Abstract
Priming of soybean seeds with static magnetic field exposure of 200 mT (1 h) and 150 mT (1 h) resulted in plants with enhanced performance index (PI). The three components of PI i.e the density of reaction centers in the chlorophyll bed (RC/ABS), exciton trapped per photon absorbed (φpo) and efficiency with which a trapped exciton can move in electron transport chain (Ψo) were found to be 17%, 27% and 16% higher, respectively in leaves from 200 mT (1h) treated compared to untreated seeds. EPR spectrum of O2. – - PBN adduct revealed that the O2. – radical level was lower by 16% in the leaves of plants that emerged from magnetic field treatment. Our study revealed that magnetoprimed seeds have a long lasting stimulatory effect on plants as reduced superoxide production and higher performance index contributed to higher efficiency of light harvesting that consequently increased biomass in plants from treated plants.
Keywords: Magnetopriming, Performance index, Superoxide radical
Magnetobiology is a new synthetic discipline encompassing the principles and techniques of many sciences from engineering, physics, chemistry, biology and centered around biophysics. It studies biological effects of oscillating or static and low-frequency magnetic fields on tissues without causing heating of tissues. Magnetic and electromagnetic treatments are being used in agriculture for seed priming as a non-invasive technique to improve the germination and vigor of seeds and finally yield. The results obtained by magnetopriming indicate that magnetic field acts as a bio- stimulant which can be considered as an alternative to chemical and biological methods for the pre-sowing treatment of the seeds.1,2 Several reports also show that the magnetic field exposure increases germination of low viability seeds and improves their quality and sprouting rate.2,3 These effects may be because of interaction of magnetic field with ionic current in the plant embryo cell membrane that induces changes in both osmotic pressure and ionic concentrations on both sides of the membrane.4 Changes in the ionic fluxes across cell membrane cause alterations in the mechanism of water uptake, as osmoregulation in embryo cells is controlled by the ionic transport across the membrane.5 The hypotheses that explain biological effects of magnetic field are based on fragmentary studies and in plants cryptochromes are believed to be the possible candidate of magnetoreception.6 Two mechanisms of magnetoreception that are currently receiving attention are (1) the “radical-pair mechanism” consisting of modulation of singlet-triplet interconversion rates of a radical pair by weak magnetic fields, (2) the “ion cyclotron resonance” that revolves around the fact that ions should circulate in a plane perpendicular to an external magnetic field with their Lamor frequencies, which can interfere with an alternating electromagnetic field.7
Previously, we have reported the effect of static magnetic field on the seeds of soybean [Glycine max (L) Merr. var: JS-335] by exposing the seeds to different magnetic field strength from 0 to 300 mT in steps of 50 mT for 30, 60 and 90 min. Treatment with magnetic field improved germination related parameters like water uptake, speed of germination, seedling length, fresh weight, dry weight and vigour indices of soybean seeds under laboratory conditions. Treatment of 200 mT (1 h) and 150 mT (1 h) which were more effective than others in increasing most of the seedling parameters were further explored for their effect on plant growth, leaf photosynthetic efficiency and leaf protein content under field conditions. Total soluble protein map (SDS-Polyacrylamide gel) of leaves showed increased intensities of the bands corresponding to larger subunit (53 KDa) and smaller subunit (14 KDa) of Rubisco in the treated plants.8 This addendum further expands to report the level of superoxide radical and chlorophyll a fluorescence parameters in the leaves of one month old field grown soybean plants that emerged from magnetoprimed seeds (150 and 200 mT for 1h).
An electromagnetic field generator “Testron EM-20” with variable magnetic field strength (50 to 500 mT) with a gap of 5 cm between pole pieces was used for treating seeds with 200 mT (1 h) and 150 mT (1 h) static magnetic field. The pole pieces of the electromagnet were cylindrical in shape with 9 cm diameter, 16 cm length. The total number of turns of copper coil per pole piece was 3000 and resistance of the coil was 16 Ohm. A DC power supply (80V/10A) with continuously variable output current was used for the electromagnet. A digital gauss meter model DGM-30 operating on the principle of Hall effect monitored the field strength produced in the pole gap. The seeds were placed between the pole pieces by keeping in a sample holder, cylindrical shape and made of non-magnetic thin transparent plastic sheet. Both the magnetic treatments were run along with unexposed control under similar conditions.
Light driven photosynthetic processes are the main source of free radical production in chloroplasts. The impact of pre-sowing magnetic field treatment on the superoxide radical content in the fourth trifoliate leaf of 30-d-old plants was measured by Electron paramagnetic resonance (EPR) spectrometer using Phenyl N-tert-butyl nitrone (PBN) as a spin trap. Spectra of xanthine/ xanthine oxidase (X/XO) + PBN was recorded as a standard spectra for superoxide. Instrument settings and sample preparation was as described earlier.9 Chl a fluorescence of dark adapted fourth trifoliate leaf of soybean was measured by a Handy PEA fluorimeter (Plant Efficiency Analyzer, Hansatech Instruments Ltd. King’s lynn Norfolk).
The EPR signals of O2. - - PBN adducts were recorded in soybean leaves that emerged from magnetic treated seeds. O2.- - PBN adduct gives a characteristic EPR spectrum known as triplet of doublet with hyperfine coupling constants of aN = 1.48 mT and aH = 0.28 mT (Fig. 1). EPR spectrum of O2· – - PBN adduct corresponding to the standard from the leaf extracts revealed that the O2· – radical level was lower in the leaves of plants that emerged from magnetic field treatment. The reduction in the free radical content was up to 16% and 11% after the treatment with 200 mT (1 h) and 150 mT 1 h (Fig. 1). In our earlier studies, we have reported more active reaction centers per unit area of the leaf combined with higher efficiency of electron transport after treatments of seeds with magnetic field. OJIP transients from the leaves in the treatment gave a higher fluorescence yield at J-I-P phase. The rise in fluorescence curve after magnetic field treatment was explained as the result of faster reduction of electron acceptors in the photosynthetic pathway downstream of PSII, notably plastoquinone and in particular QA.8 In general, the electron transport chain (ETC) in chloroplasts operates in an O2 sufficient environment such that if ETC is overloaded it will result in leakage of electrons and generation of free radicals.10 In case of plants from magnetoprimed seeds there is reduced electron leakage to O2 from QA and QB sites of ETC resulting in lesser generation of O2.- from the chlorophyll bed close to reaction center.
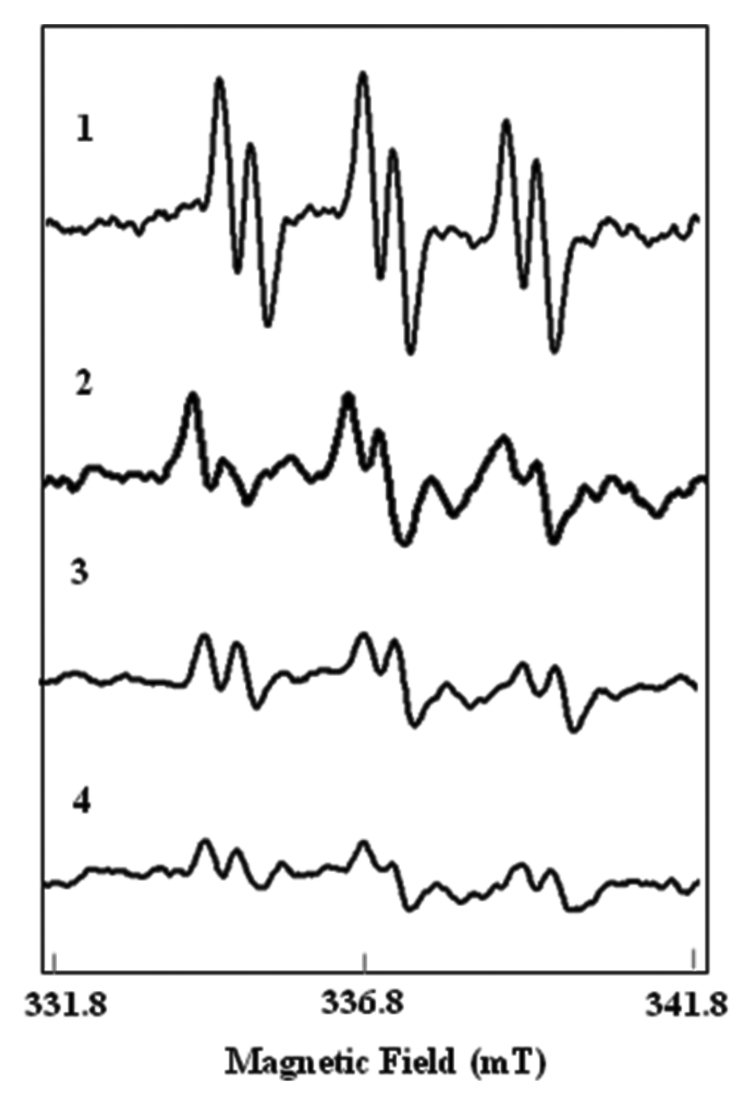
Figure 1. Effect of magnetopriming on superoxide production in soybean leaves. (1)spectra from xanthine / xanthine oxidase, (2) control, (3) 150 mT (1 h), (4) 200 mT (1 h)]. Each spectra is the representative of 18 individual spectra.
One of the most sensitive parameters calculated from Chl a fluorescent transient is the performance index (PI). This parameter encompasses fluorescent changes associated with changes in antenna conformation and energy fluctuations. Therefore the PI helps to estimate the vitality of the plants with high resolution. The PI is a function of three independent functional steps of photosynthesis, the density of reaction centers in the chlorophyll bed (RC/ABS), exciton trapped per photon absorbed (φpo) and efficiency with which a trapped exciton can move an electron into the electron transport chain further than QA- (Ψo).11 Magnetopriming of soybean seeds enhanced the PI up to 48% and 63% by 150 mT (1 h) and 200 mT (1 h) respectively over control plants.
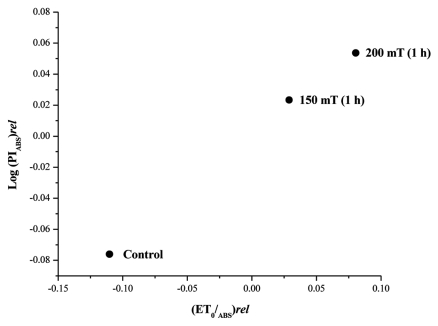
Perusal of the data on PI showed that enhancement of PI was attributable to higher efficiency of φpo. The enhancement of φpo was 27% and 20% higher in leaves from 200 mT and 150 mT treated seeds compared with control. RC/ABS and Ψo contributed equally to the enhanced PI (Table 1). The relationship between log PIABS ( = Driving force for photosynthesis) and quantum yield of electron(φEo = ET0/ABS) can be considered as a typical property of a plant’s ability to transform light energy into chemical energy (NADPH) which is subsequently directed into metabolic events during the dark reactions of photosynthesis.12 Plotting log (PIABS)rel vs (ET0/ABS)rel revealed that the higher efficiency for transforming light energy to chemical energy was expressed in plants that emerged from the seeds treated with magnetic field. The increased PI and the relationship between log PIABS ( = DF) and transport (ET0/ABS) of magnetically treated plants demonstrated that these plants exhibited a high efficiency to utilize the photosynthetically active radiation (400 -700 nm) in the natural environment. Increased Rubisco content8 and higher light harvesting efficiency in the treated plants led to 40 and 55% increase in biomass of plants from 150 mT (1 h) and 200 mT (1 h) treated seeds respectively as a strong positive correlation was observed between PI and shoot dry weight (r2 = 0.9823).
Table 1. Effect of stationary magnetic field treatment on Performance index (PI) and its different components as derived from the fluorescence induction curve.
| Treatment | The density of reaction Centers (RC/ABS) | The efficiency of light reaction ΦPo/(1-Φpo) | The efficiency of biochemical reaction ψ0/(1- ψ0) | PIABS RC/ABS. ΦPo/(1 -Φpo) . ψ0/(1 - ψ0) |
|---|---|---|---|---|
| 0 mT |
0.625 ± 0.01 |
2.802 ± 0.061 |
0.949 ± 0.029 |
1.688 ± 0.13 |
| 100mT (2h) |
0.707 ± 0.012*** |
3.385 ± 0.036*** |
1.061 ± 0.021*** |
2.50 ± 0.18*** |
| 200 mT (1h) |
0.736± 0.014*** |
3.545 ± 0.048*** |
1.105 ± 0.020*** |
2.742 ± 0.181*** |
| Data are the means of ± S.E.M. of fifteen plants (n=15). ***, ** and * indicate significance at p < 0.001, 0.01, 0.05 respectively | ||||
It is concluded that magnetopriming of soybean seeds reduced the superoxide content in the leaves of field grown soybean plants. This may help in channelizing the metabolic energy toward better growth and enhanced productivity that would have otherwise been diverted toward scavenging of the free radical. Higher performance index of plants from magnetoprimed seeds contributes to higher efficiency of light harvesting and consequently increased biomass in treated plants. The positive changes in seed physiology after magnetopriming are found to have a long lasting effect in grown up plants. This suggests that a signal transduction pathway may be operative; yet a correlation between the underlying molecular mechanisms and level of enhancement in plants from treated seeds need to be extensively studied to explain this response. AUTHOR: Please refer to Figure 2 within the article text. Please also include all authors’ full names for the affiliation section.
Figure 2. Correlation between the driving force (DFABS)rel = Log (PIABS)rel as a function of the relative yield of electron transport (ET0/ABS) rel.[ Log (PIABS)rel = [(PIABS)AVG treatment- (PIABS)all avg]/ (PIABS)all avg . (ET0/ABS) rel = [(ET0/ABS)AVG treatment- (ET0/ABS)all avg]/ (ET0/ABS)all avg ].
Acknowledgments
Indian Council of Agricultural Research [NFBSRA/PCN/AP-09/2006–07] New Delhi, India
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17720
References
- 1.Vakharia DN, Davariya RL, Parameswaran M. Influence of magnetic treatment on groundnut yield and yield attributes. Ind J Plant Physiol. 1991;34:131–6. [Google Scholar]
- 2.Alexander MP, Doijode SD. Electromagnetic field, a novel tool to increase germination and seedling vigor of conserved onion (Allium cepa,L.) and rice (Oryza sativa L.) seeds with low viability. Plant Genet Resour Newsl. 1995;104:1–5. [Google Scholar]
- 3.Carbonell MV, Martínez E. Flόrez M, Maqueda R, Lόpez Pintor A, Amaya JM. Magnetic field treatments improve germination and seedling growth in Festuca arundinacea Schreb. and Lolium perenne L. Seed SciTechnol. 2008;36:31–7. [Google Scholar]
- 4.Yaycili O, Alikamanoglu S. The effect of magnetic field on Paulownia tissue cultures. Plant Cell Tissue Organ Cult. 2005;83:109–14. doi: 10.1007/s11240-005-4852-0. [DOI] [Google Scholar]
- 5.Reina FG, Pascual LA. Influence of a stationary magnetic field on water relations in lettuce seeds. Part I: Theoretical considerations. Bioelectromagnetics. 2001;22:589–95. doi: 10.1002/bem.88. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W. Magnetic intensity affects cryptochrome-controlled response in Arabidopsis thaliana. Planta. 2007;225:615–24. doi: 10.1007/s00425-006-0383-0. [DOI] [PubMed] [Google Scholar]
- 7.Galland P, Pazur A. Magnetoreception in plants. J Plant Res. 2005;118:371–89. doi: 10.1007/s10265-005-0246-y. [DOI] [PubMed] [Google Scholar]
- 8.Shine MB, Guruprasad KN, Anjali A. Enhancement of germination, growth and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics. 2011 doi: 10.1002/bem.20656. In press. [DOI] [PubMed] [Google Scholar]
- 9.Shine MB, Guruprasad KN. Impact of presowing magnetic field exposure of seeds to stationary magnetic field on growth, reactive oxygen species and photosynthesis of maize under field conditions. Acta Physiol Plant. 2011 doi: 10.1007/s11738-011-0824-7. In press. [DOI] [Google Scholar]
- 10.Edreva A. Generation and scavenging of reactive oxygen species in chloroplasts: a submolecular approach. Agric Ecosyst Environ. 2005;106:119–33. doi: 10.1016/j.agee.2004.10.022. [DOI] [Google Scholar]
- 11.Srivastava A, Strasser RJ. Govindjee. Greening of peas: parallel measurements of 77K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica. 1999;37:365–92. doi: 10.1023/A:1007199408689. [DOI] [Google Scholar]
- 12.Hermans C, Smeyers M, Rodriguez RM, Eyletters M, Strasser RJ, Delhaye JP. Quality assessment of urban trees: A comparative study of physiological characterisation, airborne imaging and on site fluorescence monitoring by the OJIP-test. J Plant Physiol. 2003;160:81–90. doi: 10.1078/0176-1617-00917. [DOI] [PubMed] [Google Scholar]