Abstract
A single institutional outcome of the biventricular repair for congenital heart disease with interrupted aortic arch between 1982 and 2010 were retrospectively reviewed. There were 48 consecutive patients with a mean follow-up of 10.0 ± 7.9 years. The staged repair was applied in 27 patients, and primary complete repair was applied in 21. The actuarial survival was 79.0% at 10 years. There was a significant difference in survival between the patients operated before 2000 and after 2001 (65.2 vs. 100% at 10 years, P = 0.005), but not in survival between the staged repair and the primary complete repair (77.4 vs. 81.0% at 10 years, P = 0.793). There was no significant difference in freedom from unplanned reoperation between the staged repair and the primary complete repair (47.9 vs. 70.6% at 5 years, P = 0.249). No patients with primary complete repair had reoperation for left ventricular outflow tract obstruction, whereas five patients with staged repair did. The patients with interposition graft placement between ascending and descending aorta had significantly low freedom from reoperation for the aortic arch compared with other techniques (7.2 vs. 90.0% at 10 years, P = 0.001). In conclusion, surgical outcomes for interrupted aortic arch have been significantly improved in the last decade and the staged repair remains an effective option in selected patients.
Keywords: Congenital heart disease, Aortic arch, Surgery
INTRODUCTION
Interrupted aortic arch (IAA) is a rare congenital heart disease and is often associated with other cardiovascular anomalies, including ventricular septal defect (VSD), truncus arteriosus, aorto-pulmonary window and various types of single ventricle [1]. In the presence of two ventricles, varying degrees of left ventricular outflow tract (LVOT) obstruction are often observed.
Historically, the staged repair was the preferred approach in most institutions before 1990s [2, 3]. As outcomes of neonatal cardiac surgery have been improved, primary complete repair of IAA and concomitant congenital heart disease in neonatal period were gradually applied in many institutions [4, 5], and in our institution in 2001. However, even with better surgical techniques for the neonates, additional structural anomalies or comorbidities remained as risk factors for worse outcomes. In particular, primary complete repair has not eliminated the need for subsequent reoperation for LVOT obstruction [1].
The purpose of this study was to review our experience of surgical repair for IAA.
MATERIALS AND METHODS
Study design
Between January 1982 and December 2010, all patients who had surgical repair for IAA with biventricular physiology were identified using the institutional cardiothoracic database at the Arkansas Children's Hospital. This study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences and the need for patient consent was waived due to its retrospective nature.
The medical records of each subject were reviewed with regard to demographic data, past medical history, primary and secondary diagnoses, intraoperative data, postoperative outcomes including immediate and late complications and unplanned reoperation. Follow-up data were obtained from the last clinic visit.
Statistical analysis
The endpoints examined in this study were reoperation and death. Continuous values were expressed as mean ± 1 SD for normally distributed variables and as median with range for non-normally distributed variables. Groups were compared with χ2-text and Student's t-test. Survival estimates were made with Kaplan–Meier method, and comparisons between survival distributions were made with log-rank test. All data were analysed using SPSS software, version 18.0.0 (SPSS, Inc., Chicago, IL, USA) and a P-value <0.05 was considered statistically significant.
RESULTS
Patient characteristics
The study cohort included 48 patients, and the patients' profile was summarized in Table 1. The intracardiac morphology included 29 patients (60%) with VSD and 19 (40%) with complex congenital cardiac defects (eight patients with truncus arteriosus, seven with severe subaortic and aortic stenosis which required surgical intervention, one with aorto-pulmonary window, one with common atrioventricular canal, one with transposition of the great arteries and one with right aortic arch and VSD).
Table 1:
Patients' profile
| All patients (n = 48) | Patients before 2000 (n = 29) | Patients after 2001 (n = 19) | P-value | Staged repair (n = 27) | Primary complete repair (n = 21) | P-value | |
|---|---|---|---|---|---|---|---|
| Male | 25 | 15 | 10 | 0.95 | 14 | 11 | 0.97 |
| Age at surgery (days) | 7 (2–250) | 6 (2–250) | 8 (3–27) | 0.57 | 6 (2–27) | 8 (3–250) | 0.29 |
| Body weight at surgery (kg) | 3.4 (1.8–4.3) | 3.2 (1.8–4.3) | 3.5 (2.1–4.2) | 0.09 | 3.2 (1.8–4.2) | 3.5 (2.6–4.3) | 0.003 |
| Aortic arch anatomy | |||||||
| Type A | 8 | 8 | 0 | 7 | 1 | ||
| Type B | 39 | 20 | 19 | 19 | 20 | ||
| Type C | 1 | 1 | 0 | 1 | 0 | ||
| Intracardiac anatomy | |||||||
| VSD | 29 | 18 | 11 | 17 | 12 | ||
| Complex | 19 | 11 | 8 | 0.77 | 10 | 9 | 0.28 |
| DiGeorge syndrome | 10 | 2 | 8 | 0.003 | 4 | 6 | 0.24 |
VSD: ventricular septal defect.
Operative intervention
The standard surgical strategy for IAA at our institution before 2000 was staged repair (29 patients), and it was switched to primary complete repair during the neonatal period after 2001 (19 patients).
Among the 29 patient who underwent initial surgery before 2000, 22 patients had expanded polytetrafluoroethylene (ePTFE) interposition graft placement between the ascending and descending aorta via left thoracotomy with or without pulmonary artery banding (PAB), 5 patients had primary complete repair (3 with VSD and 2 with truncus arteriosus) via median sternotomy, 1 had IAA repair by direct anastomosis with PAB via left thoracotomy and 1 had Blalock–Park procedure via left thoracotomy. Among 19 patients who underwent initial surgery after 2001, 16 patients had primary complete repair via median sternotomy and 3 patients had staged repair including IAA repair by direct anastomosis with PAB, ePTFE tube interposition graft with PAB and placement of a stent in the ductus arteriosus with bilateral PAB due to right side duct and descending aorta [6], complete atrioventricular canal and small size (2.1 kg) and preoperative necrotizing enterocolitis with acute renal failure and coagulopathy. As a result, staged repair was applied in 27 patients, and primary complete repair was applied in 21 patients. The details of the initial surgery were summarized in Table 2.
Table 2:
Initial surgery
| Patients before 2000 (n = 29) | Patients after 2001 (n = 19) | Staged repair (n = 27) | Primary complete repair (n = 21) | |
|---|---|---|---|---|
| Interposition graft with ePTFE graft | 22 | 1 | 23 | 0 |
| With PAB | 18 | 1 | 19 | 0 |
| Without PAB | 4 | 0 | 4 | 0 |
| Arch repair with PAB | 1 | 1 | 2 | 0 |
| Blalock–Park procedure | 1 | 0 | 1 | 0 |
| Bilateral PAB with ductal stent placement | 0 | 1 | 1 | 0 |
| IAA repair with VSD closure | 3 | 10 | 0 | 13 |
| IAA repair with complex cardiac repair | 2 | 6 | 0 | 8 |
IAA: interrupted aortic arch; PAB: pulmonary artery banding; ePTFE: expanded polytetrafluoroethylene; VSD: ventricular septal defect.
Among 21 patients with primary complete repair, 8 patients operated before 2003 had deep hypothermic circulatory arrest and 13 patients after 2004 had regional cerebral perfusion. In the presence of severe LVOT obstruction, the modified Yasui procedure was performed in four patients in staged or primary fashion [7].
Operative outcomes
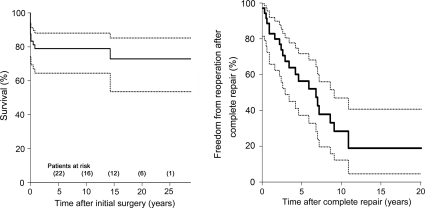
There were eight early deaths and three late deaths with a mean follow-up of 10.0 ± 7.9 years. All early deaths and two late deaths were cardiac-related and one late death was non-cardiac-related (lymphoma). Two patients were lost for follow-up shortly after the initial surgery. The actuarial survival for all patients was 79.0 and 72.9% at 10 and 20 years, respectively [10-year 95% confidence interval (CI): 67.4–90.6, Fig. 1]. A total of 40 unplanned reoperations with 45 procedures were required in 24 patients. The details of reoperation were shown in Table 3. Five patients who underwent staged repair before 2000 required five unplanned reoperations for LVOT obstruction, including two LVOT muscle resection, two Damus–Kaye–Stansel anastomosis and one Ross–Konno procedure. The actuarial freedom from unplanned reoperation among early survivors was 56.4 and 28.4% at 5 and 10 years (5-year 95% CI: 37.3–71.7, Fig. 1).
Figure 1:
Overall patient survival (left) and freedom from unplanned reoperation (right) after initial surgery. Solid lines indicate patient survival and freedom from reoperation and dashed lines indicates 95% CI
Table 3:
Reoperations
| All patients (n = 45) | Patients before 2000 (n = 33) | Patients after 2001 (n = 12) | Staged repair (n = 33) | Primary complete repair (n = 12) | |
|---|---|---|---|---|---|
| PAB adjustment | 3 | 3 | 0 | 3 | 0 |
| Arch repair | 18 | 15 | 3 | 16 | 2 |
| Surgery for LVOTO | 5 | 5 | 0 | 5 | 0 |
| RV-PA conduit replacement | 8 | 4 | 4 | 3 | 5 |
| Truncal valve surgery | 4 | 2 | 2 | 0 | 4 |
| Heart transplantation | 3 | 3 | 0 | 3 | 0 |
| RVOTO relief | 1 | 0 | 1 | 1 | 0 |
| Pacemaker placement | 1 | 0 | 1 | 0 | 1 |
| PA augmentation | 1 | 1 | 0 | 1 | 0 |
| Mitral valve surgery | 1 | 0 | 1 | 1 | 0 |
LVOTO: left ventricular outflow tract obstruction; PA: pulmonary artery; PAB: pulmonary artery banding; RVOTO: right ventricular outflow tract obstruction; RV-PA: right ventricle to pulmonary artery.
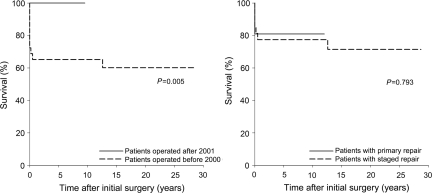
The survival estimate and free from reoperation were further analysed by dividing the patients into two groups with era (before 2000 vs. after 2001), surgical strategy (staged repair vs. primary complete repair) and intracardiac anatomy (VSD vs. complex intracardiac anomaly). There were significant differences in survival between the patients operated before 2000 and after 2001 (65.2 vs. 100% at 10 years, P = 0.005) and between the patients with VSD and with complex intracardiac anomaly (89.4 vs. 63.2% at 10 years, P = 0.006). However, there was no significant difference in survival between the patients with staged repair and with primary complete repair (77.4 vs. 81.0% at 10 years, P = 0.793, Fig. 2).
Figure 2:
Actuarial survival between the patients who were operated before 2000 and after 2001 (left) and between the patients with staged repair and primary complete repair (right)
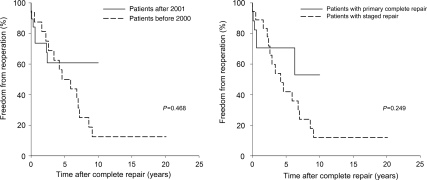
There was no significant difference in freedom from unplanned reoperation after the complete repair between the patients operated before 2000 and after 2001 (56.3 vs. 60.8% at 5 years, P = 0.468) and between the patients with staged repair and with primary complete repair (47.9 vs. 70.6% at 5 years, P = 0.249, Fig. 3). There was a significant difference in freedom from unplanned reoperation after the complete repair between the patients with VSD and with complex intracardiac anomaly (66.6 vs. 31.8% at 5 years, P = 0.049).
Figure 3:
Freedom from unplanned reoperation between the patients operated before 2000 and after 2001 (left) and between the patients with staged repair and primary complete repair (right)
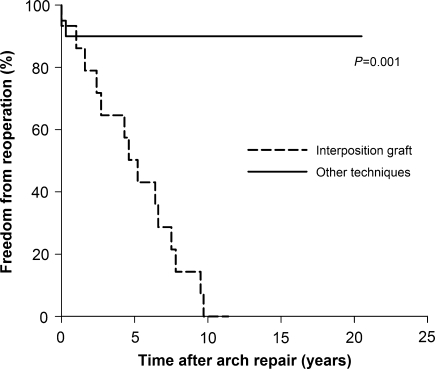
The freedom from aortic arch reoperation was compared between the patients with ePTFE interposition graft placement and other operative technique, which showed significant differences (7.2 vs. 90.0% at 10 years, P = 0.001, Fig. 4). Among 15 long-term survivors with ePTFE interposition graft, 14 had replacement of interposition graft to the larger size with the median interval of 4.9 years (0.3–11.4 years) after initial surgery (two had graft replacement twice).
Figure 4:
Freedom from reoperation for aortic arch between the patients with tube graft placement and other techniques
DISCUSSION
This single institutional review of surgical treatment of IAA demonstrated a remarkable improvement in early and late survival over the period of study, although reoperation remained a significant problem.
The standard surgical approach for IAA was switched from the staged repair until early 1990s [2, 3] to the primary complete repair in the current era [4, 5, 8, 9], even with the complex intracardiac anomaly [10, 11]. At our institution, neonatal primary complete repair was introduced in 2001, and the better early and late survival of the patients who had initial surgery after 2001 may, in part, be attributable to this change. This improvement is not likely due to an altered case mix since the characteristics of the patients in the two eras studied are similar in size and proportion. However, it must be conceded that since there have been no early or late deaths in our series since 1998, 3 years before our policy changed, there is evidence of general improvement in neonatal cardiac care independent of surgical strategy. Even if the change of the surgical strategy can be credited with some reduction in overall mortality, the change in management cannot eliminate the need for reoperation, which remains substantial. Among the patients who had initial surgery after 2001, seven patients (37%) ultimately required unplanned reoperation due to coexisting conditions (truncal valve pathology or right ventricle to pulmonary artery conduit obstruction). Only one patient with primary complete repair after 2001 required reoperation for recurrent arch obstruction, which is quite similar to report by Morales et al. [8] with a very low rate of recurrent arch obstruction after primary direct anastomosis.
LVOT obstruction has been a challenge for patients with IAA [1, 8, 12]. Among patients treated with staged repair in this series, two patients required intervention to LVOT obstruction at the time of complete repair and additional five required unplanned reoperation for LVOT obstruction. In contrast, among the patients with primary complete repair, no patient required reintervention on LVOT, although two patients had a modified Yasui procedure as the initial repair. Our policy has been not to intervene as long as the sub-aortic diameter in millimetres is larger than the patient weight in kilograms and there is no important valvar aortic stenosis. In our series, no patients had the technique including placing the upper portion of the VSD patch on the left ventricular side of the septum as described by Luciani et al. [13] to avoid LVOT obstruction.
One notable finding in this study and numerous other series is the occurrence of late death and necessity of cardiac transplantation. We speculate that some of the late deaths and late cardiomyopathy requiring transplantation may be attributable to the incomplete relief of arch obstruction, which was inherent in staged approach. The ePTFE interposition graft repair was frequently applied among the staged repair patients operated before 2000. However, it should be avoided unless absolutely necessary in the current era, due to the necessity of multiple reoperations and potential risk of incomplete relief of arch obstruction over time. Other left heart obstructive lesions such as congenital aortic stenosis and coarctation of the aorta have been demonstrated to carry significantly increased risk of premature death in late follow-up [14], and it is not unreasonable to suggest that IAA patients may be at similar or increased risk.
We have selectively employed staged repair in three patients with complicated medical conditions or particularly difficult anatomy after 2001. One of these patients had acute renal failure and shock at the time of anaesthesia induction at 10 days of age for planned primary complete repair. The patient was palliated by bilateral PAB, followed by ductal stent placement as described by Akintürk et al. [15]. Peritoneal dialysis was necessary for several weeks. Elective complete repair was accomplished at 7 months of age. In another small patient with a right-sided ductus and descending aorta, an ePTFE interposition graft and PAB were placed as the initial surgery, and complete repair was accomplished at 4 months of age.
Limitations of this study include its retrospective and non-randomized nature. It is possible that improved outcomes in the latter portion of the study are importantly related to general improvements in neonatal cardiac care. Furthermore, the study groups are relatively small, limiting the statistical power of any conclusions. An additional consideration is significantly shorter follow-up period for the patients who had initial surgery after 2001.
In conclusion, survival after surgical treatment of IAA has improved significantly. This study supports the current strategy of preferential primary complete repair of IAA and associated lesions in the neonatal period, although in selected patients of unusual complexity or in tenuous medical condition, staged management can produce similarly excellent results.
Conflict of interest: none declared.
References
- 1.McCrindle BW, Tchervenkov CI, Konstantinov IE, Williams WG, Neirotti RA, Jacobs ML Congenital Heart Surgeons Society. Risk factors associated with mortality and interventions in 472 neonates with interrupted aortic arch: a Congenital Heart Surgeons Society study. J Thorac Cardiovasc Surg. 2005;129:343–50. doi: 10.1016/j.jtcvs.2004.10.004. doi:10.1016/j.jtcvs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Sell JE, Jonas RA, Mayer JE, Blackstone EH, Kirklin JW, Castaneda AR. The results of a surgical program for interrupted aortic arch. J Thorac Cardiovasc Surg. 1988;96:864–77. [PubMed] [Google Scholar]
- 3.Brown JW, Ruzmetov M, Okada Y, Vijay P, Rodefeld MD, Turrentine MW. Outcomes in patients with interrupted aortic arch and associated anomalies: a 20-year experience. Eur J Cardiothorac Surg. 2006;29:666–73. doi: 10.1016/j.ejcts.2006.01.060. doi:10.1016/j.ejcts.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 4.Karl TR, Sano S, Brawn W, Mee RB. Repair of hypoplastic or interrupted aortic arch via sternotomy. J Thorac Cardiovasc Surg. 1992;104:688–95. [PubMed] [Google Scholar]
- 5.Oosterhof T, Azakie A, Freedom RM, Williams WG, McCrindle BW. Associated factors and trends in outcomes of interrupted aortic arch. Ann Thorac Surg. 2004;78:1696–702. doi: 10.1016/j.athoracsur.2004.05.035. doi:10.1016/j.athoracsur.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Roughneen PT, Wallach D, Ott DO. Interrupted right-sided aortic arch. Tex Heart Inst J. 1993;20:112–14. [PMC free article] [PubMed] [Google Scholar]
- 7.Yasui H, Kado H, Nakano E, Yonenaga K, Mitani A, Tomita Y, et al. Primary repair of interrupted aortic arch and severe aortic stenosis in neonates. J Thorac Cardiovasc Surg. 1987;93:539–45. [PubMed] [Google Scholar]
- 8.Morales DL, Scully PT, Braud BE, Booth JH, Graves DE, Heinle JS, et al. Interrupted aortic arch repair: aortic arch advancement without a patch minimizes arch reinterventions. Ann Thorac Surg. 2006;82:1577–83. doi: 10.1016/j.athoracsur.2006.05.105. doi:10.1016/j.athoracsur.2006.05.105. [DOI] [PubMed] [Google Scholar]
- 9.Tlaskal T, Vojtovic P, Reich O, Hucin B, Gebauer R, Kucera V. Improved results after the primary repair of interrupted aortic arch: impact of a new management protocol with isolated cerebral perfusion. Eur J Cardiothorac Surg. 2010;38:52–8. doi: 10.1016/j.ejcts.2010.01.052. doi:10.1016/j.ejcts.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Huber C, Mimic B, Oswal N, Sullivan I, Kostolny M, Elliott M, et al. Outcomes and re-interventions after one-stage repair of transposition of great arteries and aortic arch obstruction. Eur J Cardiothorac Surg. 2011;39:213–20. doi: 10.1016/j.ejcts.2010.05.009. doi:10.1016/j.ejcts.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Tlaskal T, Chaloupecky V, Hucin B, Gebauer R, Krupickova S, Reich O, et al. Long-term results after correction of persistent truncus arteriosus in 83 patients. Eur J Cardiothorac Surg. 2010;37:1278–84. doi: 10.1016/j.ejcts.2009.12.022. doi:10.1016/j.ejcts.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Ohye RG, Devaney EJ, Ishizaka T, Nathan PN, Goldberg CS, et al. Selective management of the left ventricular outflow tract for repair of interrupted aortic arch with ventricular septal defect: management of left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg. 2006;131:779–84. doi: 10.1016/j.jtcvs.2005.11.038. doi:10.1016/j.jtcvs.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Luciani GB, Ackerman RJ, Chang AC, Wells WJ, Starnes VA. One-stage repair of interrupted aortic arch, ventricular septal defect, and subaortic obstruction in the neonate: a novel approach. J Thorac Cardiovasc Surg. 1996;111:348–58. doi: 10.1016/s0022-5223(96)70444-0. doi:10.1016/S0022-5223(96)70444-0. [DOI] [PubMed] [Google Scholar]
- 14.Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32:245–51. doi: 10.1016/s0735-1097(98)00187-9. doi:10.1016/S0735-1097(98)00187-9. [DOI] [PubMed] [Google Scholar]
- 15.Akintürk H, Michel-Behnke I, Valeske K, Mueller M, Thul J, Bauer J, et al. Hybrid transcatheter-surgical palliation: basis for univentricular or biventricular repair: the Giessen experience. Pediatr Cardiol. 2007;28:79–87. doi: 10.1007/s00246-006-1444-7. doi:10.1007/s00246-006-1444-7. [DOI] [PubMed] [Google Scholar]