Abstract
Tricuspid regurgitation (TR) is common, but neglected. We evaluated the prognostic implications of TR in a cohort of 756 patients with severe aortic regurgitation (AR). A cohort of 756 patients with AR was identified from our echocardiographic database. Chart reviews were performed. Survival as a function of TR severity was analysed. Of the 756 patients with severe AR, 264 (35%) had ≥2+ TR. Univariate correlates of TR were older age (P < 0.0001), female gender (P < 0.0001), lower left ventricular ejection fraction (P < 0.0001), atrial fibrillation (P < 0.0001), presence of a pacemaker (P < 0.0001), higher PASP (P < 0.0001), presence of 3 or 4+ mitral regurgitation (P < 0.0001) and not being on a beta-blocker (P < 0.0001) or statins (P = 0.007). After adjusting for group differences, ≥2+ TR was an independent predictor of higher mortality (RR 1.47, P = 0.005). Aortic valve replacement (AVR) was independently associated with improved survival in patients with ≥2+ TR. (RR 0.46, 95% CI 0.36–0.60, P < 0.0001). In conclusion, in severe AR patients, ≥2+ TR is independently associated with a higher mortality. The performance of AVR in these patients with ≥2+ TR is associated with a survival benefit. Development of ≥2+ TR in these patients is a marker of decompensation and should serve as an indication for AVR.
Keywords: Tricuspid regurgitation, Aortic regurgitation, Survival, Aortic valve surgery
Tricuspid regurgitation (TR) is frequent in patients with valvular disorders, but relatively little attention is paid to it. It has been reported to confer worse prognosis in patients with mitral regurgitation (MR) [1]. But its frequency, risk factors and prognostic implications in those with severe aortic regurgitation (AR) are not known. We investigated the biological correlates and prognostic implications of TR in a large cohort of patients with severe AR.
METHODS
This is a retrospective cohort study from a large university medical centre. The study was approved by the institutional review board. Our echocardiographic database was searched for patients with severe AR in the period from 1993 to 2003. This yielded a total of 756 unique patients. Of these, 264 (35%) had ≥2+ TR assessed semiquantitatively based on the recommendations of the American Society of echocardiography [2]. Complete clinical, echocardiographic and pharmacological data were compiled on these patients from comprehensive chart review.
Various co-morbidities were defined as follows: hypertension as blood pressure ≥140/90 mmHg or a history of hypertension and being on antihypertensive medications, diabetes mellitus as fasting blood sugar >125 mg/dl or being on anti-diabetic agents, coronary artery disease (CAD) as presence of a history angina pectoris, myocardial infarction, a positive stress test, angiographic evidence CAD, coronary intervention, coronary artery bypass surgery or presence of significant Q-waves on the surface electrocardiogram.
All patients had standard two-dimensional echocardiographic examinations. The left ventricular ejection fraction (LVEF) was assessed visually by a level-3-trained echocardiographer and entered into a database at the time of the examination. This has been proven to be reliable and has been validated against contrast and radionuclide LV angiography [3, 4]. Anatomic and Doppler examinations and measurements were performed according to the recommendations of the American Society of Echocardiography [5]. Severe AR was diagnosed based on one or more criteria, including jet height to LV outflow tract diameter ratio ≥60%, AR vena contracta diameter >6 mm or prominent holodiastolic flow reversal in the aortic arch or abdominal aorta as judged by a level-3-trained echocardiographer [2]. TR was assessed semiquantitatively by examining the colour Doppler jet area, size of the vena contracta, morphology of the valve and any systolic flow reversal in the hepatic veins [2]. Based on these, TR was graded from 0 to 4, 4 being severe with a jet area >10 cm2 or vena contracta diameter >7 mm or systolic flow reversal in the hepatic vein [2]. Severe pulmonary hypertension was defined as a pulmonary artery systolic pressure of ≥60 mmHg.
Survival was analysed as a function of TR severity. Mortality data were obtained from the National Death Index using the social security numbers.
Stat View 5.01 (SAS Institute Inc., Cary, NC, USA) program was used for statistical analysis. Characteristics of patients with and without TR were compared using the Student t-test for continuous variables and chi-squared test for categorical variables. Kaplan–Meier survival curves were computed for patients as a function of TR, and survival curves were compared using the log-rank statistic. Cox proportional hazards models were employed to adjust for co-morbidities and covariate imbalances. A P-value of ≤0.05 was considered significant.
RESULTS
Patient characteristics
Of the 756 patients with severe AR, 190 (25%) had no TR, 302 (40%) had 1+ TR, 166 (22%) had 2+ TR, 66 (9%) had 3+ TR and 32 (4%) had 4+ TR. In other words, 264 (35%) patients had ≥2+ and 98 (13%) had 3+ or 4+ TR. Table 1 summarizes the characteristics of these patients as a function of degree of TR. Patients with ≥2+ TR were older (age 68 ± 16 vs. 58 ± 18 years, P < 0.0001), more likely to be women (55 vs. 23%, P < 0.0001), had a marginally lower LVEF (50 ± 20 vs. 56 ± 17%, P < 0.0001), lesser septal wall thickness (1.2 ± 0.2 vs. 1.3+0.2 cm, P = < 0.0001) and posterior wall thickness (1.1 ± 0.2 vs. 1.2+0.2 cm, P ≤ 0.0001), higher prevalence of atrial fibrillation (AF, 44 vs. 16%, P < 0.0001), greater prevalence of 3 or 4+ MR (45% vs. 15%, P < 0.0001) and a higher right ventricular systolic pressure (48 ± 14 vs. 38 ± 11 mmHg, P < 0.0001). They were also less likely to be on a statin or a beta-blocker and to undergo aortic valve surgery. Artificial pacemaker was present in 14% of those with ≥2+ TR compared with 5% in those with lesser TR (P < 0.0001).
Table 1:
Baseline patient characteristics
| Variables | <2+ TR (n = 492) | ≥2+ TR (n = 264) | P-value |
|---|---|---|---|
| Age (years) | 58+18 | 68+16 | <0.0001 |
| Males | 77% | 45% | <0.0001 |
| LV ejection fraction (%) | 56 ± 17 | 50 ± 20 | <0.0001 |
| AF | 16% | 44% | <0.0001 |
| Pacemaker | 5% | 14% | <0.0001 |
| Left bundle branch block (%) | 8% | 9% | 0.57 |
| Right bundle branch block (%) | 6% | 10% | 0.04 |
| CAD | 32% | 38% | 0.16 |
| Hypertension | 63% | 66% | 0.46 |
| Diabetes mellitus | 12% | 16% | 0.14 |
| Chronic renal insufficiency | 19% | 23% | 0.20 |
| Heart failure | 66% | 78% | 0.0007 |
| LV end-diastolic diameter (cm) | 5.8 ± 1.0 | 5.6 ± 1.1 | 0.07 |
| LV end-systolic diameter (cm) | 3.9 ± 1.1 | 3.9 ± 1.3 | 0.8 |
| LV posterior wall thickness (cm) | 1.2 ± 0.2 | 1.1 ± 0.2 | <0.0001 |
| Thickness of ventricular septum (cm) | 1.3 ± 0.2 | 1.2 ± 0.2 | <0.0001 |
| MR grade 3 or 4+ | 15% | 45% | <0.0001 |
| Mitral E/A velocity ratio | 1.2 ± 0.6 | 1.4 ± 0.7 | 0.02 |
| Mitral E-wave deceleration time (ms) | 209 ± 78 | 220 ± 105 | 0.20 |
| Chronic pulmonary disease | 11% | 14% | 0.19 |
| RV systolic pressure (mmHg) | 38 ± 11 | 48 ± 14 | <0.0001 |
| RV systolic pressure ≥60 mmHg | 8% | 25% | <0.0001 |
| Beta blocker therapy | 54% | 35% | <0.0001 |
| Statin therapy | 23% | 15% | 0.007 |
| Aspirin therapy | 38% | 39% | 0.9 |
| Aortic valve replacement | 46% | 26% | 0.0001 |
LV, left ventricular; RV, right ventricular.
The aetiology of AR included bicuspid in 78, aortic root dilatation in 79, degenerative in 219, infective endocarditis in 77, mixed in 134 and unknown in 151. Over a mean period of 4.4 years, there were 321 deaths in the entire cohort of 756 patients, 107 in those with ≥2+ TR. Of those with ≥2+ TR, 67 (26%) patients underwent aortic valve replacement (AVR) compared with 46% in those with lesser degrees on TR (P = 0.0001).
Analysis of predictors of TR
Logistic regression analysis was carried out to look at the predictors of ≥2+ TR. The strongest independent predictor was the level of pulmonary artery pressure (chi squared 42, P < 0.0001), followed by AF (chi squared 18, P < 0.0001), female gender (chi squared 16.8, P < 0.00010) and age (chi squared 9.2, P = 0.002).
Effect of TR on survival in patients with severe AR
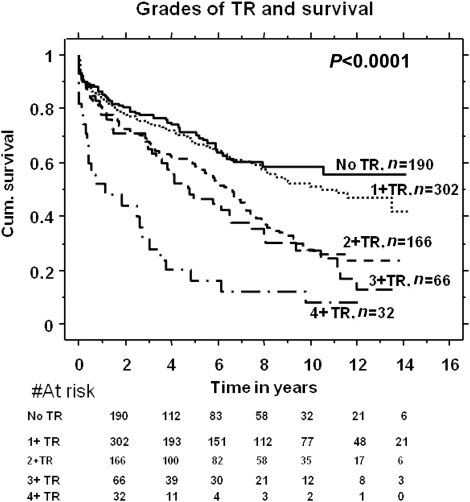
There was a graded increase in mortality with increasing TR severity as shown in Fig. 1, the best survival in those with 0–1+ TR and worst in those with 4+ TR. Patients with ≥2+ TR had worse survival compared with those with <2+TR. One- and 5-year survivals in those with ≥2+ TR were 80 and 50%, respectively, compared with 90 and 70% in patients with <2+ TR (P < 0.0001) (Fig. 2).
Figure 1:
Survival in different grades of TR in patients with severe aortic regurgitation (AR).
Figure 2:
Survival in TR grades <2 vs. ≥2 in patients with severe AR. This is compared with expected survival in age-matched US population.
Survival impact of TR in patients with severe AR stratified by the AF status
A total of 193 patients had AF. In patients with AF, patients with ≥2+ TR (n = 116) had worse survival compared with those without (n = 77), 88 and 50% survival at 1 and 5 years vs. 90 and 75%, respectively (P = 0.03). The presence of TR seemed to have a greater impact on mortality in those with AF.
Survival stratified by gender
Effect of TR on survival was seen in both genders to a similar degree. Female patients with ≥2+ TR had worse survival (n = 145) compared with those without (n = 62), 82 and 54% survival at 1 and 5 years vs. 90 and 72%, respectively (P = 0.009). Similarly, male patients with ≥2+ TR had worse survival (n = 117) compared with those without (n = 328), 78 and 42% survival at 1 and 5 years vs. 85 and 70%, respectively (P < 0.0001).
Survival as a function of CAD
In patients with no CAD, ≥2+ TR (n = 167) was associated with worse survival, 80 and 60% at 1 and 5 years vs. 90 and 78% in those without (n = 335) (P = < 0.001). In patients with CAD, 1- and 5-year survival in those with ≥2+ TR (n = 97) was 82 and 42% compared with 82 and 62% in those without (n = 156) (P = 0.001).
Survival excluding severe MR
Excluding the patients with severe MR, ≥2 TR (n = 134) was associated with 5- and 10-year survival of 68 and 30%, respectively, compared with 80 and 70% in patients without ≥2+ TR (n = 373) (P < 0.0001).
Survival after excluding infective endocarditis or pacemakers
The overall impact of TR on survival remained unchanged excluding these patient subgroups.
Cox proportional hazards models
TR was an independent predictor of higher mortality (RR 1.5, 95% CI. 1.1–1.9, P = 0.005) after adjusting for group differences.
Univariate predictors of survival in those with severe AR and >2± TR
The significant univariate predictors of higher mortality in the whole cohort of patients with severe AR and TR ≥2+ included greater age (P < 0.0001), a larger LV end-diastolic dimension (P = 0.0006), female gender (P ≤ 0.0001), AF (P < 0.0001), EF (<0.0001), not on statin therapy (0.007) and severe pulmonary hypertension (P ≤ 0.0001). The univariate predictors of better survival included beta-blocker therapy (P = 0.0001) and performance of AVR (P < 0.0001).
Effect of AVR in patients with ≥2+ TR and severe AR
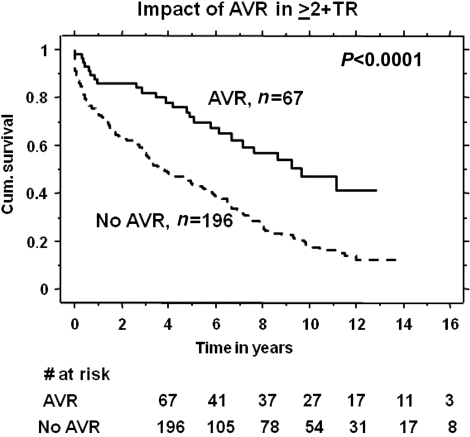
In patients with ≥2+ TR, AVR (n = 67) was associated with a better survival, 90% and 78% at 1 and 5 years, respectively, compared with 78 and 42%, respectively, in those who did not undergo surgery (n = 196) (P < 0.0001) as shown in Fig. 3. This remained significant after adjusting for group differences between AVR and non-AVR groups using the Cox regression model (RR 0.46, 95% CI 0.36–0.60, P < 0.0001). The 30-day mortality in the surgical arm was (1 out of 67). A total of 40 (15%) had mechanical valves and 26 (11%) had bioprosthetic valves, and the choice of valve type did not correlate with survival. Propensity score analysis was performed by calculating probability for AVR (propensity score) in each of the patients using logistic regression analysis. AVR remained an independent predictor of better survival after correcting for the propensity score (RR 0.45, 95% CI 0.27 −0.70, P = 0.002). We also performed time-varying Cox regression analysis to account for time to surgery from the index echocardiogram. AVR remained a significant predictor of better survival (P < 0.0001).
Figure 3:
Effect of AVR in patients with ≥2+ TR and severe AR.
DISCUSSION
The main findings of our study are that TR is common in patients with severe AR and that it negatively impacts survival in these patients and that performance of aortic valve surgery in these patients is associated with a potential survival benefit. In addition, our data indicate that the main cause of TR is development of pulmonary hypertension and gives insights into the risk factors for the genesis of TR. These novel findings have important clinical implications.
Frequency and prognostic importance of TR
Singh et al. [6] have reported on the prevalence and determinants of TR of greater than mild severity in the community. In the Framingham study, it was seen 15% in males and 18% in females. The correlates of more than mild TR were older age, higher body mass index and female gender. It has been reported that patients with mitral stenosis associated with moderate or severe TR prior are more likely to experience heart failure compared with patients with mild TR (56 vs. 14%) [7]. TR requiring surgery also predicts poor outcome in patients undergoing valve surgery [8, 9]. Our finding that TR has a significant impact on survival in severe AR patients is new.
Pathogenesis of TR in AR
Our study gives important insights into the mechanism of TR in AR. Our data suggest that the main determinant of TR is pulmonary hypertension. The correlates of TR in AR have not been described till date. Pulmonary hypertension is likely secondary to left atrial hypertension as evidenced by a higher E/A ratio and greater prevalence of 3 or 4+ MR in the ≥2+ TR group. Pulmonary hypertension caused by regurgitation of the mitral valve and perhaps contributed to by LV function and left atrial compliance has been described [10–14]. Increased left atrial size and pressure might also cause AF, which in turn leads to right atrial dilation causing tricuspid annular dilatation [15]. AF has been known to be a risk factor for the development of TR in patients with MV disease or for the persistence of TR after MV surgery. Our patients with greater degrees of TR had higher grades of MR, indicators of higher left atrial pressures, AF and higher pulmonary artery pressures in line with these reports.
Study limitations
This is a retrospective observational study. There were covariate imbalances between the two groups. Adjustments for these were made using the Cox regression model. The performance of AVR was not randomized with inevitable selection bias. A prospective randomized study addressing the management strategies including the question of tricuspid valve repair would be needed for a clearer answer on the management of these patients.
Conclusions and clinical implications
Our study indicates that TR is prognostically important in patients with severe AR and its main determinant is development of pulmonary hypertension. AF and MR may play a role in the genesis of TR. It would be prudent to evaluate for any signs of left heart failure or pulmonary hypertension in severe AR patients in order to time the performance of aortic valve surgery. The presence of TR should not be a deterrent to AVR in view of a large survival benefit with AVR.
Conflict of interest: none declared.
REFERENCES
- 1.Varadarajan P, Pai RG. Tricuspid regurgitation in patients with severe mitral regurgitation and normal left ventricular ejection fraction: risk factors and prognostic implications in a cohort of 895 patients. J Heart Valve Dis. 2010;19:412–9. [PubMed] [Google Scholar]
- 2.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 3.Van Royen N, Jaffe CC, Krumholz HM. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–50. doi: 10.1016/s0002-9149(97)89179-5. [DOI] [PubMed] [Google Scholar]
- 4.Amico AF, Lictenberg GS, Reisner SA, Stone CK, Schwartz RG, Meltzer RS. Superiority of visual versus. computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J. 1989;118:1259–65. doi: 10.1016/0002-8703(89)90018-5. [DOI] [PubMed] [Google Scholar]
- 5.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 6.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 7.Boyaci A, Gokce V, Topaloglu S, Korkmaz S, Goksel S. Outcome of significant functional tricuspid regurgitation late after mitral valve replacement for predominant rheumatic mitral stenosis. Angiology. 2007;58:336–42. doi: 10.1177/0003319707302495. [DOI] [PubMed] [Google Scholar]
- 8.Roques F, Nashef SA, Michel P. Risk factors for early mortality after valve surgery in Europe in the 1990s: lessons from the Euroscore pilot program. J Heart Valve Dis. 2001;10:572–7. [PubMed] [Google Scholar]
- 9.Turina J, Stark T, Seifert B, Turina M. Predictors of long term outcome after combined aortic and mitral valve surgery. Circulation. 1999;100:II48–II53. doi: 10.1161/01.cir.100.suppl_2.ii-48. [DOI] [PubMed] [Google Scholar]
- 10.Roberts WC, Eways EA. Clinical and anatomic observations in patients having mitral valve replacement. Am J Cardiol. 1991;68:1107–111. doi: 10.1016/0002-9149(91)90509-j. [DOI] [PubMed] [Google Scholar]
- 11.Ubago JL, Figueroa A, Ochoteco A, Colman T, Duran RM, Duran CG. Analysis of the amount of tricuspid annular dilation required to produce functional tricuspid regurgitation. Am J Cardiol. 1983;52:155–8. doi: 10.1016/0002-9149(83)90087-5. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto T, Okada M, Ozaki N, Hatakeyama T, Kawahira T. Long term evaluation of treatment for functional tricuspid regurgitation with regurgitant volume: characteristic differences based on primary cardiac lesion. J Thorac Cardiovasc Surg. 1999;117:463–71. doi: 10.1016/s0022-5223(99)70325-9. [DOI] [PubMed] [Google Scholar]
- 13.Sagie A, Schwammenthal E, Padial LR, Vasquez de Prada JA, Weyman AE, Levine RA. Determinants of functional tricuspid regurgitation in incomplete tricuspid closure: Doppler color flow study in 109 patients. J Am Coll Cardiol. 1994;24:446–53. doi: 10.1016/0735-1097(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda S, Song JM, Gillinov AM, McCarthy PM, Daimon M, Kongsaerepong V, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005;111:975–9. doi: 10.1161/01.CIR.0000156449.49998.51. [DOI] [PubMed] [Google Scholar]
- 15.Matsuyama K, Matsumoto M, Sugita T, Nishizawa J, Tokuda Y, Matsu T. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg. 2003;75:1826–8. doi: 10.1016/s0003-4975(03)00028-6. [DOI] [PubMed] [Google Scholar]