PE) is a rare but well-known complication following pulmonary resections with an incidence of 1.3% [1]. Massive PE has a mortality up to 50% and it is still debated if surgical embolectomy or thrombolysis is the appropriate treatment [2].
CASE REPORT
A 62-year old female previous smoker with a 3.5 cm left upper lobe adenocarcinoma was referred for surgical resection. She had a FEV1 of 2.5 l (92%) and an FVC of 3.2 l (103%). She had no comorbidity and did not receive any medications preoperatively. She was scheduled for VATS-lobectomy but was converted to thoracotomy because of enlarged lymph nodes (11 l) that unsuspectingly infiltrated the pulmonary artery, which required a 2 cm perpendicular vascular wedge resection and was sutured with running 5-0 Prolene with no visible diameter reduction. The operation lasted 145 min and postoperatively pain management was an epidural catheter with continuous infusion of bupivacain. She did not receive thromboembolic prophylaxis in accordance with our postoperative routine because she was completely mobilized on postoperative day (POD) 1. Her chest tube was pulled POD2 when she started to complain of dyspnoea on exercise. Chest X-ray demonstrated pulmonary congestion and her ECG revealed atrial fibrillation with a heart rate of 130 beats/min. Saturation was 92% with high-flow oxygen supply, D-dimer = 4.88 nmol/l, Hb = 6.7 mmol/l, CRP = 122 mg/L, Potassium = 3.1 mmol/l, pH = 7.45, pO2 = 8.4 kPA and pCO2 = 5.4 kPA. An immediate transthoracic echocardiography by a cardiologist showed no signs of right ventricular dysfunction, dilatation or septal defects. Low molecular weight heparin (LMWH) (Enoxaparin-Klexane®80 mg ×2) was prescribed because of her atrial fibrillation and increasingly poor mobilization.
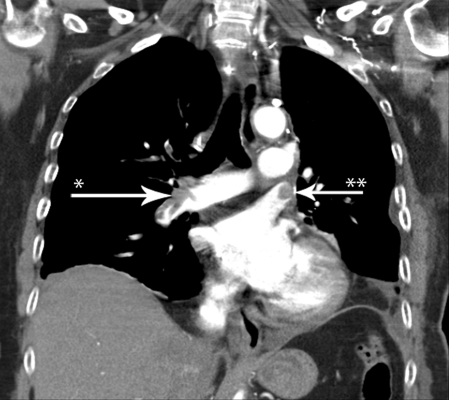
She had two brief episodes of suspected peripheral embolism; both events had spontaneous remission within 60 min. Because her dyspnoea progressed on POD5, she was scheduled for lung perfusion scintigraphy, which demonstrated a classic ‘mismatch’. A subsequent acute chest CT revealed major PE in the segmental arteries bilaterally (Fig. 1) as well as a contrast defect in the left upper lobe vein stump. The patient underwent successful intravenous thrombolysis with alteplase (Actilyse®10 mg as a bolus and continuous infusion of 90 mg over the following 2 h) with prompt improvement in her respiratory distress and had no signs of bleeding on chest X-ray nor decrease in haemoglobin.
Figure 1:
Chest CT demonstrating contrast defect from an embolus in the right pulmonary artery (arrow*) and a thrombus in the left upper lobe vein stump (arrow**).
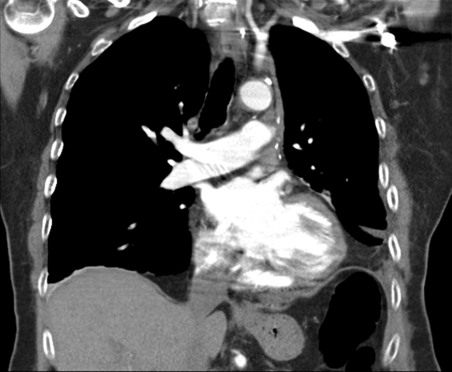
A repeat chest CT POD10 confirmed that all pulmonary emboli had disappeared (Fig. 2) and repeat echocardiography was also normal. The patient was started on oral warfarin (Marevan®) for 3 months and was discharged from the hospital POD12. At follow-up in the outpatient clinic she was well with no respiratory symptoms.
Figure 2:
Chest CT demonstrating that all the contrast defects have disappeared after aggressive intravenous thrombolysis.
DISCUSSION
Acute PE is a rare but well-known complication following pulmonary resections with an incidence of 1.3% [1]. Massive PE has a mortality up to 50%, and it is still debated if surgical embolectomy or thrombolysis is the optimal treatment [2]. There is very little published data on treatment of postoperative PE after thoracic surgery and it remains controversial whether or not systemic thrombolytic agents should be used to treat newly operated patients because of an assumed high risk of haemorrhage [3].
Patients with suspected PE are stratified into high-risk and low-risk patients according to the guidelines from the European Society of Cardiology [4]. In general, circulatory unstable high-risk patients are considered for either embolectomy or aggressive intravenous thrombolysis, whereas circulatory stable high-risk patients should receive thrombolysis or LMWH while low-risk patients are treated with LMWH. Thus, it may be argued that aggressive thrombolysis was not necessary in our patient and that she would have done well with LMWH. However, randomized trials have consistently shown that thrombolytic therapy rapidly resolves thromboembolic obstruction and exerts beneficial effects on haemodynamic parameters [4]. In the present case, perfusion scintigraphy demonstrated PE despite 3 days of treatment with LMWH and, as the patient's clinical course deteriorated, we decided that aggressive thrombolysis was necessary despite normal haemodynamic parameters. One previous report on treatment of PE in newly operated lung cancer patients used a strategy of simple anticoagulant therapy in patients with PE that developed before POD4 because of a fear of bleeding, while patients with PE that developed after POD4 received aggressive thrombolysis [3]. Of all the different aggressive thrombolytic agents, there is no evidence that any one is superior to the others [5]. With regard to heparinization, several trials compared the efficacy and safety of subcutaneous LMWH with those of unfractionated heparin and concluded that LMWH was at least as efficacious as unfractionated heparin [6].
According to the Danish Lung Cancer Registry [7], the incidence of PE in our institution where thromboembolic prophylaxis is used only in patients with high comorbidity and poor ability to mobilize was <0.1% in 1376 consecutive patients who underwent thoracic surgery for lung cancer during the last 10 years. Theoretically, our low incidence of PE could result from under-diagnosing PE or from under-reporting complications to our national registry. In the first case, it is indeed likely that some patients with minor PE are not diagnosed because symptoms are vague and mistaken from a slow but otherwise common postoperative course. Secondly, we assume that under-reporting was not a major problem because complications were reported in 25% of all the patients operated on during the same period. The reported complications were predominately prolonged air leakage, pneumonia and cardiac arrhythmias, confirming that surgeons were indeed willing to report on complications after lung cancer surgery.
Nevertheless, for medico-legal reasons our practice is now changing towards thromboembolic prophylaxis in all patients because of the recently published recommendations of the American College of Chest Physicians [8], but we do believe that this issue should be investigated further. In the present case, we must assume that lack of thromboembolic prophylaxis was an important contributing factor to the development of PE. We also suspect that atrial fibrillation was the cause of PE due to formation of clots in both atria with subsequent embolism to the pulmonary arteries peripherally and because echocardiography did not demonstrate septal defects.
In conclusion, the present case report demonstrates that high-dose intravenous thrombolytic therapy may be used safely, even in a patient who has just undergone major thoracic surgery.
Conflict of interest: none declared.
REFERENCES
- 1.Dentali F, Malato A, Ageno W, Imperatori A, Cajozzo M, Rotolo N, et al. Incidence of venous thromboembolism in patients undergoing thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2008;135:705–6. doi: 10.1016/j.jtcvs.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Barrett NA, Byrne A, Delaney A, Hibbert M, Ramakrishnan N, Barrett NA, et al. Management of massive pulmonary embolism: a retrospective single-centre cohort study. Crit Care Resusc. 2010;12:242–7. [PubMed] [Google Scholar]
- 3.Sakuragi T, Sakao Y, Furukawa K, Rikitake K, Ohtsubo S, Okazaki Y, et al. Successful management of acute pulmonary embolism after surgery for lung cancer. Eur J Cardiothorac Surg. 2003;24:580–7. doi: 10.1016/s1010-7940(03)00392-0. [DOI] [PubMed] [Google Scholar]
- 4.Torbicki A, Perrier A, Stavros K, Giancarlo A, Nazzareno G, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism. G Ital Cardiol (Rome) 2009;10:303–47. [PubMed] [Google Scholar]
- 5.Capstick T, Henry MT, Capstick T, Henry MT. Efficacy of thrombolytic agents in the treatment of pulmonary embolism. Eur Respir J. 2005;26:864–74. doi: 10.1183/09031936.05.00002505. [DOI] [PubMed] [Google Scholar]
- 6.Quinlan DJ, McQuillan A, Eikelboom JW, Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004;140:175–83. doi: 10.7326/0003-4819-140-3-200402030-00008. [DOI] [PubMed] [Google Scholar]
- 7.Jakobsen E, Palshof T, Osterlind K, Pilegaard H, Jakobsen E, Palshof T, et al. Data from a national lung cancer registry contributes to improve outcome and quality of surgery: Danish results. Eur J Cardiothorac Surg. 2009;35:348–52. doi: 10.1016/j.ejcts.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]