Abstract
Ethylene and abscisic acid (ABA) have compact effects on plant development and stress responses. It is not well understood about the mechanism of ABA modulation in ethylene biosynthesis. In our recent research, HY5-AtERF11 regulon was evidenced to connect the ABA action and ethylene biosynthesis. In this paper, by analyzing the expression of ABA biosynthesis genes and the ABA concentration in ethylene over-production mutants, we demonstrated that ethylene production affected by HY5-AtERF11 regulon targeted gene increased the expression of ABA biosynthesis genes and its contents. In addition, we discussed that HY5 might function as a convergence point of multiple hormones in response to light.
Keywords: ABA, AtERF11, ethylene biosynthesis, hormone, HY5, light
Introduction
Abscisic acid (ABA) plays an important role in seed maturation and dormancy, stomatal closure, and adaptation to environmental stress. In addition, ethylene is another stress-induced hormone with fundamental roles in germination, sex determination, leaf abscission, flower senescence, fruit ripening, and responses to biotic and abiotic stress,1 showing that a subset of the functions of ethylene overlaps with those of ABA. Thus the interaction of the ethylene and ABA pathways has been found to be crucial in regulating the development of plants and their responses to environmental stresses.2-4 Genetic studies on the components of ethylene and ABA pathways have suggested the antagonistic interaction between the two signaling pathways.2,3,5 For example, etr1, ein2, ein3, the mutants of ethylene signaling, and aba1, aba2, abi1, abi2, the mutants of ABA pathway, have been found to regulate antagonistically the expression of defense and stress-responsive genes, and subsequently to modulate plant biotic and abiotic stress responses.2,3,5,6 Furthermore, growing evidence indicates the antagonistic interaction between the ethylene and ABA pathways in regulating ethylene and ABA production. Arabidopsis ethylene insensitive mutants ein2/era3 and etr1 are shown to contain higher level of endogenous ABA, while the ABA deficient mutant aba2 displays an increase of ethylene production.5,7,8 Despite the existence of antagonistic interactions, increasing evidence also strongly suggests a synergistic interaction between the ethylene and ABA pathways in regulating plant development and defense responses. For instance, both ethylene and ABA can inhibit root growth, mutants ein2 and etr1 show enhanced resistance to ethylene and ABA, while root growth in the ABA-deficient mutant abi1 only displays resistance to ABA but not ethylene,2,5,9 indicating that ABA-inhibited root growth depends on the ethylene signaling. Hence, the ABA and ethylene signaling pathways have a close interplay in plant growth, development, and stress response. However, it remains unknown whether their respective biosyntheses have any convergent points. In this report we will briefly present an overview on the current knowledge about ABA regulation in ethylene biosynthesis.
ABA initiates a transcriptional cascade of HY5-AtERF11 to repress ethylene biosynthesis
ABA, auxins, cytokinins, ethylene, gibberellins, brassinosteroids, jasmonates and salicylic acid are essential for the regulation of plant growth, development, reproduction and survival. The complex network of cross-communication of hormone signaling pathways controls plant growth, development and defense, and changes in hormone concentration or sensitivity, which can be triggered under biotic and abiotic stress conditions, mediate a whole range of adaptive plant responses.10,11 The relationship of ABA and ethylene was extensively discussed in the past years.2,3,5,12 Though ABA affects the ethylene production in etiolated seedlings, the roots of the eto1-1 mutants were highly insensitive to ABA,5,13 the mechanism of ABA-modulated ethylene biosynthesis is much less clear. In a recent study, we reported a crucial transcriptional cascade in ABA-modulated ethylene biosynthesis. Our work demonstrated that HY5 binds to the G-box of AtERF11 to activate its transcription; AtERF11 as an EAR repressor in turn binds to the DRE of ACS genes to repress their transcription, thereby causing a reduction in ethylene emission.14 Our work evidenced that (1) HY5-AtERF11 as one of crucial transcriptional cascades under ABA action controls ethylene biosynthesis, providing new insight into the integration of ABA and ethylene biosynthesis; (2) the light signaling regulator, HY5 is involved in ethylene biosynthesis, raising interesting questions on the interaction of light signaling and ethylene biosynthesis; (3) post-transcriptional regulation of ACS proteins in ethylene biosynthesis process was pivotal for ethylene production, more than one reports showed that transcriptional regulation also played a key role in ethylene biosynthesis, our work evidenced that AtERF11 directly binds to the promoter to negatively regulate the expression of ACS genes, revealing the role of EAR motif containing ERF genes in Arabidopsis ethylene biosynthesis.14
The dynamic balance of ABA and ethylene is required for Arabidopsis development
It is reported that ACS genes have a distinct spatial and temporal expression pattern in different stages of Arabidopsis growth and development, and under various stresses.15,16 Most recent study revealed that the mutation of ACS7 that displayed increased expression of ABA biosynthesis genes and accumulation, enhanced ABA sensitivity.17 Oppositely, the knockout mutant acs4-1, which displayed 40% increase of ethylene,15 enhanced ABA tolerance in root growth as eto mutants in the early seedling stage (data not shown). These differential observations reveal that different expression pattern of each ACS gene in the different developmental stages and organs leads to diverse responses to ABA, which decides the character of ACS genes in the interaction of ABA with ethylene. However, the deficiency of ethylene mutant heptuple results in more tolerant response to ABA in root and shoot growth,15 indicating a more complex interaction of ethylene and ABA in the regulation of the expression of ACS genes.
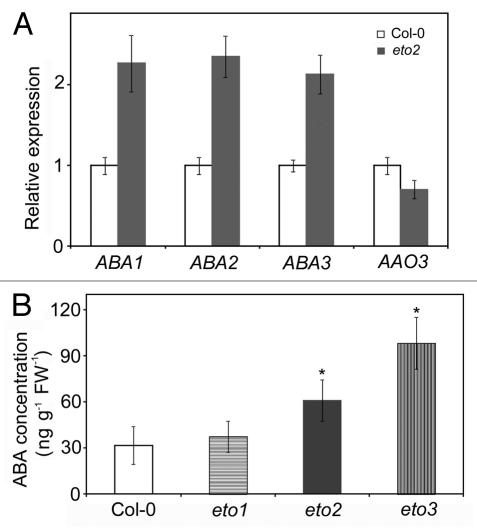
Accumulating evidence indicates that the interaction of ABA and ethylene signaling pathways extensively regulates plant growth, development and stress responses, less research was reported in the relationship of ethylene biosynthesis and ABA biosynthesis/metabolic. After checking the microarray data of eto2 (data not shown), we found that transcript levels of ABA biosynthesis genes (ABA1, ABA2 and ABA3, except AAO3) in the eto2 were obviously increased (Fig. 1A). Moreover, the endogenous ABA levels were significantly elevated in the mutation mutants of ACS5 (eto2) and ACS9 (eto3) genes, compared with these in Col. But there were no significantly different ABA contents in Col and an E3 ligase mutation mutant (eto1) (Fig. 1B), indicating that the ethylene biosynthesis, triggered at least by HY5-AtERF11 regulon targeted gene,14 stimulates ABA biosynthesis for balance the hormone through the regulation of specific ABA biosynthesis genes.
Figure 1.
Ethylene overproduction elevates endogenous ABA levels. (A) Transcript levels of ABA biosynthesis genes in Col-0 and eto2. Seedlings were grown on MS plates for 4 d under normal growth conditions. After RNA isolation and reverse transcription, Real time PCR analyses were performed. Expression levels were normalized to that of TUB4. Each value is the mean ± SD of three independent biological determinations. (B) ABA content of Col-0 and eto1, eto2 and eto3. Seedlings were grown on MS plates for 4 d then subjected to ABA immunoassay. ABA content was measured by the method described by Wu et al.47 Each value is the mean ± SD of three independent biological determinations. The P-value (eto mutants vs. Col-0) was determined by a two-tailed Student’s t-test assuming equal variances (*p < 0.05).
Although ethylene plays key roles in seed germination, seedling growth, flowering and seed mature, the increased ethylene also inhibits the growth and development.12,18 Additionally, high level of ABA was regarded as a growth inhibitor;9 and both ethylene and high level of ABA inhibit the root length, low level ABA, however, promotes root growth by promoting quiescent center quiescence,18,19 indicating that the inhibition of root length is the balance of ABA and ethylene hormones. Both endogenous developmental and exogenous environmental signals increased one kind of the hormones, others would be responded. Moreover, the increase of endogenous ABA levels in eto mutants might be act as a compensation for high level of ethylene in vivo, and endogenous ethylene changes and exogenous ABA stimulation would cause the dynamic balance of the two hormones in plant.
HY5 participates in multiple hormone biosyntheses and signaling pathways
HY5 plays positive regulatory role in photomorphogenesis by controlling the expression of multiple genes including light-responsive and hormone biosynthesis genes.20-23 Also, the phenotype of hy5 showing longer hypocotyl and lateral roots supports that hormone related genes might be involved in HY5 regulatory network,22,24 suggesting that HY5 is an important crosstalk point of gibberellins, cytokinin, auxin and ABA in Arabidopsis development and stress response.20,24-30 For instance, defection of gibberellin metabolism or signaling partially repressed photomorphogenesis, which is attributed to the fact that gibberellins reduced the abundance of HY5 via COP1-mediated pathway.25,29,30 In addition, the interaction of LIP1 and LONG1, the pea ortholog of Arabidopsis thaliana COP1 and HY5, respectively, regulated the expression of gibberellin catabolism gene GA2ox2 and gibberellin levels.26 Distinctive to gibberellins, cytokinin induced HY5 protein accumulation likely mediating COP1 under light conditions.27 And HY5 and its homolog HYH (HY5 HOMOLOG) negatively regulate AUXIN RESISTANT2 (AXR2) and SOLITARY ROOT (SLR) genes, possibly directly targeting the potential AUX/IAAs and AUXIN RESPONSE FACTOR genes in auxin signaling.24,28 And the fact that HY5 directly transcriptionally regulated the expression of ABA responsive ABI5 reveals that the HY5 integrates ABA and light signal transduction pathways.20 More importantly, we found that HY5 was a novel negative regulator of ethylene biosynthesis, and this regulation was through ABA-dependent activation of AtERF11 expression, evidencing that HY5 was a key factor in the regulation of ethylene synthesis. However, differences were observed between eto1, eto2 and eto3 in terms of the growth phenotypes and ABA responsiveness, and the observation of ACS different expression pattern in hy5 suggests that HY5-regulated ethylene biosynthesis may be occurred in numerous pathways. Actually, a genome survey of the in vivo targets of HY5 using chromatin-immunoprecipitation identified the potential ethylene biosynthesis and ERF genes,31 which probably occurred in three pathways: (1) HY5 directly transcriptionally regulates the expression of ACS genes; (2) ERF proteins, such as AtERF4, AtERF7, Sub1A and LeERF2/TERF2 downstream of ethylene signaling pathway, regulate ethylene biosynthesis,32-38 but the possibility that HY5 transcriptionally activates the expression of other ERF genes that mediate the ABA response remains to be effectively approved; (3) HY5 is a key factor in normal development under light conditions to integrate hormone balance, other regulators conceivable transcriptionally regulated by HY5 might participate in ethylene biosynthesis. Further investigation of HY5 regulation in the expression of ACS genes would be important for revealing the mechanism of integration in ethylene biosynthesis and light signaling pathway.
Perspective
Considering that both light and ethylene stimulate seed germination in normal or stress conditions, it is of great importance to note the components involved in the two signaling pathways. For example, light promotes ethylene biosynthesis through the action of PIF5 (PHYTOCHROME INTERACTING FACTOR 5);39 and our recent report also evidenced that HY5 regulates this process.14 The low extend of red to far-red light ratio regulated the emission of ethylene,40 indicating that light modulates ethylene biosynthesis. More than that, ethylene and light are critical regulators of plant responses to salt stress. In Arabidopsis, STO interacted with COP1 to enhance root growth in high salinity stress;41,42 in rice, overexpression of OsHAL3 increased seedling growth under normal light/dark cycles and enhanced salt tolerance and Na+/K+ homeostasis.43 These studies, combined with the regulation of ethylene in salt response,44 indicate that ethylene and components of the light signaling pathway are key regulators of salt stress. Furthermore, the EIN2 interacted with a putative COP9 signalosome (CSN) component EER5 (Ethylene Enhanced Response 5),45 implying a role of EIN2 in the regulation of SCF activity through the modulation of the CSN. In addition, the evidence that EIN3/EIL1 downstream of COP1 activates the ethylene-induced seedling greening reveals that compounds of ethylene signal pathway participate in photomorphogenic development,46 implying a complex mode of interactions between ethylene and light signaling pathways in Arabidopsis. Further investigations that excavate the cross-talk of the two signal pathways would be significantly important.
Acknowledgment
This work was supported by the National Science Foundation of China (90917018) and the National Basic Research Program of China (2012CB114204).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17756
Reference
- 1.Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–54. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- 2.Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;12:1103–15. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, et al. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–79. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo H, Ecker JR. The ethylene signaling pathway: new insights. Curr Opin Plant Biol. 2004;7:40–9. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–26. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagisawa S, Yoo SD, Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature. 2003;425:521–5. doi: 10.1038/nature01984. [DOI] [PubMed] [Google Scholar]
- 7.LeNoble ME, Spollen WG, Sharp RE. Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J Exp Bot. 2004;55:237–45. doi: 10.1093/jxb/erh031. [DOI] [PubMed] [Google Scholar]
- 8.Chiwocha SD, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross AR, et al. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J. 2005;42:35–48. doi: 10.1111/j.1365-313X.2005.02359.x. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14(Suppl):S15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–16. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 11.Jaillais Y, Chory J. Unraveling the paradoxes of plant hormone signaling integration. Nat Struct Mol Biol. 2010;17:642–5. doi: 10.1038/nsmb0610-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomann A, Lechner E, Hansen M, Dumbliauskas E, Parmentier Y, Kieber J, et al. Arabidopsis CULLIN3 genes regulate primary root growth and patterning by ethylene-dependent and -independent mechanisms. PLoS Genet. 2009;5:e1000328. doi: 10.1371/journal.pgen.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woeste KE, Ye C, Kieber J. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999;119:521–30. doi: 10.1104/pp.119.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Zhang L, Yu Y, Quan R, Zhang Z, Zhang H, et al. Ethylene response factor AtERF11 that is transcriptionally modulated by bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J. 2011 doi: 10.1111/j.1365-313X.2011.04670.x. In press. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchisaka A, Yu G, Jin H, Alonso JM, Ecker JR, Zhang X, et al. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics. 2009;183:979–1003. doi: 10.1534/genetics.109.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang NN, Shih MC, Li N. The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot. 2005;56:909–20. doi: 10.1093/jxb/eri083. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Zhen Z, Peng J, Chang L, Gong Q, Wang NN. Loss of ACS7 confers abiotic stress tolerance by modulating ABA sensitivity and accumulation in Arabidopsis. J Exp Bot. 2011 doi: 10.1093/jxb/err143. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega-Martínez O, Pernas M, Carol RJ, Dolan L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science. 2007;317:507–10. doi: 10.1126/science.1143409. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A, et al. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 2010;64:764–74. doi: 10.1111/j.1365-313X.2010.04367.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Zhang J, Neff MM, Hong SW, Zhang H, Deng XW, et al. Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci USA. 2008;105:4495–500. doi: 10.1073/pnas.0710778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, et al. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell. 1998;1:213–22. doi: 10.1016/S1097-2765(00)80022-2. [DOI] [PubMed] [Google Scholar]
- 22.Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997;11:2983–95. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002;16:1247–59. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday GK, Hardtke CS. Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet. 2006;2:e202. doi: 10.1371/journal.pgen.0020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alabadí D, Gallego-Bartolome J, Orlando L, Garcia-Carcel L, Rubio V, Martinez C, et al. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2008;53:324–35. doi: 10.1111/j.1365-313X.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 26.Weller JL, Hecht V, Vander Schoor JK, Davidson SE, Ross JJ. Light regulation of gibberellin biosynthesis in pea is mediated through the COP1/HY5 pathway. Plant Cell. 2009;21:800–13. doi: 10.1105/tpc.108.063628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandenbussche F, Habricot Y, Condiff AS, Maldiney R, Van der Straeten D, Ahmad M. HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana. Plant J. 2007;49:428–41. doi: 10.1111/j.1365-313X.2006.02973.x. [DOI] [PubMed] [Google Scholar]
- 28.Cluis CP, Mouchel CF, Hardtke CS. The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 2004;38:332–47. doi: 10.1111/j.1365-313X.2004.02052.x. [DOI] [PubMed] [Google Scholar]
- 29.Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–9. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–4. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, et al. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–49. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Tian L, Latoszek-Green M, Brown D, Wu K. Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Mol Biol. 2005;58:585–96. doi: 10.1007/s11103-005-7294-5. [DOI] [PubMed] [Google Scholar]
- 33.Song CP, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, et al. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell. 2005;17:2384–96. doi: 10.1105/tpc.105.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–34. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukao T, Yeung E, Bailey-Serres J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell. 2011;23:412–27. doi: 10.1105/tpc.110.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z, Zhang Z, Zhang X, Zhang H, Huang D, Huang R. Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Lett. 2004;573:110–6. doi: 10.1016/j.febslet.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Zhang Z, Chen J, Chen Q, Wang XC, Huang R. Expressing TERF1 in tobacco enhances drought tolerance and abscisic acid sensitivity during seedling development. Planta. 2005;222:494–501. doi: 10.1007/s00425-005-1564-y. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Zhang H, Quan R, Wang XC, Huang R. Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol. 2009;150:365–77. doi: 10.1104/pp.109.135830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khanna R, Shen Y, Marion CM, Tsuchisaka A, Theologis A, Schafer E, et al. The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell. 2007;19:3915–29. doi: 10.1105/tpc.107.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierik R, Cuppens ML, Voesenek LA, Visser EJ. Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol. 2004;136:2928–36. doi: 10.1104/pp.104.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Indorf M, Cordero J, Neuhaus G, Rodriguez-Franco M. Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J. 2007;51:563–74. doi: 10.1111/j.1365-313X.2007.03162.x. [DOI] [PubMed] [Google Scholar]
- 42.Nagaoka S, Takano T. Salt tolerance-related protein STO binds to a Myb transcription factor homologue and confers salt tolerance in Arabidopsis. J Exp Bot. 2003;54:2231–7. doi: 10.1093/jxb/erg241. [DOI] [PubMed] [Google Scholar]
- 43.Sun SY, Chao DY, Li XM, Shi M, Gao JP, Zhu MZ, et al. OsHAL3 mediates a new pathway in the light-regulated growth of rice. Nat Cell Biol. 2009;11:845–51. doi: 10.1038/ncb1892. [DOI] [PubMed] [Google Scholar]
- 44.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, et al. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–4. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 45.Christians MJ, Robles LM, Zeller SM, Larsen PB. The eer5 mutation, which affects a novel proteasome-related subunit, indicates a prominent role for the COP9 signalosome in resetting the ethylene-signaling pathway in Arabidopsis. Plant J. 2008;55:467–77. doi: 10.1111/j.1365-313X.2008.03521.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhong S, Zhao M, Shi T, Shi H, An F, Zhao Q, et al. EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc Natl Acad Sci USA. 2009;106:21431–6. doi: 10.1073/pnas.0907670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L, Chen X, Ren H, Zhang Z, Zhang H, Wang J, et al. ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta. 2007;226:815–25. doi: 10.1007/s00425-007-0528-9. [DOI] [PubMed] [Google Scholar]