Abstract
Vacuoles of different leaf cell-types vary in their capacity to store specific mineral elements. In Arabidopsis thaliana potassium (K) accumulates preferentially in epidermal and bundle sheath cells whereas calcium (Ca) and magnesium (Mg) are stored at high concentrations only in mesophyll cells. Accumulation of these elements in a particular vacuole can be reciprocal, i.e. as [K]vac increases [Ca]vac decreases. Mesophyll-specific Ca-storage involves CAX1 (a Ca2+/H+ antiporter) and Mg-storage involves MRS2-1/MGT2 and MRS2-5/MGT3 (both Mg2+-transporters), all of which are preferentially expressed in the mesophyll and encode tonoplast-localised proteins. However, what controls leaf-cell [K]vac is less well understood. TPC1 encodes the two-pore Ca2+ channel protein responsible for the tonoplast-localised SV cation conductance, and is highly expressed in cell-types that not preferentially accumulate Ca. Here, we evaluate evidence that TPC1 has a role in maintaining differential K and Ca storage across the leaf, and propose a function for TPC1 in releasing Ca2+ from epidermal and bundle sheath cell vacuoles to maintain low [Ca]vac. Mesophyll-specific Ca storage is essential to maintain apoplastic free Ca concentration at a level that does not perturb a range of physiological parameters including leaf gas exchange, cell wall extensibility and growth. When plants are grown under serpentine conditions (high Mg/Ca ratio), MGT2/MRS2-1 and MGT3/MRS2-5 are required to sequester additional Mg2+ in vacuoles to replace Ca2+ as an osmoticum to maintain growth. An updated model of Ca2+ and Mg2+ transport in leaves is presented as a reference for future interrogation of nutritional flows and elemental storage in plant leaves.
Keywords: Apoplast, calcium, CAX1, cell-specific, compartmentation, GLR, magnesium, mesophyll, MGT, MRS2, nutrition, TPC1, vacuole
Introduction
The storage pool for mineral elements in the vacuoles of different leaf cell-types is compositionally distinct despite a ubiquitous need for all nutrients in each cell, or a constitutive toxicity for all cells when heavy metals and NaCl are accumulated at high concentrations.1 For instance, it is generally observed that phosphate and calcium (Ca) do not co-localize to high concentrations in the same cell vacuole; if they did it would be expected that a large proportion of both elements would exist as insoluble calcium phosphate.1 In contrast, magnesium (Mg), potassium (K), chloride and nitrate may share similar cellular locations but can be at very different concentrations in different cells.1,2 Furthermore, the cellular location of a particular element is robust within an individual plant, but the cell-type that accumulates each element can vary between species.1-3
Until recently, the mechanisms and physiological role for element compartmentation in different cell-types was unknown. In two papers published in 2011 Single-Cell analysis and SAmpling (SiCSA) was used to reveal the genetic basis underpinning mesophyll-specific Ca and Mg storage in Arabidopsis thaliana leaves. In addition, a variety of physiological assays were used to uncover the fundamental importance of cell-specific nutrient compartmentation with respect to plant productivity.4,5
Cell-specific Ca storage in leaves
In Conn et al.,4,5 Arabidopsis leaves were observed to preferentially store Ca in vacuoles of palisade and spongy mesophyll cells at concentrations over 60 mM, whereas epidermal and bundle sheath cell vacuoles accumulated a [Ca] ([Ca]vac) of less than 10 mM. For Ca2+ to be accumulated in the vacuole it first enters the cell across the plasma membrane, presumably through Ca2+-permeable channels. It must then be transported against an electrochemical gradient across the tonoplast because [Ca2+]cyt is normally in the nM range. In an attempt to identify the mechanism underpinning the observed mesophyll cell-preferential compartmentation of Ca, adaxial epidermal and palisade mesophyll cell transcriptomes were compared. Although no known or hypothesized Ca2+-channels were differentially expressed between these cell-types it was revealed that certain Ca2+-transporters were differentially expressed. A vacuolar-localized Ca2+/H+ exchanger (CAX1) was the most highly represented Ca2+-transporter transcript present in the mesophyll and the most differentially expressed between epidermal and mesophyll cells, ~375-fold higher in the mesophyll (Fig. 1A).4 The T-DNA insertional mutant of CAX1 (cax1–1) did not have a [Ca]vac phenotype.4 However, the T-DNA insertion mutant cax1–1/cax3–1 (cax1/cax3),6 lacking expression of both CAX1 and CAX3, had 42% lower Ca stored in the palisade mesophyll but a 3-fold higher free apoplastic Ca concentration ([Ca]apo).4 CAX3 is not normally highly expressed in leaves, however, the CAX3 transcript encodes a tonoplast-localized Ca2+/H+-transporter closely related to CAX1 that is pleiotropically upregulated in cax1 leaves when CAX1 is non-functional.4,6
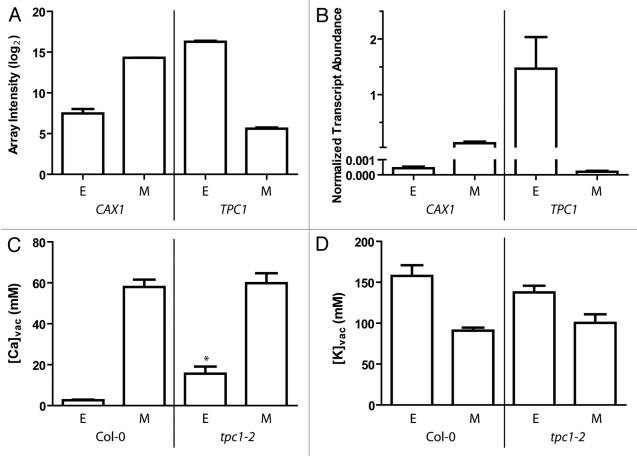
Figure 1.
(A) Expression of TPC1 and CAX1 in epidermal (E) and mesophyll (M) cells. Sampled using SiCSA and subsequent microarray (mean log2 array spot intensity + S.E.M.) or (B) qPCR (mean transcript abundance + S.E.M.) normalized against EF1-α and β-Tubulin5; n = 3 for each cell-type isolated from three different plants. For methods see Conn et al.4,5 Mean transcript abundance was statistically different between epidermal and mesophyll samples between genotypes for both genes (p < 0.05, using a One-way ANOVA and tukey’s posthoc test). (C) Vacuolar [Ca] in Col-0 and tpc2–1, n = 7 per cell type across 3 plants. Asterisk indicates a statistically significant larger value p < 0.01 in epidermal [Ca] of tpc1–2 compared with Col-0 using an unpaired t-test with Welch’s correction (GraphPad Prism). (D) Vacuolar [K] in Col-0 and tpc2–1, n = 7 per cell type across 3 plants. No statistically significant differences were detected between cell-types.
Despite the reduction in mesophyll [Ca]vac in cax1/cax3 plants there was still a preferential Ca accumulation in the mesophyll over the epidermis. This was hypothesized to be due to the presence of other Ca2+-transporters located on the tonoplast as there was an observed increase in transcript abundance for genes that encode tonoplast localized proteins (CAX2, CAX4, ACA4 and ACA11).4,6 However, in terms of Ca2+ accumulation a principal role for the tonoplast localized Ca2+-ATPases ACA4 and ACA11, which are also highly expressed in the mesophyll, can be ruled out as single and double knockout plants did not have a reduced leaf Ca content. Instead, ACA4 and ACA11 have been found to have a role in salicylic acid (SA) signaling and systemic acquired response.7
In cax1/cax3 plants, in addition to an increase in transcript abundance for several genes that encode tonoplast localized Ca2+-transporters, there was an increase in abundance of transcripts that encode Ca2+-transporters that are expressed on other membranes.4,7 Most notably this included ACA1 and ACA2 localized on plastid membranes and ACA10 on the plasma membrane.4 This suggests that when the ability to deposit [Ca2+]cyt into the vacuole is compromised, the cell may maintain a low [Ca2+]cyt by sequestering Ca2+ into other compartments including the apoplast. High apoplastic Ca2+ ([Ca2+]apo) has been implicated in a range of physiological processes such as stomatal closure and cell wall modification,8,9 therefore these processes were examined in the cax1/cax3 mutant.
Physiological processes regulated by [Ca2+]apo
Lower gas exchange and cell wall extensibility, and higher cell wall thickness and demethylesterified pectin content, was found in cax1/cax3 leaves compared with those from the parental background Columbia-0 (Col-0) when grown under standard nutrient supply. All these parameters were hypothesized to underpin the slower growth rate of cax1/cax3 compared with Col-0.4 By growing plants in low Ca solution (LCS; aCa = 25 µM) compared with basal nutrient solution (BNS; aCa = 1 mM), free [Ca]apo in the leaf could be equalized between both genotypes. Under such conditions all of the underlying parameters mentioned above and growth were equalized between both genotypes.4 A preliminary investigation into the transcriptional basis for the modification in cell wall strength elicited by changes in free [Ca]apo was also performed and results were consistent with the increase in growth of cax1/cax3 when [Ca]apo was reduced. For instance, transcript abundance of genes from the PECTINMETHYLESTERASE family (PMEPCRB, PME1 and PME2) were higher in cax1/cax3 leaves than Col-0. Proteins of the PME family are believed to demethylesterify pectin in order for Ca2+ to bind, thereby strengthening the wall by crosslinking cellulose microfibrils. Also the transcript abundance of EXPANSIN genes (EXP15 and EXP16), believed to be involved in cell wall expansion, and POLYGALACTURONASE (PGA3) believed to breakdown demethylesterified pectin were lower in cax1/cax3 leaves than Col-0 in BNS.4 However, for all the above cell wall-related genes, their expression in cax1/cax3 plants grown in LCS was similar to Col-0 grown in BNS.4 Such a result is consistent with the equalization of growth of the two genotypes under these conditions. Therefore, the importance of controlling free [Ca]apo, and by extension the role of sequestration of apoplastic Ca2+ into mesophyll cell vacuoles, can be seen to be central to optimal leaf productivity.4
The control of passive efflux of Ca2+ out of vacuoles
Despite the differences in Ca accumulation within the different cells of the leaf, all viable cells have an electrochemical gradient for Ca2+ across the tonoplast that will favor passive movement of Ca2+ out of the vacuole into the cytoplasm, even during Ca2+ signaling events when [Ca2+]cyt rises to micromolar values. Therefore this process must be tightly controlled.10 As such, in addition to a greater presence of CAX1 it is possible that cells in which high [Ca]vac accumulates there are differences in the activity of Ca2+-permeable channels in the tonoplast compared with cells that do not accumulate high [Ca]vac. This may take the form of either differentially regulated tonoplast localized Ca2+ permeable channels or a different complement of Ca2+-permeable channels.
Electrophysiological studies have identified at least four Ca2+-permeable conductances in the tonoplast11 but the SV conductance is the only one encoded by a known gene, TPC1.12,13 TPC1 has been implicated in a number of roles including [K+]vac homeostasis, Ca accumulation in the vacuole, extracellular Ca2+ sensing and stomatal closure, wounding and jasmonic acid signaling.11-13 However, there is considerable conflicting data that demonstrates, at physiologically relevant tonoplast potentials and ion concentrations, the SV channel/TPC1 can either catalyze transport of alkali earth metals with a radius the same size or smaller than Mg2+ (e.g., Ca2+, K+ and Na+) or can only transport K+ and Na+ across the tonoplast.11,13 For these ions there are interesting differences in the features of transport, for instance the rectification by luminal Na+ is such that it can enter the vacuole via the channel, but interacts on the luminal side so that the channel is not able to transport Na+ out of the vacuole.14 In the case of Ca2+, despite there being a measurable permeability to Ca2+ through the SV channel,15 luminal Ca2+shifts the voltage range of activation such that the open probability of SV channel opening is very low at physiological tonoplast membrane potentials.11,13 It has been proposed that Ca2+ is prevented from leaking out of the vacuole through the SV channel via this mechanism (as summarized in13). However, in other reports it has been calculated that the fA range Ca2+-current detected through single SV-channels would result in a physiologically relevant Ca2+-efflux out of the vacuole even at very low open probabilities due to the large abundance of this protein.11
Interestingly, we found that TPC1 was differentially expressed between epidermal and mesophyll cells, being enriched in the epidermis (Fig. 1A,B). In fact, TPC1 was more highly expressed in the epidermis than CAX1 was in the mesophyll (Fig. 1A,B). In addition, the differential transcript abundance between cell types of TPC1 was > 1000-fold while CAX1 was only ~375 fold more abundant in the mesophyll compared with the epidermis (Fig. 1A,B). This is considerably more than the ~5-fold difference found for TPC1 between guard cell and mesophyll protoplast preparations.16,17 As low [K+]vac and high [Ca2+]vac reduce the open probability of the SV channel/TPC1, and as the transcript abundance of TPC1 in mesophyll cells is low, the activity of the SV channel in intact mesophyll cells is likely to be relatively low in these conditions.11,17 However, in epidermal cells where the TPC1 transcript is highly abundant and [Ca]vac is low, the SV channel is likely to be more active and could contribute to an elemental accumulation phenotype. As a result we quantified the [K]vac and [Ca]vac in epidermal and mesophyll cells of the T-DNA insertion line tpc1–2 that lacks expression of TPC112 using SiCSA (as described by Conn et al.4).
Reconciling phenotypes associated with altered TPC1 function
Despite no visible phenotype in tpc1–2, epidermal [Ca]vac was higher than in Col-0 wildtype plants whereas no significant difference in [K]vac was found (Fig. 1C; Fig. 1D). Previously it was found that fou2 plants, which harbour a D454N point mutation in TPC1, have a significantly lower mesophyll [K]vac and higher [Ca]vac.18 The fou2 mutation in TPC1 results in a SV conductance that is insensitive to, and active at, a higher [Ca2+]vac when compared with wildtype.18 In an attempt to reconcile these phenotypes we have developed three hypotheses.
The K-shunt hypothesis
On the one hand, the fou2 phenotype may suggest that the SV channel/TPC1 functions to facilitate accumulation of Ca2+, and one proposal is that the SV channel provides a K+ shunt conductance so that the V-ATPase proton pump and CAX-mediated Ca2+/H+ will have higher transport rates.13 However, this explanation is inconsistent with our observations in that: 1) TPC1 is expressed predominantly in cells that have low [Ca]vac; and, 2) tpc1–2 plants have higher [Ca]vac in epidermal cells, a cell-type in which TPC1 is normally highly expressed. Unless considerable pleiotropic effects are occurring in tpc1–2, these observations require that a different explanation is sought for how TPC1 influences vacuolar Ca2+ accumulation consistent for with the phenotype of both fou2 and tpc1–2.
The Ca leakage hypothesis
Conceivably, if TPC1 conducts passive movement of Ca2+ across the tonoplast, the epidermal preferential expression pattern of TPC1 could help to sustain lower epidermal [Ca]vac in wildtype plants. This would occur if TPC1 allows leak of Ca2+ from the epidermal vacuoles into the cytoplasm down the large electrochemical gradient. This is consistent with the higher epidermal [Ca]vac phenotype we observed in tpc1–2 plants, since the ability of epidermal vacuoles to lose Ca2+ in tpc1–2 plants may be compromised (Fig. 1C). To explain the fou2 phenotype the relatively high mesophyll [Ca]vac of fou2 may result from excessive release of Ca2+ from epidermis leading to more available apoplastic Ca2+ for mesophyll cells to sequester. As TPC1 is much more highly expressed in the epidermis, the fou2 mutation will have a relatively larger effect in these cells compared with mesophyll cells. Furthermore, we investigated TPC1 expression in other leaf cell-types that do not accumulate high [Ca]vac and found that in vascular-associated cells (including the bundle sheath) TPC1 was highly expressed (Fig. 2; Fig. 1B in ref. 4). As [K]vac is very high in these cell-types this adds further weight to the ‘Ca leakage’ hypothesis that TPC1 may be involved in preventing the build up of vacuolar Ca2+ in both the epidermis and the bundle sheath (Fig. 2). To further investigate the feasibility of this hypothesis it would be interesting to measure transcript abundance and activity of ACA10 and other PM-Ca2+-transporters in vascular-associated cells of tpc1–2 (see Figs. 2 and3) to see if they are more abundant and upregulated to cope with the release of Ca2+ into the cytoplasm. It would also be expected that apoplastic [Ca2+] of tpc1–2 may be elevated, though perhaps not to the extent that was observed with the cax1/cax3 phenotype and this may become more evident when high Ca2+ is supplied to the plant. A fou2/cax1/cax3 mutant would be expected to be even more sensitive to high apoplastic [Ca2+] and the tpc1/cax1/cax3 would be less sensitive than the cax1/cax3 based on this hypothesis.
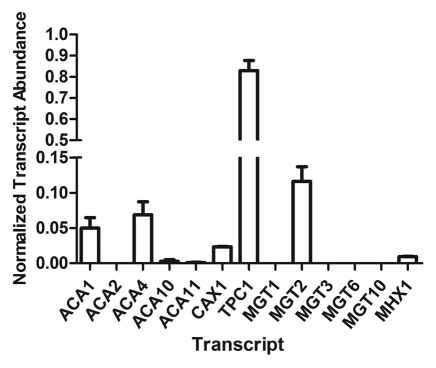
Figure 2.
PCR of selected Ca2+ and Mg2+ transporters in vascular-associated tissue of Arabidopsis thaliana ecotype Col-0, prepared by LCM (as described in Conn et al.4). qPCR (mean transcript abundance + S.E.M.) normalized against EF1-α and Actin2, n = 3 (3 biological replicates with 3 technical replicates per qPCR reaction). For space constraints all Mg2+-transporters have been referred to using their MGT nomenclature only: MGT1/MRS2–10; MGT2/MRS2–1; MGT3/MRS2–5; MGT6/MRS2–4; MGT10/MRS2–11.
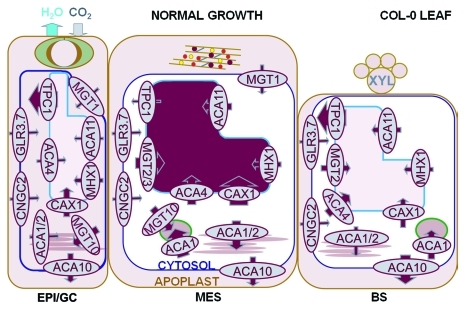
Figure 3.
Proposed model for vacuolar control of Ca2+- and Mg2+-content through expression of key transporters. The sequestration of Ca2+ into the mesophyll vacuole is important to maintain low [Ca2+]apo in order to maintain optimal gas exchange and growth rates. High [Ca2+]apo will close stomata which reduces transpiration and carbon assimilation. In addition high [Ca2+]apo will bind to demethylesterifed pectin within the cell wall crosslinking cellulose microfibrils and reducing extensibility. A reduction in either parameter could reduce growth. Magnesium is also sequestered selectively into the mesophyll vacuole and is required for maintaining turgor and growth especially when Ca2+ supply to the plant is low. (Data combined from Conn et al.,4,5 Figure 1 and 2 and data reviewed in text.) Note plasma membrane-localized Ca2+-and Mg2+-permeable channels other than GLR3.7 and CNGC2 have not been included at this stage due to paucity of information. MGT6/MRS2–4 has not been included here but its respective protein is localized to the mitochondria and was detected as more highly expressed in the mesophyll than the epidermis (S. Conn, unpublished results). Size of arrow indicates proposed size of flux through transporter, intensity of purple shading indicates concentration of Ca and Mg; transporters with M prefix are proposed to be Mg2+-permeable and other transporters are Ca2+-permeable. Model contains apoplast, chloroplast, cytoplasm, endoplasmic reticulum, vacuole, but not mitochondria, golgi or other major organelles until further information is acquired. Cell wall components indicated in apoplast of mesophyll (brown rods, cellulose; gray lines, rhamnogalacturonan II; purple circles, Ca-pectate; yellow circles polygalacturonase, red circles, expansins; guard cell shown in apoplast of epidermis. EPI, epidermis; MES, mesophyll; BS, bundle sheath. For space constraints, Mg2+-transporters have been referred to using their MGT nomenclature only instead of MRS2. MGT1/MRS2–10; MGT2/MRS2–1; MGT3/MRS2–5; MGT10/MRS2–11.
The Ca compensation hypothesis
Another explanation for the phenotype in fou2 also exists. Hedrich and coworkers have presented considerable in vitro evidence that TPC1 cannot conduct Ca2+ currents at physiological potentials.13 In light of this an alternative hypothesis for the above fou2 phenotype could be the reduced inhibition of TPC1 by [Ca]vac leading to more K+ release from the vacuole, and a greater build up of [Ca]vac to osmotically compensate.17 This could only occur if a gradient for K+ across the tonoplast could be maintained. To this end it would be informative to measure the [K]vac and [K]cyt of epidermal cells in fou2 plants.
The “Ca compensation” hypothesis and ‘K-shunt’ hypothesis could also be examined using the triple mutants suggested above since CAX1 would presumably be needed for accumulating Ca2+ within the vacuole. Although it is difficult to reconcile a K+-based transport hypothesis with tpc1–2 mutants, as no [K]vac phenotype was observed using cell-specific XRMA (Fig. 1D), it is possible there is an upregulation of other vacuolar K+-transporters to maintain cellular K+-homeostasis in these mutants. Regardless, further elemental analysis of both mutants under a variety of nutritional regimes is warranted to resolve these hypotheses.
Cell-specific Mg storage
Magnesium is also stored preferentially in mesophyll cells compared with epidermal cells and this is more apparent when plants are grown in LCS or high [Mg2+]ext (HMS, aMg = 7 mM), or detached leaves are transpirationally-fed high [Mg2+].5 Interestingly, cax1/cax3 mutants also had higher mesophyll [Mg]vac than Col-0 plants.5
Roles of cell-specific Mg storage
Given the high concentration of Ca in the mesophyll vacuole it is likely to contribute significantly to the osmotic potential of the cell. The decrease seen in [Ca]vac in cax1/cax3, or for Col-0 in serpentine or low calcium growth conditions, is partially compensated by an increase in both [K]vac and [Mg]vac.5 The increase in [Mg]vac in the mesophyll is dependent upon presence of MGT2/MRS2–1 and MGT3/MRS2–5, which encode vacuolar localized Mg2+-transporters, and an increase in transcript abundance of these genes under such conditions plays a key role in maintaining growth.5 Interestingly, leaf cells of T-DNA insertional mutants of either MGT2/MRS2–1 or MGT3/MRS2–5 grown in LCS actually increased the osmotic potential of their tissues and this was found to be due to a hyper-accumulation of K in vacuoles and a reduction in growth.5 Another consequence of knockout of MGT2/MRS2–1 and MGT3/MRS2–5 was a greater reduction in chlorophyll content under LCS conditions than in wildtype plants, but interestingly not in BNS. The reason for this is unknown but may be related to the lower [Mg]vac available for remobilization and chlorophyll synthesis.5 The significance of this work for Mg accumulation in plants and nutrition was summarized by Waters (2011)19 in a commentary on the roles of the MGT/MRS2 gene family in plants.
Expression of Mg and Ca transporters within vascular-associated cells
In addition to the mesophyll and epidermal transcriptomes gathered by SiCSA microarray and qPCR,4,5 we have isolated vascular tissue cDNA (including the bundle sheath cells) of Col-0 Arabidopsis thaliana leaves via laser capture microdissection and performed qPCR for specific Ca2+- and Mg2+-transporter genes, as well as TPC1 (Fig. 2). In vascular associated cells CAX1 is relatively lowly expressed when compared with mesophyll cells and TPC1 is highly expressed (Fig. 2). The negative correlation between cell-type specific TPC1 abundance and [Ca]vac, and the positive correlation between CAX1 expression and [Ca]vac, adds further weight to their respective proposed roles in cell-type specific Ca2+ leakage and Ca2+ accumulation. The only other two Ca2+-transporter transcripts detected in vascular associated cells were ACA1 and ACA4, albeit at relatively low levels; it was previously noted that these transcripts are widely expressed.4
In terms of Mg2+ accumulation, the tonoplast-localized Mg2+/H+ exchanger, MHX1, which was equally expressed between all cell-types (Fig. 2;5), is known to be inducible by increasing Mg2+ supply to plants and increases in expression in different ecotypes of Arabidopsis with an increase in Mg content of leaves.5 It is likely that it contributes to Mg2+ accumulation in all cell-types whereas MGT2/MRS2–1 and MGT3/MRS2–5 contribute more toward mesophyll-specific Mg2+ accumulation.5 MGT10/MRS2–11 was found to be equally abundant in epidermal and mesophyll SiCSA samples but is reported to be predominantly chloroplastic.5,20 However, Bräutigam and Weber21 found MGT10/MRS2–11 highly abundant in protoplastids so may be present in other plastid membranes in the epidermis, therefore this appears similar to ACA1 in that it shares both a chloroplastic and unspecified plastid localization.4
Model of Ca and Mg storage in leaves
A model of Ca2+-transport and storage in the epidermis and mesophyll of Arabidopsis leaves has been presented (see Figure 6 in ref. 4), here we expand that model to include vascular tissues and also Mg2+-transporters in each of these tissues (Fig. 3). This model provides a basis for further interrogation.
In the future, when the identity of additional Ca2+-channels in plasma membranes and Ca2+-permeable proteins of other organelles are better resolved, whole cell-type transport networks could be constructed and be used as the basis of models of cellular homeostasis that takes in large differences between cell-types in the expression of transporters. Both glutamate receptor-like proteins (GLRs) and cyclic-nucleotide gated channels (CNGCs) have been implicated as plasma membrane Ca2+-channels.1,22 Interestingly, cngc2 has a similar Ca2+-sensitive growth phenotype to cax1/cax3 so, as well as its well documented roles in pathogen defense signaling, may be a key entry point of apoplastic Ca2+ into cells for plant nutrition.23,24 Additionally, a recent report has confirmed that GLR3.7 and GLR2.1 act as Ca2+-channels in pollen tubes.25 The expression of GLRs in epidermal and mesophyll cells was previously investigated in Arabidopsis and despite a preference of expression of 5–6 members per cell-type, all members lacked both discernible co-expression partner(s) and cell-specificity, with GLR3.7 being the only one found in all cells.26 Therefore, GLR3.7 is likely to form a plasma membrane localized Ca2+-channel in all cell types of the leaf. Our data also shows no difference in CNGC2 expression between cell-types and this data agrees with early studies that suggest that the Ca2+-conductance properties across the plasma membrane are equal in all leaf cell-types.2
Final Remarks
The vacuole plays a key role in co-ordinating fluxes across the plasma membrane and controlling apoplastic solute concentrations, as such this represents a paradigm shift in the understanding of nutritional-related physiology of plants. Why Ca2+ and Mg2+ should be preferentially sequestered into the mesophyll in Arabidopsis and not other cell-types has not been directly tested, however it may be a consequence of the need to keep certain elements apart.1 For example, phosphorous (P) is accumulated preferentially in the epidermal cell vacuoles of Arabidopsis. If both Ca2+ and inorganic P were sequestered into the same vacuole, CaP precipitate would form making both elements potentially less available to the plant. It is attractive to speculate that the mixed results obtained through attempting Ca biofortification of food crops through constitutive misexpression of transporters are due to the need to segregate certain elements and the mechanisms that control cell-preferential accumulation of solutes.1,27 Future biofortification studies therefore may benefit from taking cell-specific approaches to improve nutrient content without being deleterious to plant physiology.27 Water flow across membranes will also be affected by [Ca2+]apo and must also be considered.28
Acknowledgments
We thank Dale Sanders for supply of tpc1–2 seeds. Financial support for this work was provided from ARC Discovery Project (reference DP0774063) awarded to Prof. Roger Leigh, Prof. Steve Tyerman and Dr. Brent Kaiser whose input and contributions were invaluable for this work.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17797
References
- 1.Conn S, Gilliham M. Comparative physiology of elemental distributions in plants. Ann Bot. 2010;105:1081–102. doi: 10.1093/aob/mcq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karley AJ, Leigh RA, Sanders D. Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends Plant Sci. 2000;5:465–70. doi: 10.1016/S1360-1385(00)01758-1. [DOI] [PubMed] [Google Scholar]
- 3.Storey R, Leigh RA. Processes modulating calcium distribution in citrus leaves. An investigation using x-ray microanalysis with strontium as a tracer. Plant Physiol. 2004;136:3838–48. doi: 10.1104/pp.104.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conn SJ, Gilliham M, Athman A, Schreiber AW, Baumann U, Moller I, et al. Cell-specific vacuolar calcium storage mediated by AtCAX1 regulates apoplastic calcium concentration, gas exchange and plant productivity. Plant Cell. 2011;23:240–57. doi: 10.1105/tpc.109.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conn SJ, Conn V, Tyerman SD, Kaiser BN, Leigh RA, Gilliham M. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol. 2011;190:583–94. doi: 10.1111/j.1469-8137.2010.03619.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng N-H, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, et al. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 2005;138:2048–60. doi: 10.1104/pp.105.061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, Chung WS, et al. Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol. 2010;154:1158–71. doi: 10.1104/pp.110.159038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb AAR, Larman MR, Montgomery LT, Taylor JE, Hetherington AM. The role of calcium in ABA-induced gene expression and stomatal movements. Plant J. 2001;26:351–62. doi: 10.1046/j.1365-313X.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–61. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 10.Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 11.Pottosin II, Schönknecht G. Vacuolar calcium channels. J Exp Bot. 2007;58:1559–69. doi: 10.1093/jxb/erm035. [DOI] [PubMed] [Google Scholar]
- 12.Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–8. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 13.Hedrich R, Martin I. TPC1 – SV channels gain shape. Mol Plant. 2011;4:428–41. doi: 10.1093/mp/ssr017. [DOI] [PubMed] [Google Scholar]
- 14.Ivashikina N, Hedrich RK. +currents through SV-type vacuolar channels are sensitive to elevated luminal Na+ levels. Plant J. 2005;41:606–14. doi: 10.1111/j.1365-313X.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- 15.Allen GJ, Sanders D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996;10:1055–69. doi: 10.1046/j.1365-313X.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rienmüller F, Beyhl D, Lautner S, Fromm J, Al-Rasheid KA, Ache P, et al. Guard cell-specific calcium sensitivity of high density and activity SV/TPC1 channels. Plant Cell Physiol. 2010;51:1548–54. doi: 10.1093/pcp/pcq102. [DOI] [PubMed] [Google Scholar]
- 18.Beyhl D, Hörtensteiner S, Martinoia E, Farmer EE, Fromm J, Marten I, et al. The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J. 2009;58:715–23. doi: 10.1111/j.1365-313X.2009.03820.x. [DOI] [PubMed] [Google Scholar]
- 19.Waters BM. Moving magnesium in plant cells. New Phytol. 2011;190:510–3. doi: 10.1111/j.1469-8137.2011.03724.x. [DOI] [PubMed] [Google Scholar]
- 20.Drummond RSM, Tutone A, Li Y-C, Gardner RC. A putative magnesium transporter AtMRS2-11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006;170:78–89. doi: 10.1016/j.plantsci.2005.08.018. [DOI] [Google Scholar]
- 21.Bräutigam A, Weber APM. Proteomic analysis of the proplastid envelope membrane provides novel insights into small molecule and protein transport across protoplastid membranes. Mol Plant. 2009;2:1247–61. doi: 10.1093/mp/ssp070. [DOI] [PubMed] [Google Scholar]
- 22.Gilliham M, Campbell M, Becker D, Dubos C, Davenport RJ. The Arabidopsis thaliana glutamate-like receptors (AtGLR). In: Communication in Plants: Neuronal Aspects of Plant Life. Baluška F, Mancuso S, Volkmann D (eds), Berlin, Germany: Springer-Verlag, 2006; pp187-204. [Google Scholar]
- 23.Chan CW, Schorrak LM, Smith RK, Jr., Bent AF, Sussman MR. A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol. 2003;132:728–31. doi: 10.1104/pp.102.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma W, Smigel A, Walker RK, Moeder W, Yoshioka K, Berkowitz GA. Leaf Senescence Signaling: The Ca2+-conducting Arabidopsis cyclic nucleotide gated channel 2 acts through nitric oxide to repress senescence programming. Plant Physiol. 2010;154:733–43. doi: 10.1104/pp.110.161356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michard E, Lima PT, Borges F, Silva AC, Carvalho JE, Gilliham M, Liu LH, et al. Glutamate-receptor-like genes control pollen tube Ca2+ influx and morphogenesis. Science. 2011;332:434–7. doi: 10.1126/science.1201101. [DOI] [PubMed] [Google Scholar]
- 26.Roy SJ, Gilliham M, Berger B, Essah PA, Cheffings C, Miller AJ, et al. Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant Cell Environ. 2008;31:861–71. doi: 10.1111/j.1365-3040.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 27.Dayod M, Tyerman SD, Leigh RA, Gilliham M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma. 2010;247:215–31. doi: 10.1007/s00709-010-0182-0. [DOI] [PubMed] [Google Scholar]
- 28.Gilliham M, Dayod M, Hocking BJ, Xu B, Conn SJ, Kaiser BN, et al. Calcium delivery and storage in plant leaves; exploring the link with water flow. J Exp Bot. 2011;62:2233–50. doi: 10.1093/jxb/err111. [DOI] [PubMed] [Google Scholar]