Abstract
The objective of this study was to identify and evaluate predictors of postoperative atrial fibrillation (POAF) in a large coronary artery bypass grafting (CABG) cohort. This was a single centre study of 7115 consecutive patients with preoperative sinus rhythm who underwent isolated CABG between January 1996 and December 2009. Independent risk factors for POAF were identified with multiple logistic regression. The predictive quality of the final model was evaluated by comparing predicted and observed events of POAF, in an effort to find patients at high risk of developing POAF. After CABG, 2270 patients (32%) developed POAF during hospital stay. Independent risk factors of POAF included advancing age (odds ratio, OR 2.0–7.3), preoperative S-creatinine ≥150 µmol/l (OR 1.6), male gender (OR 1.2), New York Heart Association class III/IV (OR, 1.2), smoking (OR 1.1), prior myocardial infarction (OR 1.1) and absence of hyperlipidemia (OR 0.9). The final prediction model was moderate (area under curve, 0.62; 95% confidence interval, 0.61–0.64). Patients with POAF had more postoperative complications, including a higher incidence of stroke and increased length of hospital stay. In conclusion, several risk factors for POAF were identified, but the moderate value of the prediction model confirms the difficulty of identifying patients at high risk of developing POAF after CABG.
Keywords: Postoperative atrial fibrillation, Coronary artery bypass grafting, Ageing
INTRODUCTION
Postoperative atrial fibrillation (POAF) remains the most common complication after cardiac surgery and affects approximately one-third of all patients undergoing coronary artery bypass grafting (CABG) [1–5]. The identification of risk factors of developing POAF has produced inconsistent results, with the exception of increasing age [1–3, 6, 7] and the prediction quality has seldom been assessed. A clinically useful prediction model for POAF in CABG patients is still lacking.
If patients at high risk of developing POAF could be identified, prophylactic efforts would be more focused. With targeted treatment with, for example, anti-arrhythmic medication or atrial pacing possible side effects could be avoided for patients not likely to benefit from this treatment.
Previous studies have proposed an association between POAF and prolonged hospitalization [1, 2, 4–8] and increased postoperative morbidity [1, 2, 4, 6, 8] and mortality [1, 2, 4–6, 8] after cardiac surgery; however, further knowledge is required.
The aim of this study was to identify predictors of POAF through preoperative and surgical variables in a large CABG cohort. We further wanted to evaluate the predictive quality of the final model by comparing predicted and observed events of POAF, in an effort to find patients at high risk of developing POAF.
MATERIALS AND METHODS
Patients
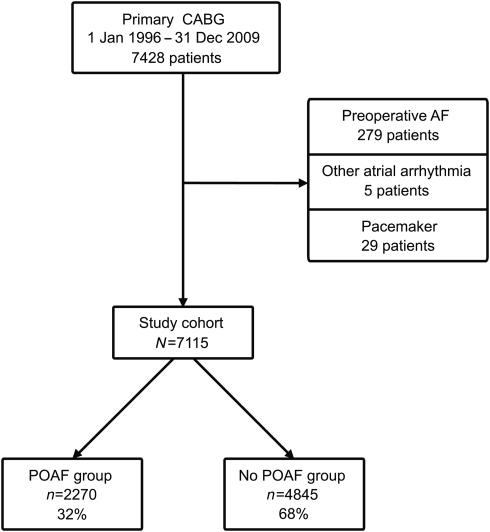
Between January 1996 and December 2009, 7428 consecutive patients underwent primary isolated CABG at the Department of Cardiothoracic Surgery, Uppsala University Hospital, Uppsala, Sweden. All patients with preoperative sinus rhythm were included in the study, including patients with a history of atrial fibrillation (AF). Patients with AF, other atrial arrhythmia or permanent pacemaker at admission for surgery were excluded. The final study cohort included 7115 patients (Fig. 1).
Figure 1:
Study design. AF: atrial fibrillation; CABG: coronary artery bypass grafting; POAF: postoperative atrial fibrillation.
No particular AF prophylaxis was used during the study period. Patients underwent CABG either with cardiopulmonary bypass (CPB) according to the departmental clinical routines, with moderate hypothermia (32–34°C) or without CPB (off-pump surgery).
Patients were monitored by five-lead telemetry until the third postoperative day, longer if any arrhythmia was detected. On admission and on postoperative day 4, all patients had a twelve-lead electrocardiogram (ECG). Patients who developed POAF were treated medically, typically with amiodarone or sotalol, and/or cardioversion.
Data collection and postoperative complications
All data were prospectively registered in the department's database. The definition of POAF was an episode of AF ≥30 s recorded postoperatively on continuous telemetry or a twelve-lead ECG.
Hyperlipidemia was defined as preoperative treatment with statins or levels of total cholesterol and low-density lipoprotein (LDL) exceeding criteria for treatment according to guidelines. Sub-classification of New York Heart Association (NYHA) functional classification into IIIA and IIIB was used: IIIA included patients who experienced slight discomfort during normal activities, but able to walk a mile on a flat surface at their own speed and able to climb stairs slowly without undue discomfort [9]. Left ventricular ejection fraction (LVEF) was classified as ‘good’ (>50%), ‘reduced’ (30–50%) or ‘poor’ (<30%), based on preoperative echocardiography or left ventricular angiography. Haemodynamics was classified as unstable if inotropic drugs were required to sustain circulation.
The database did not include information about medical treatment including β-blockers, and we therefore gathered this information from 100 random patient charts, half of them with POAF and half without POAF. Ninety-eight out of 100 patients received treatment with β-blockers. They were reinstated the day after surgery if permitted by the clinical status of the patient. Other anti-arrhythmic medication was rarely used. Since there was no difference relating to prediction of POAF, no further attempts of adjustment were made.
Re-operation included reopening of the sternum for any reason, including bleeding. Stroke was defined as severe neurological symptoms lasting 6 h or more and verified by computer tomography. Postoperative heart failure was a continuous need for inotropic drugs to sustain circulation, a need for an intra-aortic balloon pump, or death due to heart or multiple organ failure during hospital stay. Decreased postoperative renal function included dialysis or a 50% increase in serum creatinine (µmol/l), compared with the preoperative value. Perioperative myocardial injury was a plasma creatine kinase-myocardial band value of >50 µg/l on the first postoperative day.
Length of stay (LOS) was the number of days spent in the intensive care unit (ICU) and the cardiothoracic hospital ward during initial hospitalization after surgery. In-hospital mortality was defined as death from any cause during LOS.
Statistical methods
The incidence of POAF with respect to preoperative and surgical variables was compared by logistic regression. A multiple logistic regression model was created by including variables with a P-value of 0.1 or less in univariate analyses and/or of clinical interest (hypertension, aortic cross-clamp (ACC) and CPB time), in order to select the variables best predicting the incidence of POAF. The variables were evaluated for linearity and categorized for nonlinearity and used in the format that provided the best predictive value. Results of the logistic regression analyses are presented as odds ratio (OR) and 95% confidence intervals (CI).
In order to assess potential confounding due to association between LOS and other risk factors, we performed a logistic regression analyses conditioning on LOS (≤6, 7, 8, >8 days). This model resulted in essentially unchanged estimates. We also assessed the multiplicativity between risk factors by testing all two-factor interactions between the variables in the final model. These analyses showed a significant interaction (P < 0.05 after Bonferroni correction of multiple testing) between smoking and NYHA class IIIB/IV. The nature of this interaction suggests that the effect of smoking is confined to patients with a lower NYHA class than IIIB.
It could be expected, based on clinical experience and similar studies, that the incidence of POAF would be increased in patients with one or several other postoperative complications. Therefore, the robustness of the model was checked by a risk factor analysis of POAF where all patients with other complications (heart failure, intra-aortic balloon pump, re-operation, infection, haemodynamic instability and need for inotropic drugs) were excluded.
The calculated risk of developing POAF from the multiple logistic regression model was compared with the observed outcome with the area under the receiver operating characteristics (ROC) curve (AUC).
The incidence of postoperative complications was compared using the χ2-test. LOS and ICU stay were compared using the Wilcoxon–Mann–Whitney two-sample rank-sum test, with results presented as median and interquartile range. A P-value of 0.05 or less was considered statistically significant.
The SAS statistical software (version 9.2, SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses and data processing.
All authors had full access to the data and take responsibility for its integrity, and have read and agreed to the manuscript as written. The study was approved by the local Ethics Review Board.
RESULTS
The mean age was 66.5 years (range 33–86 years) and men comprised the majority (77%). Most patients (90%) were NYHA functional class III or IV, half (51%) had hypertension and one in five (21%) suffered from diabetes. Of the 7115 patients enrolled in the study, 2270 patients (32%) developed POAF during hospital stay (Fig. 1): preoperative and surgical variables by occurrence of POAF are presented in Tables 1 and 2.
Table 1:
Preoperative data of CABG patients by postoperative atrial fibrillation
| Preoperative variable | Total n = 7115 | POAF n = 2270 (32%) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| n (%)a | n (%)b | OR | 95% CI | OR | 95% CI | |
| Age (years) | ||||||
| ≤50 | 315 (4.4) | 34 (11) | Ref. | – | Ref. | – |
| 51–60 | 1358 (19) | 271 (20) | 2.0 | 1.4–3.0 | 2.0 | 1.4–3.0 |
| 61–70 | 2622 (37) | 819 (31) | 3.7 | 2.6–5.3 | 3.8 | 2.6–5.4 |
| 71–80 | 2598 (37) | 1040 (40) | 5.4 | 3.7–7.8 | 5.5 | 3.8–8.0 |
| >80 | 222 (3.1) | 106 (48) | 7.4 | 4.7–11.5 | 7.3 | 4.7–11.4 |
| Male gender | 5496 (77) | 1783 (32) | 1.1 | 1.0–1.3 | 1.2 | 1.1–1.4 |
| Current smoker | 833 (12) | 195 (23) | 1.1 | 1.0–1.2 | 1.1 | 1.0–1.3 |
| Prior MI | 3795 (54) | 1270 (34) | 1.2 | 1.1–1.3 | 1.1 | 1.0–1.2 |
| Hypertension | 3623 (51) | 1179 (33) | 1.1 | 1.0–1.2 | – | – |
| Diabetes | 1518 (21) | 473 (31) | 1.1 | 0.8–1.1 | – | – |
| Hyperlipidemia | 3822 (54) | 1141 (30) | 0.8 | 0.7–0.9 | 0.9 | 0.8–1.0 |
| S-creatinine ≥150 µmol/l | 227 (3.2) | 98 (43) | 1.6 | 1.3–2.1 | 1.6 | 1.2–2.1 |
| NYHA class | ||||||
| I/II | 684 (9.6) | 183 (27) | Ref. | – | Ref. | – |
| IIIA | 2806 (39) | 877 (31) | 1.3 | 1.1–1.6c | 1.2 | 1.0–1.4c |
| IIIB | 2977 (42) | 986 (33) | 1.4 | – | – | – |
| IV | 646 (9.1) | 223 (35) | 1.4 | – | – | – |
| LVEF | ||||||
| Good (>50%) | 4899 (69) | 1548 (32) | Ref. | – | – | – |
| Reduced (30–50%) | 1894 (27) | 612 (32) | 1.0 | 0.9–1.2 | – | – |
| Poor (<30%) | 322 (4.5) | 110 (34) | 1.1 | 0.9–1.4 | – | – |
| Unstable angina | 2336 (33) | 795 (34) | 1.2 | 1.0–1.3 | – | – |
| CCS class | ||||||
| 0 | 152 (2.1) | 42 (28) | 0.8 | 0.6–1.2 | – | – |
| 1 | 425 (6.0) | 126 (30) | 1.0 | 0.8–1.2 | – | – |
| 2 | 1780 (25) | 542 (30) | Ref. | – | – | – |
| 3 | 3768 (53) | 1225 (33) | 1.0 | 0.9–1.2 | – | – |
| 4 | 951 (13) | 328 (34) | 1.1 | 0.9–1.3 | – | – |
| Number of diseased vessels | ||||||
| 1 | 329 (4.6) | 97 (29) | Ref. | – | – | – |
| 2 | 1460 (21) | 455 (31) | 1.1 | 0.8–1.4 | – | – |
| 3 | 5326 (75) | 1718 (32) | 1.1 | 0.9–1.5 | – | – |
| Left main stem stenosis | 2342 (33) | 771 (33) | 1.1 | 1.0–1.2 | – | – |
| Priority of surgery | ||||||
| Elective | 5870 (83) | 1850 (32) | Ref. | – | – | – |
| Urgent | 955 (13) | 317 (33) | 1.1 | 0.9–1.2 | – | – |
| Emergency | 290 (4.1) | 103 (36) | 1.2 | 0.9–1.5 | – | – |
| Haemodynamically unstable | 180 (2.5) | 71 (40) | 1.4 | 1.0–1.9 | – | – |
aPercentage of patients.
bPercentage with POAF. cNYHA class III/IV.
CABG: coronary artery bypass grafting; CCS: Canadian Cardiovascular Society; CI: confidence interval; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; OR: odds ratio; POAF: postoperative atrial fibrillation.
Table 2:
Surgical data of CABG patients by postoperative atrial fibrillation
| Preoperative variable | Total n = 7115 | POAF n = 2270 | Univariate |
|
|---|---|---|---|---|
| n (%)a | n (%)b | OR | 95% CI | |
| Off-pump surgery (no CPB) | 251 (3.5) | 71 (28) | 0.8 | 0.6–1.1 |
| CPB time (min) | ||||
| <60 | 1248 (18) | 361 (29) | Ref. | – |
| 60–89 | 3373 (47) | 1070 (32) | 1.1 | 1.0–1.3 |
| 90–119 | 1848 (26) | 619 (34) | 1.2 | 1.1–1.4 |
| ≥120 | 646 (9.1) | 220 (33) | 1.3 | 1.0–1.6 |
| Aortic cross-clamp time (min) | ||||
| <30 | 1184 (17) | 349 (30) | Ref. | – |
| 30–59 | 4957 (70) | 1582 (32) | 1.1 | 1.0–1.3 |
| 60–89 | 864 (12) | 299 (35) | 1.3 | 1.0–1.5 |
| ≥90 | 110 (1.6) | 40 (36) | 1.4 | 0.9–2.1 |
| Blood cardioplegia | 1426 (20) | 463 (33) | 1.0 | 0.9–1.2 |
| Use of IMA | 6557 (92) | 2096 (32) | 1.0 | 0.9–1.2 |
| Distal anastomoses (n) | ||||
| 1 | 223 (3.1) | 60 (27) | Ref. | – |
| 2 | 1021 (14) | 307 (30) | 1.2 | 0.9–1.6 |
| 3 | 3115 (44) | 969 (31) | 1.2 | 0.9–1.7 |
| ≥4 | 2755 (39) | 934 (34) | 1.4 | 1.0–1.9 |
aPercentage of patients.
bPercentage with POAF.
None of the surgical variables were independent predictors of POAF in Multivariate analyses. CABG: coronary artery bypass grafting; CI: confidence interval; CPB: cardiopulmonary bypass; IMA: internal mammary artery; OR: odds ratio; POAF: postoperative atrial fibrillation.
Patients in the POAF group had a higher frequency of some other postoperative complications than the no-POAF group (Table 3). There was no difference in the rate of perioperative myocardial injury or in-hospital mortality between the two groups, with a total in-hospital mortality rate of 1.9%.
Table 3:
Complications after CABG by postoperative atrial fibrillation
| Postoperative variable | POAF (n = 2270) | No POAF (n = 4845) | P-value |
|---|---|---|---|
| Re-operation | 7.1 | 5.0 | <0.001 |
| Haemodynamically unstable | 15 | 8.7 | <0.001 |
| IABP | 1.3 | 1.0 | 0.280 |
| Decreased renal function | 11 | 5.9 | <0.001 |
| Perioperative myocardial injury | 9.5 | 10 | 0.523 |
| Infection | 7.3 | 5.0 | <0.001 |
| Respiratory complication | 4.4 | 2.3 | <0.001 |
| Stroke | 4.1 | 2.3 | <0.001 |
| Heart failure | 8.9 | 5.2 | <0.001 |
| Permanent PM | 0.8 | 0.7 | 0.674 |
| ICU stay (days) | 3 (2–5) | 2 (2–3) | <0.001 |
| LOS (days) | 7 (7–9) | 7 (6–8) | <0.001 |
| In-hospital mortality | 2.1 | 1.8 | 0.358 |
Data presented as percentage within the group, with P-values from χ2-tests, except ICU stay and LOS presented as median (25–75th percentile), with P-values from Wilcoxon–Mann–Whitney tests.
CABG: coronary artery bypass grafting; IABP: intra-aortic balloon pump; ICU: intensive care unit; LOS: length of stay; PM: pacemaker; POAF: postoperative atrial fibrillation.
Uni- and multivariate predictors of postoperative atrial fibrillation
Variables that influenced the incidence of POAF in univariate analyses are given in Tables 1 and 2. These variables together with hypertension, ACC-time and CPB-time were included in multivariate analyses. These identified increasing age, preoperative serum creatinine ≥150 µmol/l, male gender, NYHA class III/IV, current smoking, prior myocardial infarction (MI) and absence of hyperlipidemia as independent predictors of POAF after CABG (Table 1). The ORs were essentially unchanged after correction for other risk modifiers.
One complementary multivariate analysis stratified for LOS and one solely based on patients without other postoperative complications (n = 6264) both showed virtually unchanged estimates. Explorative analyses for all variables identified no significant interactions.
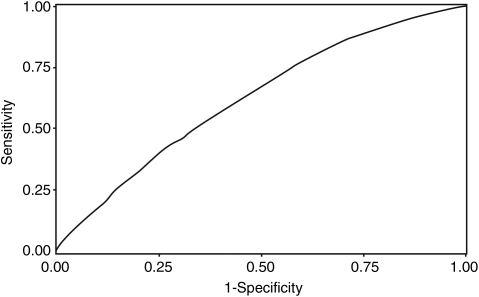
The discriminatory ability of the final prediction model was moderate, with an AUC of 0.62 (95% CI, 0.61–0.64) (Fig. 2).
Figure 2:
ROC curve for postoperative atrial fibrillation after coronary artery bypass grafting, based on preoperative and surgical data. AUC: 0.62 (95% CI, 0.61–0.64).
DISCUSSION
This study was one of the largest studies on the prediction of POAF in CABG patients, and was based on 7115 patients with sinus rhythm who underwent primary isolated CABG at a single department. The incidence of POAF after CABG was 32%, which was consistent with incidences ranging from 25 to 33% reported from other studies with isolated CABG procedures [1–5].
Advancing age was the strongest predictor of POAF in the present study, and age is the only consistent risk factor associated with POAF after CABG [1–3, 6, 7]. Our data indicate that this is more of a direct consequence of high age, for example, age-related structural changes in the atria such as fibrosis, muscle atrophy and dilation [10, 11] rather than being an indirect consequence of higher age, i.e. prolonged hospital stay or other complications that were in turn caused by high age. Crude and adjusted estimates were essentially the same, showing that the crude risks related to age are not substantially confounded by the other risk factors in the model. Tests conditioning on LOS refuted that the risk modification obtained by age was solely due to a prolonged stay in old patients that increased the possibility to capture an episode of POAF. Moreover, in an analysis based on uncomplicated cases only, the estimates were virtually unchanged implying a direct effect of age on the risk for POAF.
The other identified predictors were not as strong and have inconsistently been associated with POAF. These included elevated preoperative serum creatinine [12], prior MI, male gender and heart failure [7]. The invert impact of hyperlipidemia might be explained by treatment with statins [13], possibly by their anti-inflammatory effect.
Patients with POAF had a higher frequency of other postoperative complications and a longer LOS and ICU stay. The causative relationship has not been evaluated in this study and this may simply imply that the POAF population has a higher morbidity both before and after surgery.
Although POAF is associated with higher early mortality rates in a number of studies, ranging from 2.4 to 7.4% [1, 2, 4–6] after cardiac surgery, this was not supported in this study, as in-hospital mortality was low in the POAF group at 2.1%.
The definition and detection method of POAF could be a limitation where some POAF episodes could have been missed; however, the incidence was comparable to other published results [1–5]. Our data did not include the entire patient history, for example, COPD, which has been reported as an independent predictor in a few studies, with an OR of 1.4 [1, 4], and history of arrhythmia, where information about its importance has been inconsistent.
A sample study performed showed no difference in prediction of POAF with or without treatment with β-blockers, with a vast majority receiving treatment with β-blockers.
The poor prediction of high-risk groups, shown by the moderate AUC value of 0.62, obtained by our final model confirms the similar results previously reported for prediction models of POAF after isolated CABG, with AUC at 0.65 [2] and 0.69 [3].
CONCLUSIONS
The moderate predictive value of the final model confirms the difficulty of identifying a high-risk group before surgery, which makes it difficult to better focus prophylactic efforts. Since the number of patients with a greatly elevated risk of POAF is relatively small, the absolute number of reduced cases of POAF would be even smaller. For example, less than 5% of the patients in this study were at an age of over 80 years, which gives the highest risk of POAF. By targeted treatment with, for example, anti-arrhythmic medication or atrial pacing possible side effects could be avoided for patients not likely to benefit from this treatment. This requires a well-defined group of patients prone to develop POAF, and we are unfortunately still far from finding one.
Acknowledgements
We are grateful to Fredrik Granath for valuable statistical support.
APPENDIX. Conference Discussion
Dr A. Ahlsson (Orebro, Sweden): This is one of the largest studies so far of postoperative AF in CABG patients. I think you have shown us two things: Despite sophisticated statistical models, we can't predict which patients will develop postop AF and which patients will not. The other thing is that postoperative AF is associated with complications. The key question is: if we were able to abolish postoperative AF, would we then lower the complication rate? I don't expect you to answer that, but I have two other questions for you.
First of all, you had a higher incidence of stroke in the postoperative AF group. Do you have any information regarding the timing of the strokes and the postoperative AF episodes?
Dr Thoren: I don't have any information in this material, but a colleague at our institution has looked at stroke and it seems - this is unpublished data, of course - but it seems that about half of the strokes are caught before atrial fibrillation is registered. So even though lowering the postop A-fib rate might improve some of the strokes, I don't think it will affect all of them.
Dr Ahlsson: My second question is: you define postoperative AF as an episode of AF lasting over 30 seconds during the hospital stay. And you have longer hospital stays in the postoperative AF group, is that correct?
Dr Thoren: Yes, that's correct.
Dr Ahlsson: That means that you may have an information bias because patients with complications like mediastinitis and stroke have longer hospital stays, and by definition they will have more AF episodes the longer you observe them. So there may be an information bias issue here. But still, of course, there is a correlation between postoperative AF and complications.
Dr Thoren: That's an interesting comment. We haven't looked exactly at what day the atrial fibrillation occurred. But looking at other data, including your own, it's most common for the postop A-fib to present during day 2 or 3, and so I think that the prolonged hospitalization period has little effect on the overall conclusions.
Dr R. Yadav (London, United Kingdom): You said that patients who had AF had a greater incidence of respiratory complications. Do you have information on whether the respiratory complications preceded the AF? Because hypoxia could well be a trigger for patients who did go into AF.
Dr Thoren: No, I don't have that information. It could be, as with the stroke, that sometimes the respiratory complications precede the postop A-fib.
Dr A. Rastan (Leipzig, Germany): I missed some important risk factors in your analysis which are known to reduce possible AF. First, beta blockage medication, and second, the type of procedure. How many of these patients had off-pump procedures? You also mentioned that hyperlipidaemia was a predictive factor to reduce AF, which potentially has something to do with the statin medication the patients had preoperatively. So did you analyze these factors, too?
Dr Thoren: We have not looked at preoperative medications. Concerning beta blockers, most patients did receive beta blockers prior to surgery.
Dr Rastan: But not all patients. Did you look for this statistically?
Dr Thoren: No, we haven't studied the medication, but we found that most patients received preoperative beta blockers when we did a sample study. Concerning statins, we didn't look at the use of that either. But, as you say, patients with hyperlipidaemia are more likely to be medicated with statins and that could have influenced that finding. And what was your other point?
Dr Rastan: Off-pump procedures.
Dr Thoren: We had a relatively low percentage of off-pump surgery. I don't have the number in my head. But if you look at other studies, the results have been inconsistent about whether off-pump surgery actually lowers the postop A-fib risk. But, of course, this is interesting.
Dr A. Wahba (Trondheim, Norway): You showed us that there is an increased risk of complications following surgery in patients who have AF after surgery. Now, I wondered, you also showed that the risk of postoperative atrial fibrillation increases with age. Did you look at whether the risk of complications is an independent risk factor? Is it, for example, related to age? Is age alone explanatory?
Dr Thoren: No, we did not look at age alone. This could have influenced the outcome. We didn't look at the outcome independently.
Dr G. Wimmer-Greinecker (Bad Bevensen, Germany): Let me ask you one question. How good is your echocardiographic assessment prior to surgery? Do you have any data on moderate mitral regurgurgitation or size of atria, which could also have quite an influence on your data.
Dr Thoren: No, we did not look at those kinds of predictors. We only used easily accessible clinical variables that are easy to register for most patients.
Dr T. Kieser (Calgary, AB, Canada): I think your study is very important given how sensitive we are, as surgeons, to stroke. If you look at the SYNTAX trials, this is one of the areas of difference from PCI. Do you think there is any rationale for using prophylactic amiodarone to prevent atrial fibrillation in these patients, have you thought about doing this in your centre.
Dr Thoren: Well, the first question is, if we could lower the risk of postop A-fib whether we could indeed prevent all complications, which seems unlikely. But since it's hard to identify a high-risk group, we would have to treat almost everyone with amiodarone and then that becomes a cost question, of course. And also those patients most likely to develop postop A-fib might be more sensitive to amiodarone and it might be contraindicated for some of those patients as well.
Dr Kieser: That's very true. Our centre was the author of the PAPABEAR study which showed that there was a reduction in postoperative atrial fibrillation with use of prophylactic amiodarone. But hardly any of us use it because we find that patients go into heart block, and you're right, it's quite a problem.
Funding
This study was conducted with financial support from departmental (Department of Cardiothoracic Surgery) and institutional (Uppsala University Hospital, Sweden) funds.
Conflict of interest: none declared.
References
- 1.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. J Am Med Assoc. 2004;291:1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 2.Shen J, Lall S, Zheng V, Buckley P, Damiano RJ, Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011;141:559–70. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amar D, Shi W, Hogue CW, Jr., Zhang H, Passman RS, Thomas B, et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol. 2004;44:1248–53. doi: 10.1016/j.jacc.2004.05.078. [DOI] [PubMed] [Google Scholar]
- 4.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–13. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37:1353–9. doi: 10.1016/j.ejcts.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Villareal RP, Hariharan R, Liu BC, Kar B, Lee V-V, Elayda M, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–8. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Mahoney EM, Thompson TD, Veledar E, Williams J, Weintraub WS. Cost-effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J Am Coll Cardiol. 2002;40:737–45. doi: 10.1016/s0735-1097(02)02003-x. [DOI] [PubMed] [Google Scholar]
- 8.El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, et al. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–6. doi: 10.1016/j.jacc.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 9.Dolgin M, editor. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston: Little Brown & Co Inc; 1994. Criteria Committee of the New York Heart Association. [Google Scholar]
- 10.Cox JL. A perspective of postoperative atrial fibrillation in cardiac operations. Ann Thorac Surg. 1993;56:405–9. doi: 10.1016/0003-4975(93)90871-e. [DOI] [PubMed] [Google Scholar]
- 11.Kitzman DW, Edwards WD. Age-related changes in the anatomy of the normal human heart. J Gerontol. 1990;45:M33–9. doi: 10.1093/geronj/45.2.m33. [DOI] [PubMed] [Google Scholar]
- 12.Auer J, Lamm G, Weber T, Berent R, Ng CK, Porodko M, et al. Renal function is associated with risk of atrial fibrillation after cardiac surgery. Can J Cardiol. 2007;23:859–63. doi: 10.1016/s0828-282x(07)70839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol. 2000;86:1128–30. doi: 10.1016/s0002-9149(00)01172-3. [DOI] [PubMed] [Google Scholar]