Abstract
A reliable, relatively simple method for isolation and quantification of disaturated lecithins is described. In rabbit lung, 34% of the lecithins were disaturated, in alveolar macrophages, 19%. More than 95% of the fatty acids of the disaturated lecithins from lung and alveolar macrophages was palmitic. Hence, the disaturated lecithins from these sources were essentially all dipalmitoyl lecithin.
Both heterophils and alveolar macrophages incorporated 14C-labeled choline and palmitate into disaturated lecithins. Liver slices in which only about 1% of the lecithins were disaturated incorporated very little of these precursors into this fraction. Of the palmitate incorporated in vitro into disaturated lecithins by alveolar macrophages, heterophils, and lung slices, 37% was in the 1 position. In disaturated lecithins isolated from pulmonary lavage fluid, alveolar macrophages, and lung of rabbit 8-12 hr after a single intravenous injection of palmitic-1-14C acid, 45% of the 14C was in position 1. At earlier times, from 20-240 min after injection, the distribution of 14C was similar in the samples from lung, but in those from alveolar macrophages and lavage fluid, the percentage in position 1 was slightly lower.
Glycerol-U-14C was incorporated into disaturated lecithins by alveolar macrophages and by lung slices in vitro. Both tissues incorporated very little label from ethanolamine or from methyl-labeled methionine into this fraction. All of the data are consistent with the view that alveolar macrophages synthesize dipalmitoyl lecithin via the cytidine diphosphate-choline pathway.
Full text
PDF

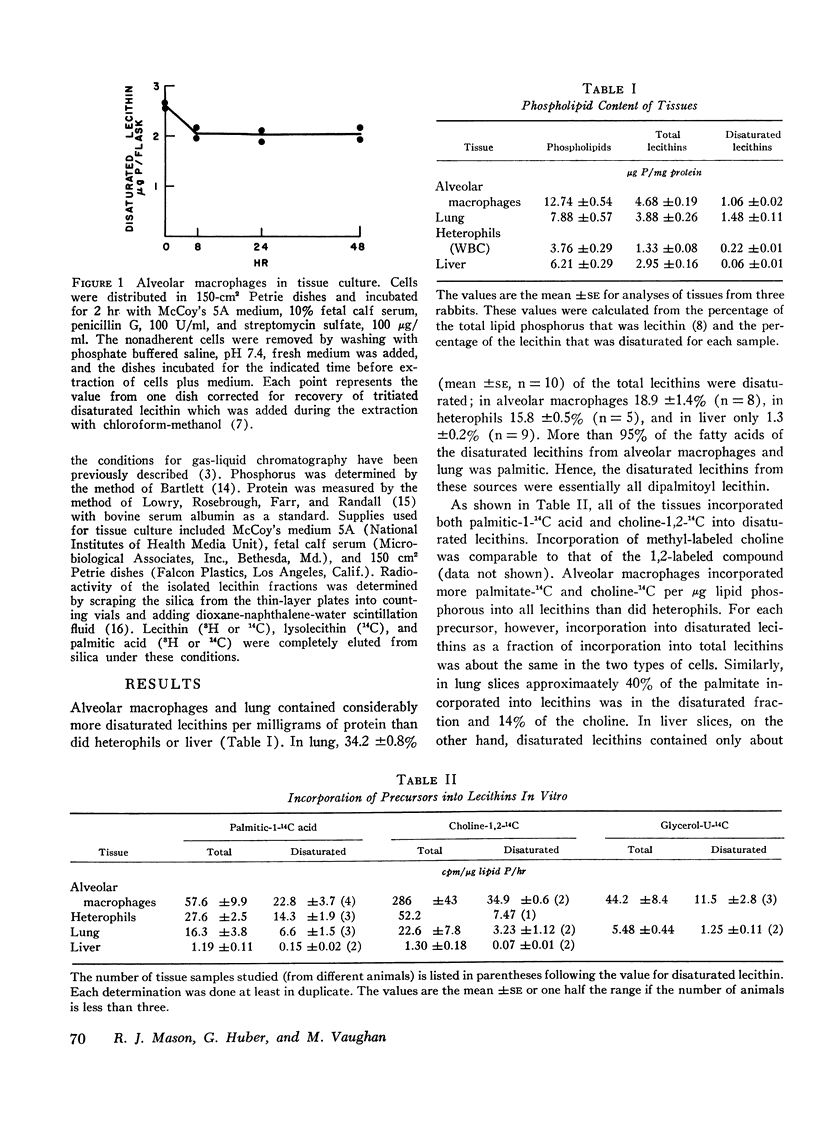
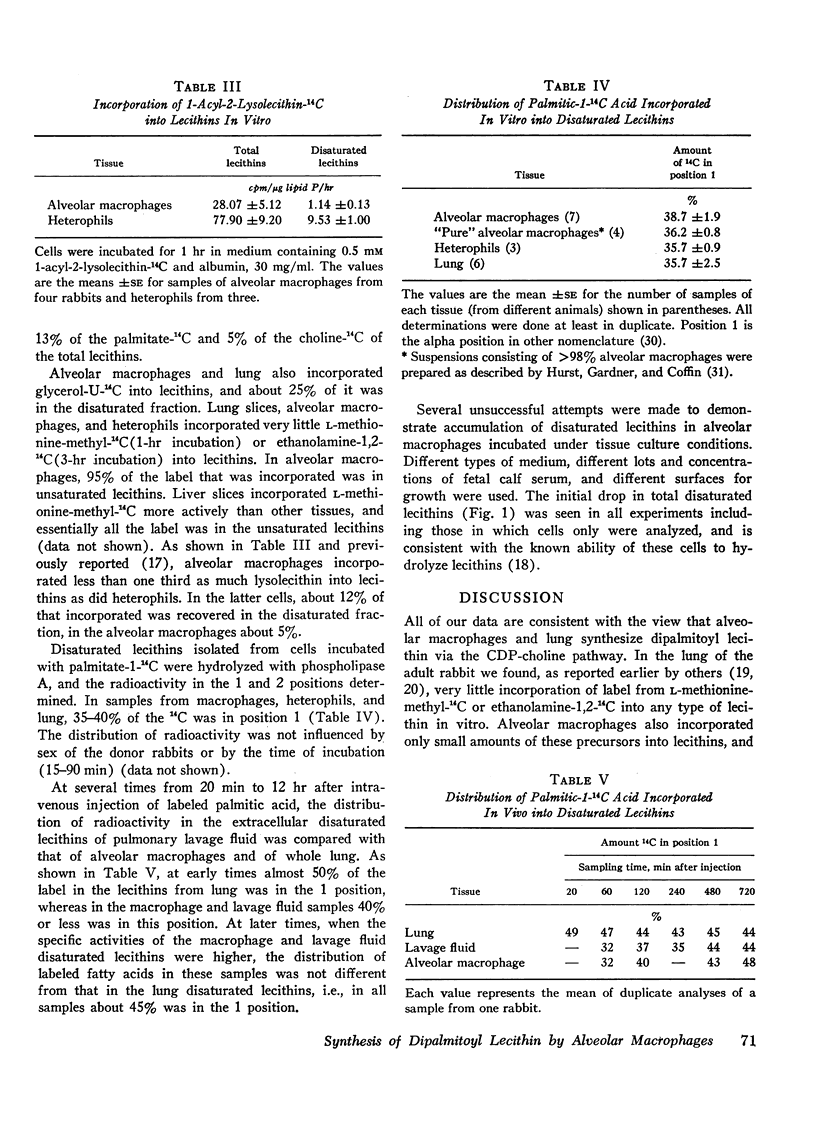


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzopardi A., Thurlbeck W. M. The oxidative enzyme pattern in developing and adult mice and adult rabbits. Lab Invest. 1967 May;16(5):706–716. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BROWN E. S. ISOLATION AND ASSAY OF DIPALMITYL LECITHIN IN LUNG EXTRACTS. Am J Physiol. 1964 Aug;207:402–406. doi: 10.1152/ajplegacy.1964.207.2.402. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM S., AVERY M. E. Time of appearance of lung surfactant in the foetal mouse. Nature. 1962 Feb 17;193:688–689. doi: 10.1038/193688a0. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Nutter L. J., Privett O. S. Determination of the structure of lecithins. Lipids. 1966 Mar;1(2):132–135. doi: 10.1007/BF02533005. [DOI] [PubMed] [Google Scholar]
- Buckingham S., Heinemann H. O., Sommers S. C., McNary W. F. Phospholipid synthesis in the large pulmonary alveolar cell. Its relation to lung surfactants. Am J Pathol. 1966 Jun;48(6):1027–1041. [PMC free article] [PubMed] [Google Scholar]
- Clements J. A., Nellenbogen J., Trahan H. J. Pulmonary surfactant and evolution of the lungs. Science. 1970 Aug 7;169(3945):603–604. doi: 10.1126/science.169.3945.603. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Levy S. Increased synthesis of phospholipid during phagocytosis. J Clin Invest. 1968 Oct;47(10):2217–2229. doi: 10.1172/JCI105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P. Phospholipid metabolism by phagocytic cells. I. A comparison of conversion of [32P]lysolecithin to lecithin and glycerylphosphorylcholine by homogenates of rabbit polymorphonuclear leukocytes and alveolar macrophages. Biochim Biophys Acta. 1966 Dec 7;125(3):510–524. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Frosolono M. F., Slivka S., Charms B. L. Acyl transferase activities in dog lung microsomes. J Lipid Res. 1971 Jan;12(1):96–103. [PubMed] [Google Scholar]
- Gluck L., Sribney M., Kulovich M. V. The biochemical development of surface activity in mammalian lung. II. The biosynthesis of phospholipids in the lung of the developing rabbit fetus and newborn. Pediatr Res. 1967 Jul;1(4):247–265. doi: 10.1203/00006450-196707000-00002. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., RODBELL M., TURNER L. D. Enzymatic formation of monopalmitoleyl- and monopalmitoyllecithin (lysolecithins). J Biol Chem. 1954 Jan;206(1):431–441. [PubMed] [Google Scholar]
- Huber G. L., Mason R. J., LaForce M., Spencer N. J., Gardner D. E., Coffin D. L. Alterations in the lung following the administration of ozone. Arch Intern Med. 1971 Jul;128(1):81–87. [PubMed] [Google Scholar]
- Hurst D. J., Gardner D. E., Coffin D. L. Effect of ozone on acid hydrolases of the pulmonary alveolar macrophage. J Reticuloendothel Soc. 1970 Sep;8(3):288–301. [PubMed] [Google Scholar]
- KARRER H. E. The ultrastructure of mouse lung: the alveolar macrophage. J Biophys Biochem Cytol. 1958 Nov 25;4(6):693–700. doi: 10.1083/jcb.4.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG C., PENNY I. F. The structure of the naturally occurring phosphoglycerides. III. Action of moccasin-venom phospholipase A on ovolecithin and related substances. Biochem J. 1957 Feb;65(2):382–389. doi: 10.1042/bj0650382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan T. E., Edmunds L. H., Jr Pulmonary artery occlusion. 3. Biochemical alterations. J Appl Physiol. 1967 May;22(5):1012–1016. doi: 10.1152/jappl.1967.22.5.1012. [DOI] [PubMed] [Google Scholar]
- SNYDER F. RADIOASSAY OF THIN-LAYER CHROMATOGRAMS: A HIGH-RESOLUTION ZONAL SCRAPER FOR QUANTITATIVE C14 AND H3 SCANNING OF THIN-LAYER CHROMATOGRAMS. Anal Biochem. 1964 Oct;9:183–196. doi: 10.1016/0003-2697(64)90102-2. [DOI] [PubMed] [Google Scholar]
- SPECTOR A. A., STEINBERG D., TANAKA A. UPTAKE OF FREE FATTY ACIDS BY EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Mar;240:1032–1041. [PubMed] [Google Scholar]
- Said S. I., Klein R. M., Norrell L. W., Maddox Y. T. Metabolism of alveolar cells: histochemical evidence and relation to pulmonary surfactant. Science. 1966 Apr 29;152(3722):657–659. doi: 10.1126/science.152.3722.657. [DOI] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Spitzer H. L., Morrison K., Norman J. R. The incorporation of L-[Me-14C]methionine and [Me-3H]choline into lung phosphatides. Biochim Biophys Acta. 1968 May 1;152(3):552–558. doi: 10.1016/0005-2760(68)90095-7. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Murad F., Mason R. J., Vaughan M. Regulation of glycogen metabolism in polymorphonuclear leukocytes. J Biol Chem. 1970 Nov 25;245(22):6228–6234. [PubMed] [Google Scholar]
- Treble D. H., Frumkin S., Balint J. A., Beeler D. A. The entry of choline into lecithin, in vivo, by base exchange. Biochim Biophys Acta. 1970 Feb 10;202(1):163–171. doi: 10.1016/0005-2760(70)90227-4. [DOI] [PubMed] [Google Scholar]
- WITTELS B., BRESSLER R. TWO-DIMENSIONAL THIN-LAYER CHROMATOGRAPHIC ISOLATION OF FATTY ACYL CARNITINES. J Lipid Res. 1965 Apr;6:313–314. [PubMed] [Google Scholar]



