Abstract
The purpose of this study was to show the long-term outcome of induction chemoradiotherapy, using docetaxel and cisplatin with concurrent radiotherapy followed by surgery for non-small-cell lung cancer (NSCLC) with mediastinal nodal metastasis. Between January 2000 and July 2006, 22 consecutive NSCLC patients with pathologically proven mediastinal nodal metastasis were treated with tri-modality therapy. The regimen consisted of docetaxel and cisplatin plus concurrent radiation at a dose of 40–46 Gy. The induction therapy was followed by surgery 4–6 weeks later. The pulmonary resections were composed of a lobectomy in 19 patients, including 3 with a sleeve lobectomy, a bilobectomy in 2 patients and a left pneumonectomy in 1 patient. With a median follow-up duration of 8.7 years, the 3-year and 7-year overall survival (OS) rates for the entire population were 72.7 and 63.6%, respectively. Our results suggest that tri-modality therapy is promising for NSCLC patients with mediastinal nodal metastasis.
Keywords: Non-small-cell lung cancer, Induction chemoradiotherapy, N2
INTRODUCTION
Mediastinal lymph node metastasis is significantly associated with a poor outcome in non-small-cell lung cancer (NSCLC). The standard treatment of N2 or N3 disease with good performance status (PS) is concomitant chemoradiotherapy with or without consolidation chemotherapy [1]. On the other hand, although surgical resection after the induction therapy is not currently considered as an established standard approach, surgery after the induction therapy is mainly performed by experienced institutions worldwide.
Two recent, large, randomized phase III trials (the Lung Intergroup trial 0139 and the European Organization for Research and Treatment of Cancer (EORTC) trial 08941) investigated the prognostic impact of surgery on patients with pN2 stage IIIA [2, 3]. Although the study designs and patient populations of each study differed, the two studies failed to demonstrate a benefit from the addition of surgery in the entire population. However, in the subset analysis of the Lung Intergroup trial 0139 for patients who underwent a lobectomy versus a matched subset undergoing chemoradiotherapy, a significant difference in the 5-year survival rate was found [2]. This result strongly suggests the possible advantage of surgical resection after induction chemoradiotherapy for a select population of patients with N2 disease.
Various kinds of chemotherapeutic regimes have been reported for the first-line treatment in patients with advanced NSCLC. We reported the feasibility and favourable prognosis of concomitant chemoradiotherapy using docetaxel and cisplatin in patients with unresectable locally advanced NSCLC with moderate, but acceptable toxicities [4, 5]. Given the success of this regimen, we selected this treatment for induction chemoradiotherapy followed by surgery, and reported the feasibility of the treatment and promising outcomes in patients with locally advanced NSCLC [6]. Here, we present the long-term survival data of tri-modality therapy for NSCLC patients with mediastinal nodal metastasis.
MATERIALS AND METHODS
Patient selection and evaluation
Previously untreated NSCLC patients with pathologically confirmed mediastinal nodal metastasis were eligible for enrolment in the study. Patients with mediastinal lymph node longer than 10 mm along the short axis as viewed on a CT scan underwent a cervical mediastinoscopy to evaluate stations 2, 4 and 7. An anterior mediastinoscopy was combined when metastasis was suspected at stations 5 or 6 [7].
The inclusion criteria were age ≤75 years, with an Eastern Cooperative Oncology Group (ECOG) PS of 0–1 [8] and adequate functional reserves of major organs as described previously. Written informed consent was obtained from all patients. This protocol was approved and amended in 2000 by the Institutional Review Board/Ethical Committee of Okayama University. Disease stage was evaluated using chest radiography, enhanced chest and abdominal CT scans, including adrenal glands, enhanced brain magnetic resonance imaging (MRI) and radionuclide bone scan, or [18-fluoro-2-deoxyglucose positron emission tomography (PET-CT) scan] and bronchoscopy [7]. After completion of tri-modality treatment, chest and abdominal CT (or PET-CT) and enhanced brain MRI were repeated every 3 months at least 2 years after completion of the tri-modality therapy. Between 3 and 5 years after the completion, chest and abdominal CT (or PET-CT) and enhanced brain MRI were repeated every 6 months. After 5 years, chest X-ray was repeated every year, and further image analyses were conducted if necessary.
Treatment plan
Docetaxel (40 mg/m2) was administered intravenously on days 1 and 8 over 1 h followed by 1-h infusion of cisplatin (40 mg/m2) before the radiation therapy [6]. Chemotherapy was repeated at 3- or 4-week intervals. Chemotherapy dose and schedule modification were as reported previously [6]. Radiotherapy was started on the first day of chemotherapy, using a linear accelerator (6–10 MV). A total radiation dose of 46 Gy was planned, in principle, using a conventional fractionation regimen (2 Gy/day). The original volume included the site of the primary tumour and mediastinum, as described previously [6].
Following induction chemoradiotherapy, patients were evaluated for response based on a chest radiograph and CT scans. Patients without progressive disease (PD) were scheduled to undergo surgery within 6 weeks of completing the induction therapy. The surgical procedure was determined, based on the disease extent, before induction treatment. While a posterolateral thoracotomy was used as the basic approach, a median sternotomy was applied for patients with contralateral mediastinal lymph node metastasis or when great vessels, such as the main pulmonary artery, needed to be secured for a safe resection. A lobectomy with mediastinal lymph nodal dissection was basically the resection of first choice; however, a bilobectomy or pneumonectomy was performed in cases requiring these procedures because of disease extension. A sleeve resection was preferred to avoid a pneumonectomy, if appropriate. A complete ipsilateral superior mediastinal and subcarinal lymphadenectomy was performed in all the cases. For patients with primary lower lobe lesions, lymph nodes from stations 8 and 9 were also resected. Patients with primary left pulmonary lesions also underwent the resection of lymph node stations 5 and 6. A sharp resection with division of the azygous vein on the right-hand side and the Botallo ligament on the left-hand side was applied to enable a complete lymph node dissection in the mediastinum, since a ‘frozen’ mediastinum was sometimes observed because of chemoradiotherapy and pre-treatment mediastinoscopy. The bronchial stump was covered with the pericardial fat tissue or the pedicled intercostal muscle. When a sleeve resection was performed, the greater omentum was basically used to wrap the anastomosis. Postoperative adjuvant treatment was left to the physician's discretion.
Response, survival and toxicity assessments
Radiological response was basically assessed using the ECOG criteria with some modification and classified into four categories: complete response (CR), partial response (PR), stable disease (SD) and PD [6, 8]. The pathological response of the induction therapy was classified into three categories: pathological CR; pathological major response, pathological minor response [9].
The overall survival (OS) and the event-free survival (EFS) were calculated from the date of initiation of chemoradiotherapy until the date of death or the last follow-up for OS, and until confirmed death of any cause or recurrence at local or distant site for EFS. The survival curve was calculated by the Kaplan–Meier method, and difference between groups was compared with the log-rank test. Toxicity was assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0. All data were analysed using JMP® 9.0.0 Program for Windows (SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-sided and probability values <0.05 were defined as being statistically significant.
RESULTS
Patient characteristics and the induction therapy
Between January 2000 and July 2006, a total of 22 NSCLC patients with pathologically proven mediastinal lymph node metastasis underwent tri-modality treatment. The patient characteristics are shown in Table 1. Among the 22 patients, 7 completed the planned induction treatment. Administration of chemotherapeutic agents was modified due to toxicity in 15 patients. The total radiation dose was 40 Gy in three patients and 46 Gy in 19 patients. The radiological response to the induction therapy was PR in 8 patients (36.4%) and SD in 14 patients (63.6%). The toxicities experienced during the induction therapy are listed in Table 2. The toxicity was manageable using standard approaches.
Table 1:
Patient characteristics
| Characteristics | No. of patients |
|---|---|
| Median and range of age (years) | 60 (31–74) |
| Sex | |
| Male | 16 |
| Female | 6 |
| ECOG performance status | |
| 0 | 12 |
| 1 | 10 |
| Histological subtypes | |
| Squamous cell carcinoma | 11 |
| Adenocarcinoma | 9 |
| Adenosquamous carcinoma | 1 |
| Large cell carcinoma | 1 |
| c-Stage | |
| IIIA | 19 |
| T1N2M0 | 2 |
| T2N2M0 | 14 |
| T3N2M0 | 3 |
| IIIB | 3 |
| T2N3M0 | 1 |
| T4N2M0 | 2 |
| Lymph node status | |
| pN2 | 21 |
| pN3 | 1 |
Table 2:
Toxicity of induction chemoradiotherapy
| Toxicity | Grade of toxicity (n) |
% of Toxicities ≥grade 3 | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Leucopenia | 2 | 2 | 8 | 8 | 2 | 45.5 |
| Neutropenia | 3 | 2 | 6 | 5 | 6 | 50.0 |
| Anemia | 0 | 9 | 11 | 2 | 0 | 9.1 |
| Thrombocytopenia | 13 | 9 | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 2 | 8 | 8 | 2 | 2 | 18.2 |
| Diarrhoea | 6 | 15 | 1 | 0 | 0 | 0 |
| Constipation | 18 | 3 | 0 | 0 | 1 | 4.5 |
| Hepatic | 10 | 9 | 2 | 1 | 0 | 4.5 |
| Renal | 19 | 3 | 0 | 0 | 0 | 0 |
| Cardiac | 19 | 2 | 0 | 0 | 1 | 4.5 |
| Pulmonary | 0 | 4 | 1 | 1 | 0 | 4.5 |
| Oesophagitis | 7 | 2 | 11 | 2 | 0 | 9.1 |
| Allergy | 20 | 1 | 0 | 0 | 1 | 4.5 |
Toxicity was assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0.
Surgery and pathological response
The median time from the end of the induction therapy until surgery was 37 days (range: 30–59 days). The surgical procedures included a lobectomy in 16 patients, a sleeve lobectomy in 3 patients, a bilobectomy in 2 patients and a left pneumonectomy in 1 patient. Twenty-one patients had a complete tumour resection with microscopically negative margins and the complete resection rate was 95.4%.
The pathological responsiveness of the resected specimens was estimated. Three (13.6%) patients exhibited a complete pathological response, 13 (59.1%) exhibited a major pathological response and 6 (27.3%) exhibited a minor pathological response. Mediastinal lymph node downstaging was obtained in eight (36.4%) patients: seven patients to pN0 and one patient to pN1.
Postoperative complications
The major postoperative complication was pulmonary toxicity. Four patients experienced radiation pneumonitis (grade 2 in three patients, and grade 3 in one patient). These four patients were successfully treated with the steroid therapy. One patient had acute pneumonia. No treatment-related deaths occurred in this series.
Postoperative treatment
Of the 22 patients, nine received postoperative adjuvant chemotherapy: four patients received docetaxel and cisplatin; three received irinotecan and cisplatin; one received gemcitabine and cisplatin; one received gemcitabine and carboplatin.
Survival and pattern of relapse
At the final data analysis in July 2011, the median follow-up period was 8.7 years, ranging from 5.1 to 11.4 years. Fourteen patients (63.6%) were alive, including one with recurrent disease. Of the 21 patients who underwent a complete resection, disease relapse was observed in five patients as only distant, two patients as both distant and locoregional and one patient as only locoregional metastases. One patient with incomplete resection had dissemination.
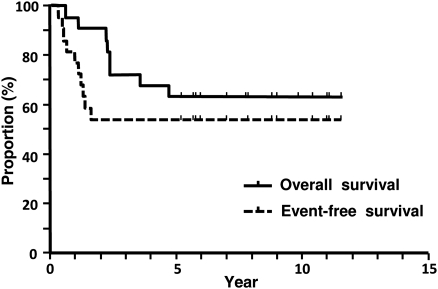
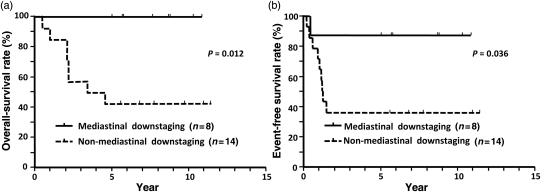
The survival curves for the entire population are shown in Fig. 1. The 3-, 5- and 7-year OS rates were 72.7, 63.6 and 63.6%, respectively. The 1- and 2-year EFS rates were 72.7 and 54.5%, respectively. Subset analyses for survival were conducted to identify factors that were related to a favourable prognosis. The results are summarized in Table 3. Mediastinal downstaging was identified as a favourable factor related to prolonged OS (P = 0.012) and EFS (P = 0.036) times (Fig. 2).
Figure 1:
Overall and event-free survival curves for 22 patients who were treated with tri-modality therapy.
Table 3:
OS and EFS rates according to clinicopathological factors
| Subsets (n) | OS (%) |
P-value | EFS (%) |
P-value | ||
|---|---|---|---|---|---|---|
| 3 years | 7 years | 1 year | 2 years | |||
| Sex | ||||||
| Male (16) | 68.6 | 62.5 | 0.71 | 75.0 | 56.3 | 0.77 |
| Female (6) | 83.3 | 66.7 | 66.7 | 50.0 | ||
| Histology | ||||||
| Non-adenocarcinoma (13) | 69.2 | 69.2 | 0.77 | 69.2 | 61.5 | 0.60 |
| Adenocarcinoma (9) | 77.8 | 55.6 | 77.8 | 44.4 | ||
| c-Stage | ||||||
| IIIA (19) | 73.7 | 68.4 | 0.20 | 63.2 | 57.9 | 0.69 |
| IIIB (3) | 33.3 | 33.3 | 66.7 | 33.3 | ||
| Radiological response | ||||||
| CR/PR (8) | 75.0 | 75.0 | 0.41 | 75.0 | 62.5 | 0.62 |
| SD (14) | 71.4 | 57.1 | 71.4 | 50.0 | ||
| Pathological response | ||||||
| Complete/major (16) | 75.0 | 75.0 | 0.12 | 81.3 | 68.9 | 0.026 |
| Minor (6) | 66.7 | 33.3 | 50.0 | 16.7 | ||
| Mediastinal downstaging | ||||||
| Downstaged (8) | 100 | 100 | 0.012 | 87.5 | 87.5 | 0.036 |
| Non-downstaged (14) | 57.1 | 42.9 | 57.1 | 35.7 | ||
OS: overall survival; EFS: event-free survival; CR: complete response; PR: partial response; SD: stable disease; P-value was calculated by log-rank test.
Figure 2:
Overall and event-free survival curves stratified by mediastinal downstaging. Overall (a) and event-free survival (b)
DISCUSSION
The rationale for induction treatment in patients with locally advanced disease is to facilitate a complete surgical resection by reducing the number of cancer cells in the primary tumour and metastatic regional nodes and to eradicate possible micrometastases [10], resulting in a high cure rate. Thus, a powerful treatment for local and distant sites is crucial. The majority of the reported tri-modality therapies used a combination of second-generation agents, including etoposide or vinblastine plus cisplatin [2, 11, 12]. The ECOG study showed that paclitaxel, a third-generation agent, plus cisplatin, was superior to etoposide plus cisplatin with regard to the outcomes of patients with advanced NSCLC [13]. Of note, Stupp et al. also reported the excellent prognosis (40% of the 5-year OS rate after a median follow-up of 58 months) of stage IIIB patients who were treated with docetaxel and cisplatin followed by accelerated radiotherapy (44Gy) and surgery [14].
Needless to say, surgery is also crucial for the tri-modality therapy. A significantly high incidence of postoperative complications after induction treatment has been reported, particularly, after a pneumonectomy [2]. In our series, we tried to avoid a pneumonectomy by using a sleeve resection whenever possible, as the safety of this procedure after the induction therapy has been confirmed despite the negative effect of chemoradiotherapy on the healing of the suture line. Among surgically related complications, bronchopleural and bronchopulmonary artery fistula are critical complications, sometimes resulting in treatment-related death. To minimize the risk of these bronchial fistulas, the bronchial suture lines were wrapped and separated from the pulmonary artery using the pericardial fat tissue, the pedicled intercostal muscle or the greater omentum in cases with a sleeve lobectomy.
To assist the proper interpretation of our study, limitations need to be considered. Some of the inherent limitations of our study are the small number of patients and the lack of a randomized design, suggesting that a significant selection bias may be present.
In conclusion, concomitant chemoradiotherapy using docetaxel and cisplatin followed by surgery is a feasible therapeutic option for NSCLC patients with mediastinal nodal metastasis.
ACKNOWLEGDEMENTS
We thank Kazuhiko Shien and Junichi Soh, Department of Thoracic Surgery, Okayama University Hospital and Kammei Rai, Department of Respiratory Medicine, Okayama University Hospital for collection of patient data.
Conflict of interest: K. Kiura and N. Takigawa had honorarium from Sanoji-Aventis. No other authors have conflict of interest to declare.
REFERENCES
- 1.Sekine I, Nokihara H, Sumi M, Saijo N, Nishiwaki Y, Ishikura S, et al. Docetaxel consolidation therapy following cisplatin, vinorelbine, and concurrent thoracic radiotherapy in patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2006;1:810–5. [PubMed] [Google Scholar]
- 2.Albain KS, Swann RS, Rusch VW, Turrisi AT, III, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–50. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 4.Kiura K, Ueoka H, Segawa Y, Tabata M, Kamei H, Takigawa N, et al. Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer. Br J Cancer. 2003;89:795–802. doi: 10.1038/sj.bjc.6601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol. 2010;28:3299–306. doi: 10.1200/JCO.2009.24.7577. [DOI] [PubMed] [Google Scholar]
- 6.Katayama H, Ueoka H, Kiura K, Tabata M, Kozuki T, Tanimoto M, et al. Preoperative concurrent chemoradiotherapy with cisplatin and docetaxel in patients with locally advanced non-small-cell lung cancer. Br J Cancer. 2004;90:979–84. doi: 10.1038/sj.bjc.6601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 8.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 9.Yokomise H, Gotoh M, Okamoto T, Yamamoto Y, Ishikawa S, Nakashima T, et al. Induction chemoradiotherapy (carboplatin-taxane and concurrent 50-Gy radiation) for bulky cN2, N3 non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007;133:1179–85. doi: 10.1016/j.jtcvs.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–92. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, III, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–92. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 12.Jaklitsch MT, Herndon JE, II, DeCamp MM, Jr, Richards WG, Kumar P, Krasna MJ, et al. Nodal downstaging predicts survival following induction chemotherapy for stage IIIA (N2) non-small cell lung cancer in CALGB protocol #8935. J Surg Oncol. 2006;94:599–606. doi: 10.1002/jso.20644. [DOI] [PubMed] [Google Scholar]
- 13.Bonomi P, Kim K, Fairclough D, Cella D, Kugler J, Rowinsky E, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–31. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 14.Stupp R, Mayer M, Kann R, Weder W, Zouhair A, Betticher DC, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol. 2009;10:785–93. doi: 10.1016/S1470-2045(09)70172-X. [DOI] [PubMed] [Google Scholar]