Abstract
The immediate complication after implantation of mechanical circulatory support (MCS) is more often bleeding than thromboembolic events. We report an unsual case of massive thrombus formation following systemic thrombolytic therapy which happened twice after implantation of MCS. Various mechanisms may contribute to this severe complication, but attention should be paid to patients who receive MCS after systemic thrombolysis because of the secondary hypercoagulability induced by this therapy.
Keywords: Acute myocarditis, Thrombus, Systemic thrombolysis, Mechanical circulatory support, Extracorporeal membrane oxygenation, Total artificial heart
BACKGROUND
Myocarditis is a disease characterized by a polymorphic clinical presentation and appears to be a major cause of sudden, unexpected death in young adults. In the case of refractory cardiogenic shock, mechanical circulatory support (MCS) should be considered as a bridge to recovery [1]. Pump thrombosis and thromboembolic events persist as major sources of morbidity and mortality of MCS [2]. Nevertheless, the immediate post-operative course after implantation is mostly complicated by haemorrhagic events, and thromboembolic events happen later on [2]. We report a case of massive thrombus formation after the implantation of MCS.
CASE PRESENTATION
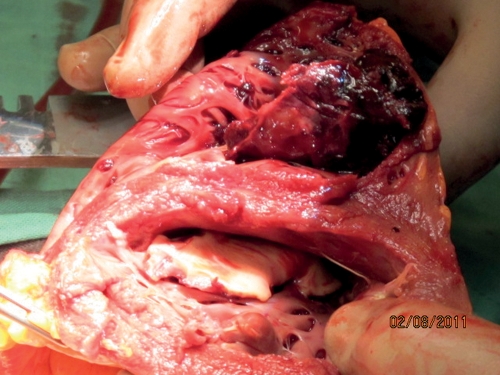
A 37-year old man presented with 1-week old gastroenteritis and was admitted due to acute pulmonary oedema, which needed mechanical ventilation. Echocardiography showed severe left ventricular dysfunction (ejection fraction 20%). The haemodynamic status deteriorated rapidly due to ventricular tachycardia. Cardioversion was performed and the patient was initially treated with systemic thrombolysis with recombinant tissue plasminogen activator because of suspected acute myocardial infarction. Coronarographic findings were normal. The patient received an intraaortic balloon pump (IABP) and peripheral venoarterial extracorporeal membrane oxygenation (ECMO) was initiated (flow 6 l min−1 with a speed of 5300 rpm) because of refractory cardiogenic shock. Pre-operative echocardiography showed no thrombus. The neurological clinical examination and the cerebral computed tomography scan were normal. The patient developed sepsis with positive blood cultures with Staphylococcus epidermidis. After 3 days, it was decided to implant a ventricular assist device. Pre-operative echocardiography revealed thrombi in the pulmonary artery, the aortic valve and the ventricles, despite systemic anticoagulation with unfractioned heparin (platelets 51 giga per liter, partial thromboplastin time 47 s, prothrombin time 69 s and antithrombin III 71%). The patient was transferred to our hospital and received a CardioWest total artificial heart (TAH). ECMO was maintained at a decreased speed (2500 rpm) because of severe lung oedema. Pathological examination of the explanted heart confirmed the massive thrombus (Fig. 1) and the diagnosis of lymphocytic myocarditis. The diagnoses of heparin-induced thrombocytopenia and disseminated intravascular coagulopathy were excluded by plasmatic tests (platelet-factor IV assays negative, schizocytes negative and fibrinogen 6.5 g l−1).
Figure 1:
Post-explantation heart showing thrombus of left and right ventricles.
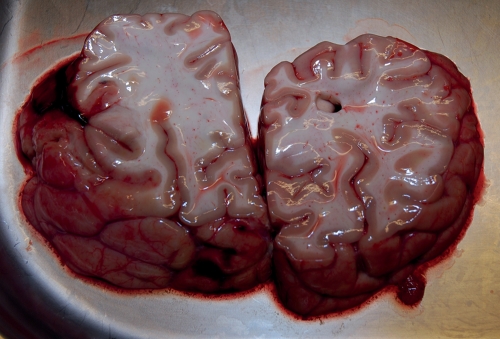
After 1 day with the TAH, the patient presented haemodynamic instability. The biochemical findings showed haemolysis (free haemoglobin 730 g dl−1 and lactate dehydrogenase 4600 U l−1). Thrombosis of the TAH was diagnosed by echocardiography. The clinical status deteriorated with asymmetry of the pupils. Cerebral computed tomography revealed multiple cerebral emboli. The patient died. The post-mortem report confirmed the multiple emboli in the brain (Fig. 2).
Figure 2:
Post-mortem analysis showing the multiple cerebral emboli.
DISCUSSION
The immediate complication following MCS implantation is more often bleeding than thromboembolic events [2]. The massive thrombus formation in our case was unexpected because the patient received systemic thrombolysis therapy before the placement of the MCS and continuous unfractioned heparin. There are few reports of thrombus formation after aggressive thrombolytic or anticoagulation therapy [3, 4]. Doepp et al. [3] reported apical thrombus formation following systemic thrombolysis in a patient with acute systemic stroke. Leontiadis et al. [4] reported a case of massive thrombus of the aortic root and ascending aorta after ECMO and IABP placement despite double antiplatelet therapy and systemic anticoagulation with heparin.
In our patient, a massive thrombus developed throughout the heart within 3 days after thrombolytic therapy, strongly suggesting a causal relationship of secondary hypercoagulability and thrombus formation.
Systemic thrombolysis could have resulted in a secondary hypercoagulability status. In fact, systemic thrombolysis is followed by an increase in procoagulant factors that activate coagulation: activation of plasminogen activator inhibitor-1, resulting in suppression of endogenous fibrinolysis, increased thrombin generation and activity, and increased thrombin–antithrombin-III-complex levels [5].
Other factors may favour massive thrombus formation. Cardiogenic shock is accompanied by the production of numerous inflammatory cytokines (interleukin (IL)-1β, IL-6, IL-8, Tumour necrosis factor alpha, C-reactive protein, soluble adhesion molecules, complement system) responsible for hypercoagulability [6]. The levels of these cytokines remain high after implantation of a ventricular assist device. The low ejection fraction contributes to thrombosis of the native heart by blood stasis.
In our patient, blood culture indicated staphylococcal sepsis, which strongly activates platelets and the coagulation system by increasing procoagulation factors (prothrombin fragments 1 and 2, fibrinopeptide A, thrombin–antithrombin complexes, fibrin monomers) [7].
We are not sure whether the aetiology of cardiomyopathy contributes to thrombus formation. In one case report of massive ventricle thrombus formation following ECMO after fulminant myocarditis [8], heparin-induced thrombopenia was suspected but not confirmed. Our patient had no heparin-induced thrombopenia.
Finally, the low flow in MCS favours thrombus formation. In our case, the ECMO flow was >6 l min−1 (speed 5300 rpm) before CardioWest TAH implantation. After implantation, the speed was reduced to 2500 rpm to optimize the functioning of the CardioWest TAH. The low flow in the TAH favours thrombus formation. Venoarterial ECMO is not safe after TAH implantation because of flow competition and of thrombosis risk.
CONCLUSION
We report a case of massive thrombus formation following systemic thrombolytic therapy which happened twice after the implantation of MCS. Various mechanisms may contribute to this severe complication (secondary multifactor hypercoagulability, sepsis, low flow), but attention should be paid to patients who receive MCS after systemic thrombolysis.
ACKNOWLEDGEMENTS
We thank Daniela Roefe and Lukasz Kizner, who contributed materials essential for the study.
Conflict of interest: none declared.
REFERENCES
- 1.Beiras-Fernandez A, Deutsch MA, Kainzinger S, Kaczmarek I, Sodian R, Ueberfuhr P, et al. Extracorporeal membrane oxygenation in 108 patients with low cardiac output—a single-center experience. Int J Artif Organs. 2011;34:365–73. doi: 10.5301/IJAO.2011.7727. [DOI] [PubMed] [Google Scholar]
- 2.Genovese EA, Dew MA, Teuteberg JJ, Simon MA, Kay J, Siegenthaler MP, et al. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann Thorac Surg. 2009;88:1162–70. doi: 10.1016/j.athoracsur.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doepp F, Sanad W, Schreiber SJ, Baumann G, Borges AC. Left ventricular apical thrombus after systemic thrombolysis with recombinant tissue plasminogen activator in a patient with acute ischemic stroke. Cardiovasc Ultrasound. 2005;3:14. doi: 10.1186/1476-7120-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leontiadis E, Koertke H, Bairaktaris A, Koerfer R. Thrombosis of the ascending aorta during mechanical circulatory support in a patient with cardiogenic shock. Interact CardioVasc Thorac Surg. 2010;11:510–1. doi: 10.1510/icvts.2010.240689. [DOI] [PubMed] [Google Scholar]
- 5.Paganelli F, Alessi MC, Morange P, Maixent JM, Levy S, Vague IJ. Relationship of plasminogen activator inhibitor-1 levels following thrombolytic therapy with rt-PA as compared to streptokinase and patency of infarct related coronary artery. Thromb Haemost. 1999;82:104–8. [PubMed] [Google Scholar]
- 6.Shpektor A. Cardiogenic shock: the role of inflammation. Acute Card Care. 2010;12:115–8. doi: 10.3109/17482941.2010.523705. [DOI] [PubMed] [Google Scholar]
- 7.Mavrommatis AC, Theodoridis T, Orfanidou A, Roussos C, Christopoulou-Kokkinou V, Zakynthinos S. Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit Care Med. 2000;28:451–7. doi: 10.1097/00003246-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto K, Yasuda S, Noguchi T, Tanimoto T, Kakuchi H, Morii I, et al. Fulminant myocarditis causing severe left heart failure and massive thrombus formation following cardiac tamponade: a case report. J Cardiol. 2005;46:25–31. [PubMed] [Google Scholar]