Abstract
The barley ROP GTPase HvRACB is a susceptibility factor of barley to powdery mildew caused by the biotrophic fungus Blumeria graminis f.sp. hordei (Bgh). In a recent publication, we reported about a MICROTUBULE-ASSOCIATED ROP GTPASE-ACTIVATING PROTEIN 1 (HvMAGAP1) of barley. Transient-induced gene silencing or overexpression of HvMAGAP1 resulted in enhanced or reduced susceptibility to Bgh, respectively, indicating a possible HvRACB-antagonistic function of HvMAGAP1 in interaction with Bgh. HvMAGAP1 also influences the polarity of cortical microtubules in interaction with Bgh. In AtROPGAP1 and AtROPGAP4, Arabidopsis homologs of HvMAGAP1, knock-out T-DNA insertions enhanced susceptibility of Arabidopsis to the virulent powdery mildew fungus Erysiphe cruciferarum, indicating functions of ROPGAPs in pathogen interaction of monocots and dicots. Here we discuss the role of AtROPGAP1 and AtROPGAP4 in Arabidopsis pathogenesis of powdery mildew in some more detail.
Keywords: Erysiphe cruciferarum, Arabidopsis, compatibility, polarity, RHO, RHOGAP
Plant RHO-like ROP proteins are multifunctional switches involved in a variety of plant signaling processes, such as growth and development, cytoskeleton organization, secretion, hormone response, generation of reactive oxygen species (ROS) and susceptibility and resistance to plant pathogens.1-4 As molecular switches, RAC/ROPs exist in a GDP-bound inactive form and a GTP-bound active form for targeting downstream effectors. The intrinsic GTPase activity of RAC/ROP proteins is low and requires specific activation by ROPGAPs (ROP GTPASE-ACTIVATING PROTEINGS. ROPGAPs are typical members of the eukaryotic RHOGAP family and contain an arginine finger in their conserved catalytic GAP domain, which is supposed to reach into the GTP-binding pocket for stimulation of the GTPase activity of RAC/ROPs, resulting in hydrolysis of GTP and ROP shutdown.5
In the monocots barley or rice, several RAC/ROP proteins are involved in plant-pathogen interactions. In rice, OsRAC1 is a positive regulator of defense against the rice blast fungus Magnaporthe grisea by stimulating ROS production and cell death through interaction with an NADPH oxidase.6,7 In barley, HvRACB was identified as susceptibility factor to penetration by Blumeria graminis f.sp hordei (Bgh), influencing actin reorganization during pathogen attack.8-12 Stable knock-down of HvRACB expression inhibits penetration success of Bgh and impairs establishment and expansion of fungal haustoria in barley epidermal cells. Furthermore, active HvRACB is required for initiation and elongation of barley root hairs and affects adult plant height similar to ROPs in Arabidopsis.13,14 HvRACB is hence a common factor of surface expansion both in epidermal root cells during formation of hairs and in epidermal leaf cells during formation of the haustorial complex. This includes formation of the extrahaustorial membrane, which is in continuum with the host plasma membrane, and of the extrahaustorial matrix a new apoplastic compartment with cell wall like constituents.
Recently, we identified the barley MICROTUBULE-ASSOCIATED GAP 1 (HvMAGAP1) protein as potential regulator of RAC/ROPs in susceptibility to Bgh. HvMAGAP1 belongs to the family of ROPGAPs containing a CRIB domain for G-protein interaction.13,15 Transient-induced gene silencing of HvMAGAP1 resulted in enhanced susceptibility of barley epidermal cells to Bgh. A similar effect was observed when a mutated version of HvMAGAP1, MAGAP-R185G, was expressed, which can still interact with HvRACB in planta but apparently exerts a dominant negative effect due to mutation of the catalytic residue. Similarly, Arabidopsis Atropgap1 and Atropgap4 knockout mutants display enhanced susceptibility in a compatible interaction with the powdery mildew fungus Erysiphe cruciferarum.13
Although many RAC/ROP-regulated processes have been identified in plants, only little is known about physiological processes controlled via RAC/ROP-regulating ROPGAP proteins. CRIB containing NtRHOGAP1 is an important factor for restriction of ROP activity to the apex of the growing pollen tube tip by lateral inactivation of ROP activity in tobacco. Overexpression of an inactive mutant NtRHOGAP1 protein version caused depolarized growth similar to overexpression of NtRAC5.16,17 Another type of ROPGAP, a pleckstrin-homology domain–containing ROPGAP called REN1 also functions in regulation of pollen tube polarity in Arabidopsis.18 Depolarization of normally tip-growing root hairs also takes place when a constitutively activated (CA) version of barley HvRACB is stably expressed in barley.12 The same CA HvRACB plants are super-susceptible to powdery mildew. However, super-susceptible Atropgap1 or Atropgap4 mutants do not display aberrant root hairs, suggesting function of other ROPGAPs in root hair growth (data not shown).
AtROPGAP4 is involved in tolerance to oxygen deprivation.19 Oxygen deprivation is suggested to cause ROS production via AtROP-mediated activation of an NADPH oxidase. Elevated levels of cellular hydrogen peroxide cause induction of ADH (ALCOHOL DEHYDROGENASE expression, which is important for alcoholic fermentation and cell survival under oxygen deprivation. In parallel to expression of ADH, expression of AtROPGAP4 increases to attenuate AtROP signaling via a negative feedback loop to balance the survival process.19
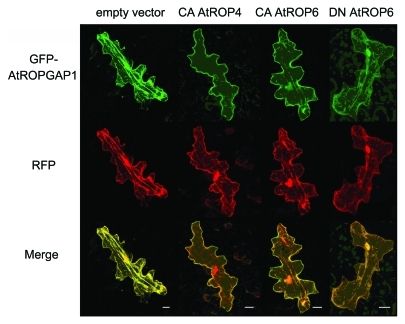
Similar to HvMAGAP1, AtROPGAP1 and AtROPGAP4 belong to the CRIB domain-containing ROPGAPs. In former studies it was shown that both Arabidopsis proteins regulate activity of AtROPs in vitro and in planta.15,19 Targeted yeast-two hybrid (Y2H) assays revealed a broad binding capacity for the AtROPGAPs to leaf-expressed AtROPs.13 Heterologous Y2H assays with barley HvRAC/ROPs revealed also interaction of AtROPGAPs with several barley RAC/ROPs in yeast with AtROPGAP4 interacting with HvRACB and CA HvRACB (data not shown). This may support conserved functions of AtROPGAPs and HvMAGAP1 although they are differently localized in epidermal leaf cells.13 In contrast to HvMAGAP1, which is associated with microtubules in barley epidermal cells, GFP-fusions of AtROPGAP1 and AtROPGAP4 show mainly cytoplasmic and nuclear localization in Arabidopsis epidermal cells.13 This can be explained because the C-terminus of HvMAGAP1, responsible for association of the protein with microtubules, greatly differs in sequence from that of Arabidopsis ROPGAPs. However, similar to barley HvMAGAP1, AtROPGAP1 and AtROPGAP4 translocate to the cell periphery or plasma membrane when corresponding membrane associated CA RAC/ROPs are co-expressed. This is evident for CA AtROP4 and CA AtROP6, but dominant negative AtROP6 does not recruit GFP-AtROPGAPs supporting interaction of AtROPGAPs with the active form of AtROPs in planta (Fig. 1).15 Hence AtROPGAPs might be readily recruited to the cell periphery when ROPs are activated thereby warranting a tight spatial control of ROP activity.
Figure 1.
Subcellular localization of GFP-AtROPGAP1 in epidermal cells of Arabidopsis. Arabidopsis epidermal cells transiently transformed with GFP-ROPGAP1 (green, first row) alone or together with CA AtROP6, CA AtROP4 or DN AtROP6 expression constructs. Soluble RFP (red, second row) was co-transformed to label the cytoplasm and nucleoplasm GFP-AtROPGAP1 alone or co-expressed with unlabeled DN AtROP6 displays cytoplasmic localization, whereas co-expression of unlabeled, membrane-associated CA AtROP4 or CA AtROP6 results in recruitment of GFP-AtROPGAP1 to the plasma membrane. Pictures are maximum projections of 20–30 optical sections at 2 μm increments. Scale bars represent 20 μm. In merged pictures signal co-localization is represented by yellow color (third row). Similar results are obtained for GFP-AtROPGAP4 (online Supplement of Hoefle et al.13).
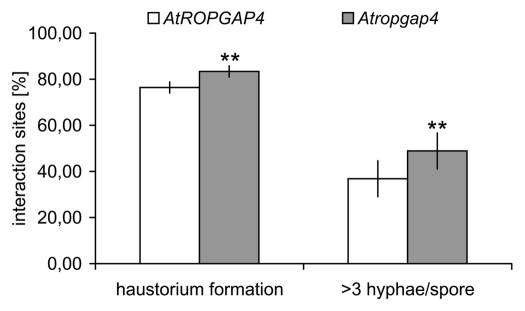
In contrast to the enhanced susceptibility of Atropgap1 and Atropgap4 mutants to the adapted powdery mildew fungus E. cruciferarum, inoculation experiments with Botrytis cinerea revealed no alteration of basal resistance to this necrotrophic fungus (data not shown). This might indicate a specific role for AtROPGAPs in limiting susceptibility to powdery mildew rather than in regulation of general or hormone-regulated defense pathways. Accordingly, expression of defense-related genes like PR1 (indicator for salicylic acid (SA)-dependent defense,20) and PDF1.2 (marker for jasmonate (JA)-dependent defense processes,21) was unchanged in Atropgaps mutants inoculated with E. cruciferarum when compared with wild type. Additionally, Atropgap1 and Atropgap4 did not show an obvious developmental phenotype (data not shown). Microscopic inspections showed that E. cruciferarum succeeds better in establishment of haustoria in epidermal pavement cells of Atropgap4 (SALK_038694) than on the wild type and develops more epiphytic hyphae 48 h after inoculation (Fig. 2). The effect appears comparatively weak, when considering the effect of knocking down HvMAGAP1 in barley.13 However, this may be due to a higher redundancy of ROPGAPs in Arabidopsis than in barley. The same Atropgap4 mutant, however, is fully resistant to penetration by the non-adapted biotrophic barley powdery mildew fungus Bgh just like the wild type (data not shown). This shows that defense to direct penetration at the cell wall is not greatly affected in this mutant. Together this suggests that functional AtROPGAPs limit compatibility with the powdery mildew fungus rather than being directly required for basal resistance. Together with the data from barley, where HvRACB and HvMAGAP1 influence leaf cell polarity, we speculate that AtROPGAP1 and AtROPGAP4 limit the activity of AtROPs, which are involved in cell polarity of epidermal pavement cells such as AtROP2, AtROP4 and AtROP6.22,23 AtROPGAP1 and AtROPGAP4 can bind to all of these AtROPs in yeast, in vitro or in planta (Fig. 1).13,15,19 However, it remains to be analyzed whether AtROP-mediated organization of microtubules, which is increasingly well understood in Arabidopsis,22-25 is involved in susceptibility to powdery mildew. Alternatively, the recently established FERONIA receptor like kinase-ROPGEF-ROP pathway, involved in polar growth of root hairs in Arabidopsis,26 might also function in leaves, because FERONIA is also required for susceptibility to powdery mildew.27 In such a scenario ROPGAPs would limit FERONIA mediated processes involved in compatibility with the invading fungus.
Figure 2.
Fungal development on Atropgap4 and on wild type at 48 h after inoculation. Each 10 Arabidopsis rosette leaves of Atropga4 (SALK_038694) and wild type AtROPGAP4 (Col-0) have been inoculation with conidia of E. cruciferarum and fixed for microscopy 48 h later according to Hoefle et al.13 Analysis of 150–200 individual interaction sites on each leaf revealed differences in the fungal success to form a haustorium in the first attacked epidermal pavement cell and to develop more than 3 epiphytic hyphae per spore at this time of the interaction. ** indicates significant differences between wild type and Atropgap4 at p < 0.01 (unpaired Student’s t-test). Repetition of the experiment led to very similar results.
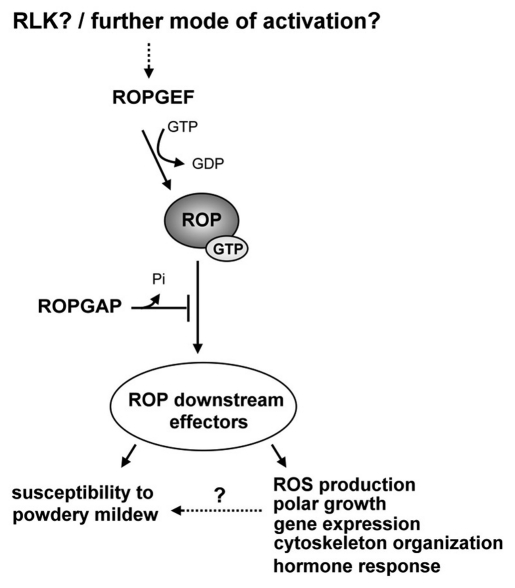
Based on these results, we propose that ROPGAPs act as antagonistic players of ROP downstream signaling that limit susceptibility to adapted powdery mildew in Arabidopsis similar to the situation in barley (Fig. 3). However, it is not yet understood which ROP downstream signaling events and cellular responses are involved in Arabidopsis susceptibility to powdery mildew. Regulation of NADPH oxidases via the ROP-ROPGAP rheostat as suggested by Baxter-Burrell et al.19 might be important for limiting susceptibility to powdery mildew because NADPH oxidase RBOHF2 of barley is involved in both susceptibility and resistance to fungal penetration depending on the plant developmental status in which gene function is analyzed.28,29 This could even involve regulation of ADH because ADH of barley was recently shown to be involved in susceptibility to powdery mildew.30,31
Figure 3.
Hypothetical model for the role of Arabidopsis ROPGAPs in ROP mediated signaling. During pathogen attack, ROPs are activated via ROPGEFs, which catalyze the exchange of ROP bound GDP for GTP. RLKs were identified as possible upstream activators of ROPGEFs, but other modes of activation should not be excluded. Active ROPs might support via downstream effectors susceptibility to powdery mildews, while ROPGAP mediated inactivation of ROPs act antagonistic to these processes. There is also a possible influence of known ROP-regulated processes such as ROS production, gene expression, cytoskeleton organization or hormone responses on susceptibility to powdery mildew (shown as dotted line).
Acknowledgments
This work was supported by a grant of the German Research Foundation (DFG) to R.H. (HU886/3). We thank the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants, and we are grateful to Verena Klingl for outstanding technical assistance.
Glossary
- Abbrevations
ROP, RHO of plants
- GAP
GTPase activating protein
- ADH
alcohol dehydrogenase
- MAGAP1
microtubule associated GTPase activating protein 1
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17943
References
- 1.Nibau C, Wu HM, Cheung AY. RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci. 2006;11:309–15. doi: 10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Berken A, Wittinghover A. Structure and function of Rho-type molecular switches in plants. Plant Physiol Biochem. 2008;46:380–93. doi: 10.1016/j.plaphy.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Wu HM, Hazak O, Cheung AY. Yalovsky S. RAC/ROP GTPases and auxin signaling. Plant Cell. 2011;23:1208–18. doi: 10.1105/tpc.111.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mucha E, Fricke I, Schaefer A, Wittinghofer A, Berken A. Rho proteins of plants - Functional cycle and regulation of cytoskeletal dynamics. Eur J Cell Biol. 2011 doi: 10.1016/j.ejcb.2010.11.009. In press. [DOI] [PubMed] [Google Scholar]
- 5.Paduch M, Jelen F, Otlewski J. Structure of small G proteins and their regulators. Acta Biochim Pol. 2001;48:829–50. [PubMed] [Google Scholar]
- 6.Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2001;98:759–64. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–34. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultheiss H, Dechert C, Kogel KH, Hückelhoven R. A small GTP-binding host protein is required for entry of powdery mildew fungus into epidermal cells of barley. Plant Physiol. 2002;128:1447–54. doi: 10.1104/pp.010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultheiss H, Dechert C, Kogel KH, Hückelhoven R. Functional analysis of barley RAC/ROP G-protein family members in susceptibility to the powdery mildew fungus. Plant J. 2003;36:589–601. doi: 10.1046/j.1365-313X.2003.01905.x. [DOI] [PubMed] [Google Scholar]
- 10.Schultheiss H, Hensel G, Imani J, Broeders S, Kumlehn J, Kogel KH, et al. Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol. 2005;139:353–62. doi: 10.1104/pp.105.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opalski KS, Schultheiss H, Kogel KH, Hückelhoven R. The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f. sp. hordei. Plant J. 2005;41:291–303. doi: 10.1111/j.1365-313X.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- 12.Pathuri IP, Zellerhoff N, Schaffrath U, Hensel G, Kumlehn J, Kogel KH, et al. Constitutively activated barley ROPs modulate epidermal cell size, defense reactions and interactions with fungal leaf pathogens. Plant Cell Rep. 2008;27:1877–87. doi: 10.1007/s00299-008-0607-9. [DOI] [PubMed] [Google Scholar]
- 13.Hoefle C, Huesmann C, Schultheiss H, Börnke F, Hensel G, Kumlehn J, et al. A barley ROP GTPase ACTIVATING PROTEIN associates with microtubules and regulates entry of the barley powdery mildew fungus into leaf epidermal cells. Plant Cell. 2011;23:2422–39. doi: 10.1105/tpc.110.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Shen JJ, Zheng ZL, Lin Y, Yang Z. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 2001;126:670–84. doi: 10.1104/pp.126.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, Li H, Yang Z. Arabidopsis RopGAPs are a novel family of Rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for Rop-specific GTPase stimulation. Plant Physiol. 2000;124:1625–36. doi: 10.1104/pp.124.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/ROP to the apex of pollen tubes. Plant Cell. 2006;18:3033–46. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klahre U, Becker C, Schmitt AC, Kost B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant J. 2006;46:1018–31. doi: 10.1111/j.1365-313X.2006.02757.x. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JU, Vernoud V, Szumlanski A, Nielsen E, Yang Z. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr Biol. 2008;18:1907–16. doi: 10.1016/j.cub.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science. 2002;296:2026–8. doi: 10.1126/science.1071505. [DOI] [PubMed] [Google Scholar]
- 20.Frye CA, Innes RW. An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell. 1998;10:947–56. doi: 10.1105/tpc.10.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM. A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol. 2003;132:1020–32. doi: 10.1104/pp.102.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Fu Y, Xu T, Zhu L, Wen M, Yang Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol. 2009;19:1827–32. doi: 10.1016/j.cub.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G, Gao P, Zhang H, Huang S, Zheng ZL. A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PLoS ONE. 2007;2:e1074. doi: 10.1371/journal.pone.0001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mucha E, Hoefle C, Hückelhoven R, Berken A. RIP3 and AtKinesin-13A - a novel interaction linking Rho proteins of plants to microtubules. Eur J Cell Biol. 2010;89:906–16. doi: 10.1016/j.ejcb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Duan Q, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci U S A. 2010;107:17821–6. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, et al. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–71. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo M, Altschmied L, Schweizer P, Kogel KH, Hückelhoven R. Respiratory burst oxidase homologue A of barley contributes to penetration by the powdery mildew fungus Blumeria graminis f.sp. hordei. J Exp Bot. 2006;57:3781–91. doi: 10.1093/jxb/erl191. [DOI] [PubMed] [Google Scholar]
- 29.Proels RK, Oberhollenzer K, Pathuri IP, Hensel G, Kumlehn J, Hückelhoven R. RBOHF2 of barley is required for normal development of penetration resistance to the parasitic fungus Blumeria graminis f. sp. hordei. Mol Plant Microbe Interact. 2010;23:1143–50. doi: 10.1094/MPMI-23-9-1143. [DOI] [PubMed] [Google Scholar]
- 30.Pathuri IP, Reitberger IE, Hückelhoven R, Proels RK. Alcohol dehydrogenase 1 of barley modulates susceptibility to the parasitic fungus Blumeria graminis f.sp. hordei. J Exp Bot. 2011;62:3449–57. doi: 10.1093/jxb/err017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proels RK, Westermeier W, Hückelhoven R. Infection of barley with the parasitic fungus Blumeria graminis f.sp. hordei results in the induction of HvADH1 and HvADH2. Plant Signal Behav. doi: 10.4161/psb.6.10.16889. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]