Abstract
Plant cells have a rigid cell wall that constrains internal turgor pressure yet extends in a regulated and organized manner to allow the cell to acquire shape. The primary load-bearing macromolecule of a plant cell wall is cellulose, which forms crystalline microfibrils that are organized with respect to a cell's function and shape requirements. A primary cell wall is deposited during expansion whereas secondary cell wall is synthesized post expansion during differentiation. A complex form of asymmetrical cellular differentiation occurs in Arabidopsis seed coat epidermal cells, where we have recently shown that two secondary cell wall processes occur that utilize different cellulose synthase (CESA) proteins. One process is to produce pectinaceous mucilage that expands upon hydration and the other is a radial wall thickening that reinforced the epidermal cell structure. Our data illustrate polarized specialization of CESA5 in facilitating mucilage attachment to the parent seed and CESA2, CESA5 and CESA9 in radial cell wall thickening and formation of the columella. Herein, we present a model for the complexity of cellulose biosynthesis in this highly differentiated cell type with further evidence supporting each cellulosic secondary cell wall process.
Keywords: cell shape, cellulose biosynthesis, embryogenesis, mucilage, radial cell wall, secondary cell wall biosynthesis, seed coat, seed development
Directional growth in plant cells is mediated, in part, by the constraint imposed on the cells internal turgor pressure by a rigid yet extensible primary cell wall. The primary structural elements of the cell wall are paracrystalline chains of β-1,4 linked glucose molecules that create a cellulose elemental microfibril (CEF).1 Cellulose biosynthesis is essential for plant growth and development as illustrated genetically2 and pharmacologically.3 Several lines of evidence suggest that cellulose synthesizing organisms produce microfibrils from plasma membrane localized cellulose synthesizing complexes, namely terminal complexes (TC) due to the attachment between CEF and freeze fracture rosettes in the plasma membrane.4 In the Arabidopsis genome, 10 cellulose synthase (CESA) genes have been identified based on sequence similarity originally to bacterial cellulose synthases2,5 and subsequently to RSW1 (CESA1).6 A combination of biochemical and genetic analyses have shown that at least three CESA subunits directly interact to form a CSC in primary7,8 and secondary (xylem) cell wall formation,9 but added complexity, such as ancillary associated proteins10,11 is expected. Indeed, the diverse range of cell shapes and complexity of cell differentiation and functional requirement in certain cell types suggests added complexity in CESA functionality may exist (Fig. 1), but this is poorly understood at the genetic level. By theory, combinations of specific CESAs may be active in different cell types at different times and may be active in the same cell type at different stages, serving different functions.
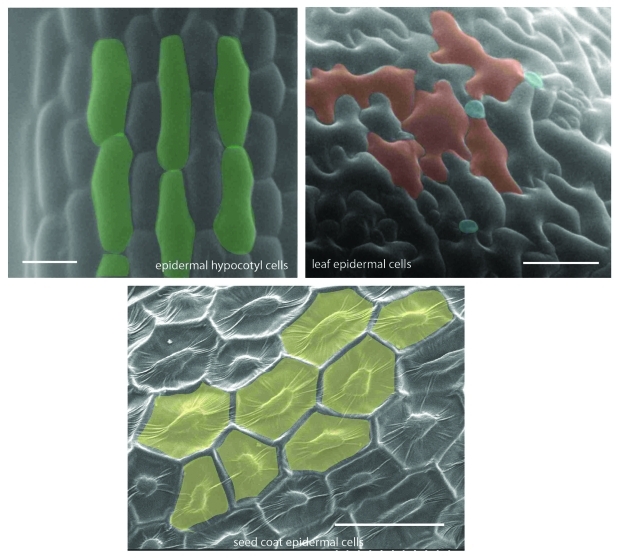
Figure 1.
Scanning electron microscopy (SEM) micrographs showing variation in epidermal cell size and shape in different plant organs. Etiolated hypocotyls, leaves and seeds from Arabidopsis were sputter coated with gold-palladium using Hummer VI sputtering system (Anatech) and visualized using Hitachi-S-800 SEM. Scale bars = 30 μm.
Functional specialization of CESAs has been examined. Our current understanding suggests that CESA1 is a necessary component of primary wall synthesis in most tissues because rsw1–1,2 a temperature sensitive allele caused disappearance of TCs from the plasma membrane at restrictive temperature. CESA3 is co-expressed with CESA1, and homozygous cesa3 alleles are also male gametophytic lethal.7 CESA6 is required for elongation of hypocotyl cells in etiolated seedlings.12,13 During xylem development, all three secondary wall CESAs (CESA4, CESA7, CESA8) are expressed in the same cells at the same time.9 The secondary radial wall of seed coat epidermal cells requires at least CESA2, CESA5 and CESA9.14,15 Secondary cell wall mucilage biosynthesis in seed coat epidermal cells requires CESA5 for attachment to the parent seed.15,16 Currently, function of CESA10, which based on sequence homology is most similar to CESA16,17 is not known.
Evidence for discreet microfibrils in a single cell type has recently been postulated18 and recently shown in differentiating xylem cells of Populus19 and in trichomes.20 In the case of xylem cells, authors identified two independent CSCs, providing evidence that different CSCs could produce structurally discrete microfibrils, capable of partitioning during cell wall synthesis.19 Functional specialization of CESAs was recently explored using genetics in the epidermal seed coat,14-16 which revealed that the distinct two secondary cell wall processes, mucilage synthesis, and secondary cell wall reinforcement requiring specific CESAs spatially and temporally (7 vs. 9 d post-anthesis: DPA) (Fig. 2). Arabidopsis epidermal seed coat cells follow a complex developmental program.21 After fertilization, the ovule outer integument cell layer differentiates into an asymmetrically structured cell type. Two of the main processes are thought to require cellulose biosynthesis.
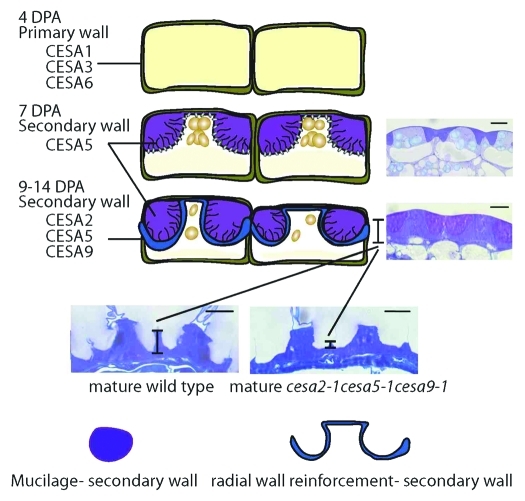
Figure 2.
Schematic representation of secondary cell wall processes in the seed coat epidermal cells during seed development. During development seed coat epidermal cells undergo a complex developmental program formation leading to the formation of an asymmetrically differentiated seed coat cell. Two secondary cell wall processes occur. First, the mucilage biosynthesis and second, the formation of a thick secondary cell wall that covers the columella and extends to the primary radial wall. Our data15 showed that at least three CESA genes (CESA2, CESA5 and CESA9) are necessary for the secondary cell wall processes. Triple mutant lacking these three CESAs showed a severe reduction or lack of radial wall formation and a defect in mucilage adherence to the parent seed.15 Inset figures were obtained from original article Mendu et al.15 Scale bar = 10 µm
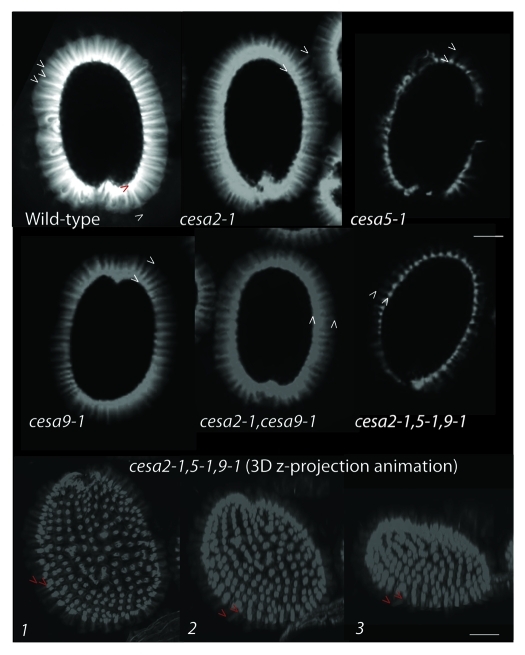
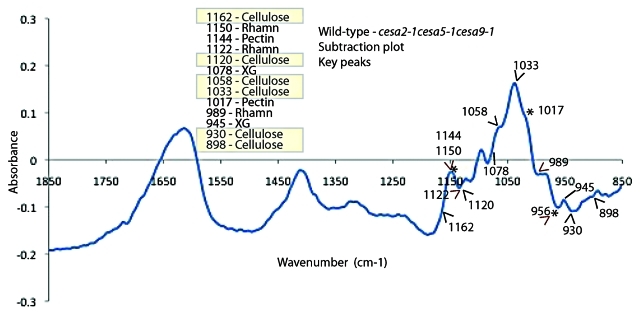
First, the production of a donut-shaped apoplastic pocket filled with pectinaceous mucilage contains linear striations of cellulose.22 Second, the formation of a thick secondary cell wall that covers the columella and extends to the radial boundary wall,23 which is thought to assist in reinforcing the cell layer that must ultimately protect the embryo while withstanding the forces of dessication and hydration. Cellulose is thought to be a key component of both these secondary cell wall processes. CESA5 is essential for the pectinaceous mucilage halo to remain attached to the parent seed.15,16 By laser scanning confocal microscopy of Calcofluor stained seeds from cesa2–1, cesa5–1, cesa9–1, cesa2–1cesa9–1 and cesa2–1cesa5–1cesa9–1 it is clearly visible that linear striations arising from the hydrated seed coat are largely intact, but the fluorescence arising from in between linear striations was greatly reduced in genetic background with loss of CESA5 (cesa5–1 and cesa2–1cesa5–1cesa9–1) (Fig. 3). Three dimensional reconstructions of the triple mutant seed illustrate the lack of fluorescence between columella (Fig. Three 3D z-projection animation). By contrast, wild-type seed exposed to Calcofluor was so bright in three dimensional reconstructions (Data not shown) that it is most effectively illustrated from a cross sectional viewpoint (Fig. 3). Cross sections reveal disperse yet bright fluorescence arising between columella striations. We interpret this as suggesting that cellulose is produced during mucilage production and integrates into the inner layer of mucilage,15,16 where is it mediates attachment. We propose that CESA5 is producing the fluorescence that is evident between columella in wild-type cross section based on Calcofluor fluorescence (Fig. 3 and Fig. 2 for a schematic). If cellulose is integrating into the mucilage, it is plausible that we could detect variation in mucilage structure linked to cellulose using Fourier Transform Infrared spectroscopy (FTIR). To attempt test this hypothesis, generated spectra for wild-type and triple mutant mucilage and used this data to create a subtraction spectra documenting the differences between wild-type and triple mutant mucilage (Fig. 4). While qualitative in nature, FTIR spectroscopy has been used effectively to detect subtle changes in cell wall carbohydrate structures in mutants compared with wild type based on signature peaks.24,25 Our observations identified several previously characterized peaks linked to crystalline cellulose as key differences between wild-type and triple mutant.24,26 The peaks at wave numbers (WN) linked to cellulose that were different between wild type and triple mutant (via subtraction plot analysis) were 898, 930, 1033, 1058, 1120 and 1162 (cm-1).24 Within the FTIR subtraction plot, we could also identify different peaks between pectin (WN-989, 1017, 1122, 1144 and 1150) and xyloglucan (WN-1078 and 945)24 between the wild type and triple mutant spectra. These data, together with biochemical data15 support histochemical analyses27 and the mucilage adherence phenotype linked to CESA5 (Fig. 4).
Figure 3.
Absence of cellulose dependent florescence arising between columella striations in mutants involving CESA5. Analysis of Calcofluor white stained seeds showed lack of florescence between striations of fluorescence arising from regions coincident with columella in the mutant seeds involving CESA5 (cesa5–1 and cesa2–1cesa5–1cesa9–1) compared with wild type and other mutant seeds. The intensity and length of the florescence halo surrounding the seed was reduced in cesa2–1, cesa9–1 and cesa2–1cesa9–1 compared with wild type. To visualize the calcofluor florescence, wild type and mutant seeds were stained with Calcofluor white for 5 min and observed under laser scanning confocal microscopy. Three dimensional reconstructions were made by taking images on a Z-series at 2 μm intervals and threading them into an animation. The images represent three different angles and illustrate the uniformity of the phenotype across a three dimensional plane. Scale bar = 250 μm.
Figure 4.
Analysis of mucilage composition using Fourier Transform Infrared analysis (FTIR) showed differences in cellulose, hemicelluloses and pectin peaks. FTIR was performed on the ammonium oxalate extracted mucilage for structural changes. Analysis of subtraction plot between wild type and cesa2–1cesa5–1cesa9–1 triple mutant showed differences in peaks related to cellulose (898, 930, 1033, 1058, 1120 and 1162, hemicellulose (WN-1078 and 945) and pectin WN-989, 1017, 1122, 1144 and 1150). Samples were prepared by dissolving exactly 2mg mucilage pellet in 50 μL water with 20mg of KBr, the overnight dried (50°) mixture was stored in a desiccator. Pellets for FT-IR were made by 2mg mucilage-KBr mixture with 23 mg of fresh KBr, pressed into 7-mm pellets and collected spectra for wild type and mutants using a Thermo Nicolet Nexus 470 spectrometer (ThermoElectric Corporation, Chicago, IL) over the range 4,000–800 cm−1. For each spectrum, 200 scans were performed at a resolution of 8 cm−1. Subtraction plot between wild type and triple mutant was made using OMNIC software (Thermo Nicolet, Madison, WI) and spectral differences were cross referenced with Kacuráková et al.24 to identify peaks linked to cellulose (indicated by black caret), xyloglucan (black line) and pectin (asterisk and red caret).
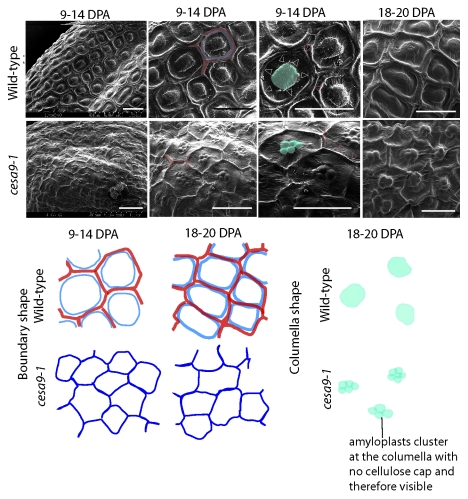
Second, to explore the cellulose biosynthetic machinery in the secondary wall that reinforces the columella and radial wall, we examined T-DNA insertional alleles for individual, double and triple mutants for CESA genes.15 While mutation in CESA6 (procuste) failed to cause aberration of cell shape or cause radial wall ridge reduction, CESA2, CESA5 and CESA9 cause suppression of radial wall integrity.14,15 By examining and quantifying the radial wall height in sectioned seed coats of wild-type and mutant lines, we found an additive reduction in radial wall height thickening suggesting that at least CESA2, CESA5 and CESA9 are involved in secondary radial wall formation (Fig. 2).15 The most severe allele among cesa2–1, cesa5–1 and cesa9–1 single mutants was cesa9–1. To examine this allele in greater detail, we excised whole seed from siliques at two developmental stages and imaged them via SEM (approximately 9–14 DPA and 18 DPA) (Fig. 5). Careful examination of micrographs from wild-type seeds excised approximately 9–14 DPA revealed that the secondary radial wall was forming as a circumference near the primary radial wall. Between neighboring cells, it was possible to distinguish a boundary ridge that remained partially fused to the thickening radial wall (Fig. 5). By 18 DPA, the radial wall appeared thicker and fused between neighboring cells, and determinate ridges could not be distinguished. By contrast, the cesa9–1 allele displayed a visually less pronounced radial wall (Fig. 5) at both 9–14 and 18 DPA. A notable observation was the visibility of the underlying amyloplasts in the cesa9–1. Consistent with past models28-31 the secondary radial wall thickening encompasses the columella (Fig. 2) and based on this model, we predicted that the amyloplasts, which are typically present in the columella vicinity during development, may be visible by SEM due to the thin outer tangential wall. Confirmation of this postulate further suggests that the cellulosic secondary cell wall encompasses the boundary radial wall, and central columella (Fig. 5).15,30 The reason this was not examined by section analysis15 was that quantification of the radial wall was the most robust means to illustrate loss of secondary wall in mutant alleles, as columella naturally display shape variability.
Figure 5.
Radial wall ridge development in wild type and cesa9–1 seed coat cells. Seeds were excised from siliques at approximately 9–14 and 18–20 DPA, sputter coated and visualized under SEM (Hitachi-S-800). Analysis of radial wall development using SEM at 9–14 DPA showed formation of secondary radial wall next to the primary cell wall and a thicker fused wall at 18 DPA in wild type while cesa9–1 showed less pronounced secondary radial wall at 9–14 DPA and thinner wall at 18 DPA. Scale bar = 30 μm.
Our data are consistent with the speculation that specific cell types may require functionally distinct cellulose deposition18 and we question whether functionally specification is a consequence of amino acid composition or transcriptional regulation. The developing seed coat represents an excellent system to dissect the composition of CSC in two separate secondary cell wall systems and an intriguing area for future exploration of mediating polarized deposition of cellulose. Moreover, while it has long been held that cellulose is the structural core of the plant extracellular matrix,1,32 its association to adherence between the cell wall and parent cell has been complicated to examine. Our results illustrate that cellulose fulfills a matrix attachment role in cell wall biogenesis.
Acknowledgments
This work was partially supported by the National Science Foundation grants EFRI-0937657 and NSF-IOS- 0922947.
Glossary
Abbreviations:
- CSC
cellulose synthase complex
- CESA
cellulose synthase-A
- WT
wild type
- SEM
scanning electron microscopy
- DPA
days post anthesis
- CEF
cellulose elemental microfibril
- TC
terminal complex
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17709
References
- 1.Brown RM., Jr. Cellulose microfibril assembly and orientation: Recent developments. J Cell Sci Suppl. 1985;2:13–32. doi: 10.1242/jcs.1985.supplement_2.2. [DOI] [PubMed] [Google Scholar]
- 2.Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science. 1998;279:717–20. doi: 10.1126/science.279.5351.717. [DOI] [PubMed] [Google Scholar]
- 3.Heim DR, Skomp JR, Tschabold EE, Larrinua IM. Isoxaben inhibits the synthesis of acid insoluble cell wall materials in Arabidopsis thaliana. Plant Physiol. 1990;93:695–700. doi: 10.1104/pp.93.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giddings TH, Brower DL, Staehelin LA. Visualization of particle complexes in the plasma membrane of micrasterias denticulata associated with the formation of cellulose fibrils in primary and secondary cell walls. J Cell Biol. 1980;84:327–39. doi: 10.1083/jcb.84.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM. Higher plants contain homologs of the bacterial cela genes encoding the catalytic subunit of cellulose synthase. Proc Natl Acad Sci USA. 1996;93:12637–42. doi: 10.1073/pnas.93.22.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richmond TA, Somerville CR. The cellulose synthase superfamily. Plant Physiol. 2000;124:495–8. doi: 10.1104/pp.124.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, et al. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:15566–71. doi: 10.1073/pnas.0706592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, et al. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:15572–7. doi: 10.1073/pnas.0706569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR. Interactions among three distinct cesa proteins essential for cellulose synthesis. Proc Natl Acad Sci USA. 2003;100:1450–5. doi: 10.1073/pnas.0337628100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Y, Kaplinsky N, Bringmann M, Cobb A, Carroll A, Sampathkumar A, et al. Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc Natl Acad Sci USA. 2010;107:12866–71. doi: 10.1073/pnas.1007092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Somerville C. Cellulose synthase interacting protein a new factor in cellulose synthesis. Plant Signal Behav. 2010;5:1571–4. doi: 10.4161/psb.5.12.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, et al. Procuste1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell. 2000;12:2409–24. doi: 10.1105/tpc.12.12.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desprez T, Vernhettes S, Fagard M, Refrégier G, Desnos T, Aletti E, et al. Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform cesa6. Plant Physiol. 2002;128:482–90. doi: 10.1104/pp.010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stork J, Harris D, Griffiths J, Williams B, Beisson F, Li-Beisson Y, et al. Cellulose synthase9 serves a nonredundant role in secondary cell wall synthesis in Arabidopsis epidermal testa cells. Plant Physiol. 2010;153:580–9. doi: 10.1104/pp.110.154062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendu V, Griffiths J, Persson S, Stork J, Downie B, Voiniciuc C, et al. Subfunctionalization of cellulose synthases in seed coat epidermal cells mediate secondary radial wall synthesis and mucilage attachment. Plant Physiol. 2011 doi: 10.1104/pp.111.179069. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan S, Ralet M-C, Berger A, Diatloff E, Bischoff V, Gonneau M, et al. Cesa5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol. 2011 doi: 10.1104/pp.111.179077. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll A, Specht CD. Understanding plant cellulose synthases through a comprehensive investigation of the cellulose synthase family sequences. Front Plant Sci. 2011;2:5. doi: 10.3389/fpls.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpita N, Vergara C. A recipe for cellulose. Science. 1998;279:672–3. doi: 10.1126/science.279.5351.672. [DOI] [PubMed] [Google Scholar]
- 19.Song D, Shen J, Li L. Characterization of cellulose synthase complexes in populus xylem differentiation. New Phytol. 2010;187:777–90. doi: 10.1111/j.1469-8137.2010.03315.x. [DOI] [PubMed] [Google Scholar]
- 20.Betancur L, Singh B, Rapp RA, Wendel JF, Marks MD, Roberts AW, et al. Phylogenetically distinct cellulose synthase genes support secondary wall thickening in Arabidopsis shoot trichomes and cotton fiber. J Integr Plant Biol. 2010;52:205–20. doi: 10.1111/j.1744-7909.2010.00934.x. [DOI] [PubMed] [Google Scholar]
- 21.Western TL, Skinner DJ, Haughn GW. Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol. 2000;122:345–56. doi: 10.1104/pp.122.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macquet A, Ralet M-C, Kronenberger J, Marion-Poll A, North HM. In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol. 2007;48:984–99. doi: 10.1093/pcp/pcm068. [DOI] [PubMed] [Google Scholar]
- 23.Arsovski AA, Haughn GW, Western TL. Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signal Behav. 2010;5:796–801. doi: 10.4161/psb.5.7.11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kacuráková M, Capek P, Sasinková V, Wellner A, Ebringerová A. Ft-ir study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr Polym. 2000;43:195–203. doi: 10.1016/S0144-8617(00)00151-X. [DOI] [Google Scholar]
- 25.Mouille G, Robin S, Lecomte M, Pagant S, Höfte H. Classification and identification of Arabidopsis cell wall mutants using fourier-transform infrared (ft-ir) microspectroscopy. Plant J. 2003;35:393–404. doi: 10.1046/j.1365-313X.2003.01807.x. [DOI] [PubMed] [Google Scholar]
- 26.Abidi N, Hequet E, Cabrales L, Gannaway J, Wilkins T, Wells LW. Evaluating cell wall structure and composition of developing cotton fibers using fourier transform infrared spectroscopy and thermogravimetric analysis. J Appl Polym Sci. 2008;107:476–86. doi: 10.1002/app.27100. [DOI] [Google Scholar]
- 27.Blake AW, McCartney L, Flint JE, Bolam DN, Boraston AB, Gilbert HJ, et al. Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J Biol Chem. 2006;281:29321–9. doi: 10.1074/jbc.M605903200. [DOI] [PubMed] [Google Scholar]
- 28.North H, Baud S, Debeaujon I, Dubos C, Dubreucq B, Grappin P, et al. Arabidopsis seed secrets unravelled after a decade of genetic and omics-driven research. Plant J. 2010;61:971–81. doi: 10.1111/j.1365-313X.2009.04095.x. [DOI] [PubMed] [Google Scholar]
- 29.Nowack MK, Ungru A, Bjerkan KN, Grini PE, Schnittger A. Reproductive cross-talk: Seed development in flowering plants. Biochem Soc Trans. 2010;38:604–12. doi: 10.1042/BST0380604. [DOI] [PubMed] [Google Scholar]
- 30.Haughn G, Chaudhury A. Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci. 2005;10:472–7. doi: 10.1016/j.tplants.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Windsor JB, Symonds VV, Mendenhall J, Lloyd AM. Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 2000;22:483–93. doi: 10.1046/j.1365-313x.2000.00756.x. [DOI] [PubMed] [Google Scholar]
- 32.Green PB. Mechanism for plant cellular morphogenesis. Science. 1962;138:1404–5. doi: 10.1126/science.138.3548.1404. [DOI] [PubMed] [Google Scholar]