Abstract
The mobilization of inorganic phosphate (Pi) in planta is a complex process regulated by a number of developmental and environmental cues. Plants possess many Pi transporters that acquire Pi from the rhizosphere and translocate it throughout the plant. A few members of the high-affinity Pht1 family of Pi transporters have been functionally characterized and, for the most part, have been shown to be involved in Pi acquisition. We recently demonstrated that the Arabidopsis Pi transporter, Pht1;5, plays a key role in translocating Pi between tissues. Loss-of-function pht1;5 mutant seedlings accumulated more P in shoots relative to wild type but less in roots. In contrast, overexpression of Pht1;5 resulted in a lower P shoot:root ratio compared with wild type. Also, the rosette leaves of Pht1;5-overexpression plants senesced early and contained less P, whereas reproductive organs accumulated more P than those of wild type. Herein we report the molecular response of disrupting Pht1;5 expression on other factors known to modulate P distribution. The results reveal reciprocal mis-regulation of PHO1, miR399d, and At4 in the pht1;5 mutant and Pht1;5-overexpressor, consistent with the corresponding changes in P distribution in these lines. Together our studies reveal a complex role for Pht1;5 in regulating Pi homeostasis.
Keywords: nutrition, Phosphorus, Pht1 transporter, Pi distribution
Phosphorus (P) is a major macronutrient for plants that is vital to a number of processes. Thus, plants must acquire large quantities of P from the soil and coordinate its distribution to satisfy the developmental requirements of each tissue. P is acquired as inorganic phosphate (Pi) via Pi transporters, which also distribute Pi throughout the plant.1 A number of plant Pi transporters have been identified based on their homology to the Saccharomyces cerevisiae Pho84p Pi transporter. These proteins, which are localized to the plasma-membrane and have 12 membrane-spanning domains, comprise the PHOSPHATE TRANSPORTER 1 (Pht1) family and are presumed to be high-affinity transporters.2 The Arabidopsis Pht1 family contains 9 members, Pht1;1 through Pht1;9.2 Of these, only Pht1;1 and Pht1;4 had previously been functionally characterized, and were shown to be involved in Pi acquisition.3 Recently, we described the characterization of Pht1;5 via analyses of loss-of-function mutants and overexpression transgenics.4 We found that Pht1;5 plays key roles in Pi translocation and remobilization. Herein we provide additional evidence that Pht1;5 is required for normal Pi homeostasis in Arabidopsis by examining the expression of other factors known to control P distribution in Pht1;5-mutant and overexpression lines.
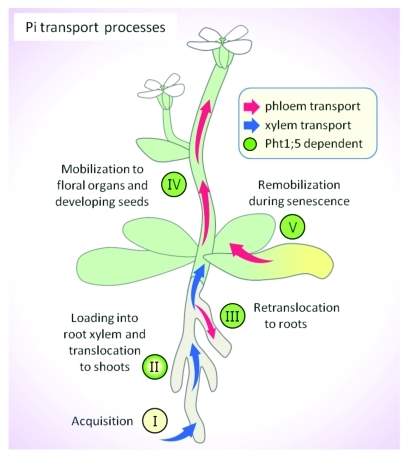
In plants, Pi acquisition occurs via Pi transporters present in root epidermal and cortical cells (Fig. 1, I).5-10 From the cortex, Pi must be loaded into the xylem for subsequent translocation to shoot tissues (Fig. 1, II). The PHO1 protein plays a role in root Pi xylem loading in Arabidopsis.11,12 Via the phloem, Pi is retranslocated to root tissues (Fig. 1, III) and across shoot tissues (Fig. 1, IV and V) in accordance with developmental cues and Pi status. Our recent report4 demonstrated that Arabidopsis Pht1;5 is involved in 1) P retranslocation between shoots and roots of young seedlings (Fig. 1, III) and 2) mobilization/remobilization of P from shoot sources (i.e., mature and senescing leaves) to sinks (i.e., young leaves and inflorescence tissues; Figure 1, IV and V).4 Interestingly, loss of Pht1;5 led to an increase in the shoot:root ratio of P relative to wild type, whereas Pht1;5-overexpression resulted in a decrease. To gain insight into the influence of Pht1;5 disruption on other factors known to impact P distribution, we measured the transcript levels of PHO1 and At4, as well as primary transcripts of miR399d, in the roots of the pht1;5–1 loss-of-function mutant and a Pht1;5-overexpression line. As shown in Figure 2, PHO1 transcript levels were lower in pht1;5–1 mutant roots compared with wild type, but were higher in roots of the Pht1;5-overexpressor. This is consistent with the mutant exhibiting downregulation of PHO1 in an attempt to decrease Pi xylem loading in roots and subsequent translocation to shoots, whereas upregulation of PHO1 in the Pht1;5-overexpression line may result from the increased mobilization of Pi to roots. The microRNA miR399 and At4 are two antagonistic components of a circuit that functions to modulate multiple Pi starvation responses including distribution of P.13-17 As with PHO1, the expression of miR399d (one representative of the miR399 family) and At4 show reciprocal changes in the pht1;5–1 mutant and Pht1;5-overexpressor (Fig. 2). Transcript levels for miR399d and At4 were higher and lower, respectfully, in pht1;5–1 roots compared with wild type, whereas the Pht1;5-overexpressor accumulated less miR399d and more At4 transcripts relative to wild type. This also indicates that loss of Pht1;5 affects the shoot-derived Pi starvation signal thereby systemically upregulating the expression of miR399d in the roots. Taken together these results suggest that disruption of Pht1;5 expression causes atypical Pi mobilization, which leads to the initiation of other signal transduction events that attempt to overcome the alterations in P distribution.
Figure 1.
Proposed role for Pht1;5 in Pi mobilization. The processes of Pi transport are shown following its acquisition from the rhizosphere. Green labels show processes in which Pht1;5 likely plays a role. The arrows indicate the movement of Pi via the xylem (blue) and phloem (red).
Figure 2.
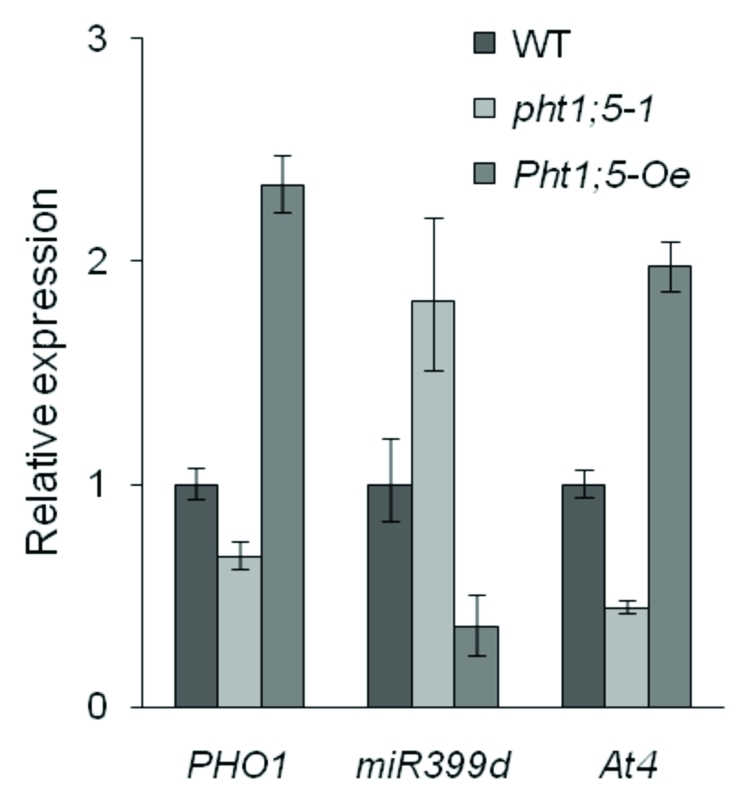
Quantitative RT-PCR analysis of genes associated with P distribution. Wild-type (WT), pht1;5–1 mutant, and Pht1;5-overexpression (Pht1;5-Oe) seedlings were grown hydroponically for three weeks in high-Pi (1.25 mM Pi) media. Total RNA isolated from these plants was used for qRT-PCR. At4g26410 was used as an internal reference gene, and the values, normalized to WT levels, are the means +/− SE of two independent biological replicates run in triplicate.
Our recent report also indicated that Pht1;5 contributes to mobilization of Pi from mature and senescing leaves to metabolically active tissues. The leaves of transgenic Arabidopsis plants overexpressing Pht1;5 senesced earlier than those of wild type and contained lower levels of P.4 However, the Pht1;5-overexpressor accumulated more P in floral stalks and siliques compared with wild type.4 These results are consistent with Pht1;5 functioning in retranslocation of Pi from P source to sink organs (Fig. 1, IV and V). A similar function was recently proposed for the rice Pi transporter, OsPht1;8. Overexpression of OsPht1;8 led to increased P content in the panicles and hulls of transgenic rice.18 OsPht1;8 was also implicated in root-to-shoot transport of Pi due to its expression at root-shoot junctions.18 Similarly, Pht1;5 is expressed in root stele cells of Pi-starved Arabidopsis,8 and mutation of Pht1;5 led to a drop in Pi root-to-shoot translocation under Pi-deficient conditions.4 These results suggest that Pht1;5 may also contribute to the loading of Pi into root xylem for subsequent translocation to shoots during low Pi conditions (Fig. 1, II).
In conclusion, our work on Pht1;5 highlights intricate regulation of Pi mobilization and distribution in higher plants. In addition to Pi acquisition from soil, high-affinity Pi transporters are also needed for internal Pi mobilization, which is controlled by the coordination of developmental and environmental (i.e., P availability) cues. Our work thus gives credence to the notion that Pi transporters are not simply downstream components of Pi signaling pathways, but rather influence gene expression and signaling events by regulating Pi homeostasis in tissues and organs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17906
References
- 1.Marschner H. Mineral Nutrition of Higher Plants. 2nd ed. Boston, MA: Academic Press1995. [Google Scholar]
- 2.Rausch C, Bucher M. Molecular mechanisms of phosphate transport in plants. Planta. 2002;216:23–37. doi: 10.1007/s00425-002-0921-3. [DOI] [PubMed] [Google Scholar]
- 3.Shin H, Shin HS, Dewbre GR, Harrison MJ. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004;39:629–42. doi: 10.1111/j.1365-313X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP. Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol. 2011;156:1149–63. doi: 10.1104/pp.111.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Muchhal US, Uthappa M, Kononowicz AK, Raghothama KG. Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiol. 1998;116:91–9. doi: 10.1104/pp.116.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiou TJ, Liu H, Harrison MJ. The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. Plant J. 2001;25:281–93. doi: 10.1046/j.1365-313x.2001.00963.x. [DOI] [PubMed] [Google Scholar]
- 7.Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG. Regulated expression of Arabidopsis phosphate transporters. Plant Physiol. 2002;130:221–33. doi: 10.1104/pp.020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mudge SR, Rae AL, Diatloff E, Smith FW. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 2002;31:341–53. doi: 10.1046/j.1365-313X.2002.01356.x. [DOI] [PubMed] [Google Scholar]
- 9.Schünmann PHD, Richardson AE, Smith FW, Delhaize E. Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.) J Exp Bot. 2004;55:855–65. doi: 10.1093/jxb/erh103. [DOI] [PubMed] [Google Scholar]
- 10.Ai P, Sun SB, Zhao JN, Fan XR, Xin WJ, Guo Q, et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009;57:798–809. doi: 10.1111/j.1365-313X.2008.03726.x. [DOI] [PubMed] [Google Scholar]
- 11.Poirier Y, Thoma S, Somerville C, Schiefelbein J. A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 1991;97:1087–93. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamburger D, Rezzonico E, Petetot JMC, Somerville C, Poirier Y. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell. 2002;14:889–902. doi: 10.1105/tpc.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol. 2005;15:2038–43. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Shin H, Shin HS, Chen R, Harrison MJ. Loss of At4 function impacts phosphate distribution between the roots and the shoots during phosphate starvation. Plant J. 2006;45:712–26. doi: 10.1111/j.1365-313X.2005.02629.x. [DOI] [PubMed] [Google Scholar]
- 15.Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a MicroRNA399 target gene. Plant Physiol. 2006;141:1000–11. doi: 10.1104/pp.106.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell. 2006;18:412–21. doi: 10.1105/tpc.105.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bari R, Pant BD, Stitt M, Scheible WR. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–99. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, et al. The Phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011;156:1164–75. doi: 10.1104/pp.111.175240. [DOI] [PMC free article] [PubMed] [Google Scholar]