Abstract
In a recent publication using an actin-visualized line of Arabidopsis (Ichikawa et al. 2011, ref. 11), we reported a detailed analysis with higher time resolution on the dynamics of chloroplast actin filaments (cp-actin filaments) during chloroplast avoidance movement and demonstrated a good correlation between the biased configuration of cp-actin filaments and chloroplast movement. However, we could not conclusively determine whether the reorganization of cp-actin filaments into a biased configuration preceded actual chloroplast movement (and, thus, whether it could be a cause of the movement). In this report, we present clear evidence that the reorganization of cp-actin filaments into a biased distribution is induced even in the absence of the actual movement of chloroplasts. When the cells were treated with 2,3-butanedione monoxime (BDM), a potent inhibitor of myosin ATPase, chloroplast motility was completely suppressed. Nevertheless, the disappearance and biased relocalization of cp-actin filaments toward the side of the prospective movement direction were induced by irradiation with a strong blue light microbeam. The results definitively indicate that the reorganization of cp-actin filaments is not an effect of chloroplast movement; however, it is feasible that the biased localization of cp-actin filaments is an event leading to chloroplast movement.
Keywords: Actin filament, Arabidopsis, Chloroplast movement, Organelle movement, Photomovement, Phototropin
Chloroplast relocation movement in response to environmental light conditions is a ubiquitous phenomenon in the plant kingdom.1-3 In Arabidopsis thaliana, phototropin (phot) 1 and 2 act as blue light photoreceptors and regulate the accumulation movement toward weak light; phot2 alone is responsible for avoidance movement away from strong light.4-6 Based on the analyses of actin dynamics during chloroplast photomovement in an actin-visualized transgenic Arabidopsis line expressing GFP-talin, we have shown previously that phot-dependent chloroplast photorelocation adopts a novel movement mechanism that utilizes short actin filaments unique to chloroplasts, which we called cp-actin filaments (chloroplast actin filaments).7 The cp-actin filaments are located between the chloroplasts and the plasma membrane and show a biased localization on the chloroplasts during movement. These filaments are abundant on the leading edge of chloroplasts, and the difference in the amount between the front and rear halves of the chloroplasts is well correlated with their speed both in avoidance and in accumulation movements. In the non-biased configuration, cp-actin filaments also function in the anchoring of chloroplasts to the site of their location within the cytoplasm. In the mutants lacking cp-actin filaments, we had consistently found that no photorelocation was induced. Furthermore, we had also observed that the chloroplasts occasionally showed rapid movement in the cell, which was indicative of passive transport along the cytoplasmic streaming as a result of detachment from the plasma membrane.7,8 In these studies, however, the temporal resolution of cp-actin filament dynamics was limited to 5 or 10 min due to technical reasons. The blue GFP excitation beam for fluorescence imaging is inevitably absorbed by phots, thus reducing their light absorption gradient in the cell under partial cell irradiation, which is essential for the induction of chloroplast avoidance and accumulation movements. Cp-actin filament systems have also been described in moss and fern cells, and similar functions in chloroplast movement and anchoring have been suggested.9,10
In a recent publication by Ichikawa et al. (2011),11 cp-actin reorganization during the avoidance movement of chloroplasts was analyzed under a higher time resolution, in the range of seconds. In the report, rather than using partial cell irradiation, chloroplast avoidance movement was induced by continuous irradiation of whole cells with a strong blue GFP excitation beam for the imaging of actin filaments. Under this condition, the cp-actin filaments in the wild type plants transiently disappeared after approximately 30 sec, and a biased configuration appeared at approximately 70 sec after induction by the blue excitation light; the chloroplast avoidance movement occurred at nearly the same time as the latter (i.e., 70 sec). In phot2 and phot1phot2 mutant plants, however, neither cp-actin filament reorganization nor chloroplast movement was observed, yet the promotion of the reorganization of the cp-actin filaments was observed in the phot1 single mutant. Consistently, the chloroplast movement in the phot1 mutants began earlier than in the wild type plants, suggesting inhibitory actions of phot1 on the cp-actin filament reorganization and, thus, the avoidance movement. Furthermore, a modulation by a background red light of the blue light-induced avoidance response was clarified; both promotive and inhibitory effects of red light were found depending on the light intensity of red light. Under a weak background red light, the timing to attain the biased configuration of the cp-actin filaments was earlier, and the chloroplasts began their movement more quickly when compared with the condition without the background red light. In contrast, strong red light delayed the timing of the chloroplast movement, and the time to achieve the biased cp-actin configuration was also significantly delayed. Therefore, all of the evidence obtained indicated a correlation between the biased configuration of cp-actin filaments and chloroplast movement.
Unfortunately, however, we could not conclusively determine whether the reorganization of cp-actin filaments into the biased configuration preceded the actual chloroplast movement. The difficult-to-detect nature of the cp-actin filament system and the low speed of the chloroplast movement prevented further quantitative analyses of the cp-actin filament reorganization and motility. Thus, the temporal relationship between the reorganization of the cp-actin filaments and the actual movement of the chloroplasts was not resolved; the possibility remained that the biased cp-actin configuration could be the results of chloroplast migration.
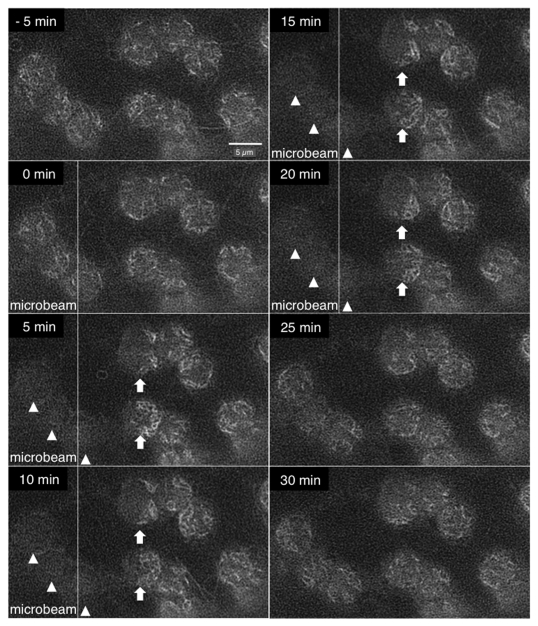
In this report, we present evidence that the reorganization of cp-actin filaments into a biased distribution is, at least, not an outcome of the movement of chloroplasts, as we demonstrate the dynamics of the filaments in the absence of chloroplast movement. Plant organelle movements are suppressed by 2,3-butanedione monoxime (BDM) or N-ethylmaleimide (NEM), potent inhibitors of myosin ATPase;12,13 however, due to the potential off-target effects of these inhibitors,11 it has remained unclear whether plant organelle movements depend on myosins. Indeed, recent genetic evidence has not provided any indication that myosins are involved in chloroplast motility, which contrasts with what has been shown for other organelles, such as the mitochondria, peroxisomes, Golgi bodies and endoplasmic reticulum.14 The contribution of class XI myosins in organellar movements has been clearly demonstrated15-19 for the above organelles but not for chloroplast movement.14,15 Regardless of whether BDM inhibited plant myosins or non-myosin proteins, the chloroplast motility in Arabidopsis cells that were treated with 25 mM BDM was suppressed, and no avoidance movements were induced under microbeam irradiation with blue light of 377 µmole m−2 sec−1 (Fig. 1). When we examined the blue light effect on the dynamics of the cp-actin filaments, we observed the same reorganization of the cp-actin filaments as had been observed under the normal condition without the inhibitor.7 The cp-actin filaments on the chloroplasts near the microbeam exhibited a biased relocalization toward the side of the direction of prospective movement upon irradiation with a microbeam blue light (arrowheads), but their biased distribution was abolished when the microbeam was turned off. In addition, the cp-actin filaments on the chloroplasts in the beam area disappeared upon blue light irradiation (arrows) but reappeared when the irradiation ceased. This evidence definitively indicates that the reorganization of the cp-actin filaments was not an effect of the chloroplast movement. Assuming each cp-actin filament has a pulling force, we propose that the biased distribution of the cp-actin filaments directly induces chloroplast movement depending on the difference in the number of filaments attached at either end.
Figure 1.
Reorganization of cp-actin filaments in the absence of movement. Arabidopsis cells were treated with 25 mM 2,3-butanedione monoxime (BDM). After 30 min of incubation in the dark, a cell was observed under background red light (89 µmol m−2 sec−1), followed by partial irradiation with a microbeam blue light (377 µmol m−2 sec−1) for 20 min in the presence of the background red light. Note that the cp-actin filaments on the chloroplasts near the microbeam exhibited a biased relocalization upon microbeam blue light irradiation (arrowheads), but their biased distribution was abolished when the light was turned off. Note also that the cp-actin filaments on the chloroplasts in the beam area disappeared upon blue light irradiation (arrows) but reappeared when the light was turned off. A control experiment without BDM can be found in Kadota et al. 2009 (Fig. 1A-C in ref. 7).
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Sports, Science and Technology of Japan and from the Japan Society for the Promotion of Science (19039027 and 22570047 to A.K.; 17084006, 20227001, and 23120523 to M.W. and 20870030 to N.S.).
Glossary
Abbreviations:
- cp-actin filament
chloroplast actin filament
- GFP
green fluorescent protein
- phot
phototropin
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17767
References
- 1.Wada M, Kagawa T, Sato Y. Chloroplast movement. Annu Rev Plant Biol. 2003;54:455–68. doi: 10.1146/annurev.arplant.54.031902.135023. [DOI] [PubMed] [Google Scholar]
- 2.Suetsugu N, Wada M. Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol Chem. 2007;388:927–35. doi: 10.1515/BC.2007.118. [DOI] [PubMed] [Google Scholar]
- 3.Suetsugu N, Wada M. Phytochrome-dependent photomovement responses mediated by phototropin family proteins in cryptogam plants. Photochem Photobiol. 2007;83:87–93. doi: 10.1562/2006-02-27-IR-817. [DOI] [PubMed] [Google Scholar]
- 4.Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–4. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 5.Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, et al. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science. 2001;291:2138–41. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 6.Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, et al. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98:6969–74. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, et al. Short actin-based mechanism in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:13106–11. doi: 10.1073/pnas.0906250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suetsugu N, Yamada N, Kagawa T, Yonekura H, Uyeda TQP, Kadota A, et al. Two kinesin-like proteins mediate actin-based chloroplast movement in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2010;107:8860–5. doi: 10.1073/pnas.0912773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita H, Sato Y, Kanegae T, Kagawa T, Wada M, Kadota A. Chloroplast actin filaments organize meshwork on the photorelocated chloroplasts in the moss Physcomitrella patens. Planta. 2011;233:357–68. doi: 10.1007/s00425-010-1299-2. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi H, Wada M. Distribution pattern changes of actin filaments during chloroplast movement in Adiantum capillus-veneris. J Plant Res. 2011 doi: 10.1007/s10265-011-0444-8. In press. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa S, Yamada N, Suetsugu N, Wada M, Kadota A. Red light, phot1 and JAC1 modulate phot2-dependent reorganization of chloroplast actin filaments and chloroplast avoidance movement. Plant Cell Physiol. 2011;52 doi: 10.1093/pcp/pcr087. In press. [DOI] [PubMed] [Google Scholar]
- 12.Williamson RE. Organelle movements. Annu Rev Plant Physiol. 1993;44:181–202. doi: 10.1146/annurev.pp.44.060193.001145. [DOI] [Google Scholar]
- 13.Shimmen T. The sliding theory of cytoplasmic streaming: fifty years of progress. J Plant Res. 2007;120:31–43. doi: 10.1007/s10265-006-0061-0. [DOI] [PubMed] [Google Scholar]
- 14.Suetsugu N, Dolja VV, Wada M. Why have chloroplasts developed a unique motility system? Plant Signal Behav. 2010;5:1190–6. doi: 10.4161/psb.5.10.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avisar D, Prokhnevsky AI, Makarova KS, Koonin EV, Dolja VV. Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol. 2008;146:1098–108. doi: 10.1104/pp.107.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avisar D, Abu-Abied M, Belausov E, Sadot E, Hawes C, Sparks IA. A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol. 2009;150:700–9. doi: 10.1104/pp.109.136853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparkes I, Runions J, Hawes C, Griffing L. Movement and remodeling of the endoplasmic reticulum in nondividing cells tobacco leaves. Plant Cell. 2009;21:3937–49. doi: 10.1105/tpc.109.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, et al. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci USA. 2010;107:6894–9. doi: 10.1073/pnas.0911482107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peremyslov VV, Prokhnevsky AI, Dolja VV. Class XI myosins are required for development, cell expansion and F-actin organization in Arabidopsis. Plant Cell. 2010;22:1883–97. doi: 10.1105/tpc.110.076315. [DOI] [PMC free article] [PubMed] [Google Scholar]