Abstract
The plant cell wall is involved in different biological processes like cell morphogenesis and response to biotic/abiotic stress. Functional integrity of the wall is apparently being maintained during these processes by changing structure/composition and coordinating cell wall with cellular metabolism. In S.cerevisiae a well-characterized mechanism exists that is maintaining functional integrity of yeast the cell wall during similar processes. During the last years it has become obvious that plants have evolved a mechanism to monitor and maintain functional integrity of their cell walls. However, our understanding of the mechanism is rather limited. The available evidence suggests that similar signaling cascades may be involved and particular protein activities may be conserved between plants and yeast. Here we review the available evidence briefly and highlight similarities between yeast and plants that could help us to understand the mode of action of the signaling cascades maintaining plant cell wall integrity.
Keywords: biotic/abiotic stress response, cell wall integrity, plant
The plant cell wall is involved in different biological processes like cell morphogenesis and biotic/abiotic stress responses.1,2 Maintaining functional integrity of the cell wall during these different processes is essential. In S. cerevisiae a dedicated mechanism monitoring and maintaining functional integrity of the cell wall has been described.3,4 Although this specialized mechanism exists, the available data shows that both osmo- and mechano-perception mechanisms are also involved in cell wall integrity (CWI) maintenance in yeast.3,4 Recently evidence has accumulated suggesting that a similar CWI maintenance mechanism exists in plants and several excellent reviews have covered this area to some extent.5,6 However, it has become increasingly obvious that plant CWI maintenance may additionally involve osmo-and damage associated molecular pattern (DAMP)-perception.2,7 DAMPs are low-molecular weight molecules like oligogalacturonides (OGs) derived from plant cell walls named in analogy to pathogen associated molecular patterns (PAMP).8 They are thought to arise during exposure of cell walls to abiotic/biotic stress and possibly during cell morphogenesis. These observations suggest that the plant CWI maintenance mechanism could actually be just one component of a matrix of signaling cascades coordinating and tailoring cellular responses to maintain plant cell wall integrity during interaction with the environment and development.9 Combining knowledge derived from yeast and plant research this review aims to highlight how different plant signaling cascades could interact to maintain functional plant CWI.
The yeast cell wall integrity matrix
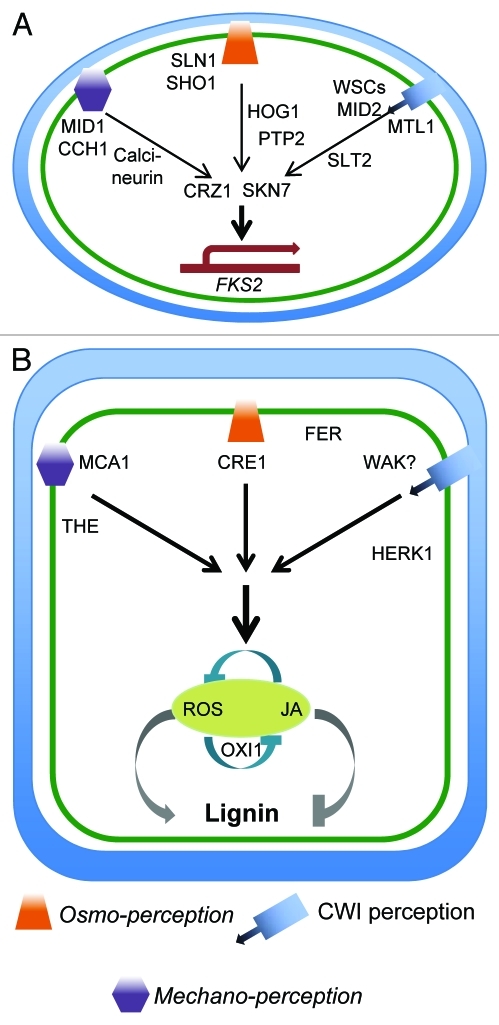
Three different sensor systems can monitor the functional integrity of the yeast cell wall and modulate responses to maintain CWI upon cell wall damage (CWD):The MID1 CCH1 based mechano-perception pathway; the high-osmolarity glycerol (HOG) pathway and the CWI maintenance mechanism.3 The signal generated by the CCH1 MID1 complex upon membrane stretch is relayed via calcineurin and CRZ1 to activate response genes like the glucan synthase FKS2.10 FKS2 activity is additionally regulated by the CWI pathway. Two different sensors (SHO1; SLN1/YPD1/SSK1) perceive hyperosmotic stress and generate signals relayed to the MAPKinase HOG1.11,12 These signals lead to activation of the transcriptional response via SKN7.3,13 SKN7 also mediates responses induced by the plasma membrane localized CWI sensor MID2 through interaction with CRZ1.3,14 Sequence similarity between the different yeast CWI sensor proteins WSC1, 2, 3, MTL1 and MID2 is limited and they appear to be required during distinct biological processes.15 Their extracellular regions, formed bycysteine rich domains (CRD) and highly O-mannosylated serine/threonine rich (STR) domains, project antenna-like into the yeast cell wall.16 The CRD domain is considered capable of interacting with glucans thus linking the extracellular domain of the sensor closely to the cell wall.17 Biophysical evidence suggests that the STR domain has properties of a nanospring thus enabling it to translate any conformational change of the extracellular domain when triggered by strain on the cell wall or the membrane, to the cytoplasmic part of the sensor.18 The cytoplasmic region of the sensors interact with the GDP/GTP exchange factor ROM2 generating a signal that is translated via protein kinase C and a MAPkinase module that includesSLT2.19 Interestingly the hyperosmotic stress activated HOG pathway interacts with the CWI pathway when induced by hypo-osmotic shock thus modulating the response of the yeast cells to low pH, heat shock and zymolyase treatment.4 During the response to zymolase (an enzyme mix consisting mostly of β-1,3glucanase activity) treatment the molecular mechanism coordinating both signaling cascades involves the MAPKinases SLT2, HOG1 and the PTP2 phosphatase.20-22 In a SLT2 deficient strain PTP2 expression is not induced by zymolase treatment, the phosphorylation level of the HOG1 MAPkinase is increased and expression of several stress response genes is induced.20 To summarize in yeast three different signaling mechanisms monitor events(membrane stretch, CWD and osmo-stress) indicative of possible CWI impairment and mediate the responses to maintain CWI. In certain situations exemplified here byzymolyase treatment different signaling mechanisms interact to modulate the response to a particular type of stress indicating a CWI signaling matrix exists in yeast.
The plant cell wall integrity maintenance mechanism
Evidence for the existence of a plant CWI maintenance mechanism has accumulated recently. A wide range of responses to different types of CWD has been described. Examples include enhanced pathogen resistance, ectopic lignin deposition, increased production of jasmonic acid, deposition of neutral cell wall sugars and changes in carbohydrate metabolism7,23-28 (Wormit et al., unpublished). Previously, three qualitatively different classes of signaling cascades have been described in the last years that could be involved in CWI monitoring and maintenance either directly or indirectly. For the sake of brevity we will focus on selected examples to represent the different signaling mechanisms capable of detecting a variety of stimuli. More detailed overviews can be found in the following reviews.5,6
The first group consists of receptor-like kinases (RLK) capable of detecting cell wall fragments (DAMPs) or changes in cell wall composition/structure. More than 600 RLKs have been identified in the Arabidopsis genome.29 They consist of an extracellular ligand-binding domain, a single trans-membrane segment and a cytosolic kinase domain. Cell wall fragments can be generated by cell wall degrading enzymes secreted by pathogens during infection. A classic example are the Endo-polygalacturonases (PGs), enzymes which cleave linkages between α-1,4 D-galacturonic acid residues in non-methylated homogalacturonan, the major component of pectin.30 During the hydrolysis of homogalacturonan, PGs supposedly release OGs from the pectin matrix, (embedded in the cellulose-hemicellulose network). WALL-ASSOCIATED KINASE1 (WAK1) from Arabidopsis has been shown to reside in the plasma membrane, bind tightly to cell walls and respondto OGs derived from pectic polysaccharides.31,32 WAKs have been shown to bind covalently to pectic homogalacturonan in plants and non-covalently to Ca2+crosslinked OGs in culture.33,34 WAK2 has been implicated in turgor pressure sensitive processes linking pectin perception with activation of an invertase that can modulate soluble sugar levels in planta, which in turn affects turgor pressure.35,36 These observations suggest that plant WAKs might be the functional analogs of the yeast CWI sensors monitoring the functional integrity of the plant cell wall through interaction with pectic polysaccharides. Downstream elements of the signaling cascade might be MAPKinases 3 and 6 but the signal transduction between these elements of cascade remains to be determined.36 THESEUS (THE), HERCULES1 (HERK1) and FERONIA (FER) belong to the Cataranthus roseus-like RLK (CrRLK1L) family and have been implicated in CWI maintenance during development.37-39 HERK1 and FER affect cell elongation but no CWD response phenotypes have been described to date.38 The seedlings exhibit defects in cell morphogenesis and CBI-induced lignin deposition suggesting that the same CWI maintenance mechanism may be active during both processes.37 While all these kinases have been implicated in CWI maintenance their specific functions and ligands remain to be characterized. To summarize, WAKs could represent the plant analogs of yeast CWI sensors. In the case of WAK2 the signals generated activate an invertase that can change soluble sugar levels, which in turn could affect turgor pressure.
Representative examples for the second group of sensors are MID1-COMPLEMENTING ACTIVITY1 and 2 (MCA1 and2).40,41 These putatively stretch-activated, plasma membrane localized Ca2+-channels can partially complement the mutant phenotype of a MID1 deficient yeast strain. mca1Arabidopsis seedling roots exhibit root growth defects, calcium influx in root cells upon mechano-stimulation is reduced and less ectopic lignin is deposited upon cellulose biosynthesis inhibition (CBI).9,40,41 CBI is a well-established method to cause highly specific cell wall damage by weakening the cellulose microfibril based exoskeleton providing most of the structural support to a plant cell.42,43 These observations implicateMCA1 and Ca2+-based signaling processes in the response to CWD. Furthermore calcium signaling inhibitors prevent CBI-induced lignin and reactive oxygen species (ROS) and JA production in a concentration dependent manner.9 CBI-induced ROS is generated by the NADPHoxidase RBOHD, which is synergistically activated by Ca2+ and phosphorylation.9,44 In rbohD seedlings, the CBI-induced ROS production is reduced and JA production enhanced.9 These observations suggest that THE is required for ROS biosynthesis and is not the only CWD sensor in Arabidopsis. In addition, it indicates that JA/ROS may form a negative feedback loop inhibiting each other’s production.9 The extent of CWD-induced lignin deposition seems to be modulated by JA/ROS signaling due to lignin being reduced in ROS signaling/production (OXI1, rbohDF) mutants while being enhanced in JA signaling and biosynthesis mutants.9 To summarize JA, ROS and Ca2+-based signaling mechanisms mediate the response to CWD in plants. Interestingly, Arabidopsis MCA1 is able to rescue the MID1 yeast mutant while also being required for CWD-induced lignin deposition in Arabidopsis.
The third group of sensors is exemplified by the ARABIDOPSIS HISTIDINE KINASES (AHK1–3, AHK4/CRE1). They form part of a two-component system consisting of a histidine kinase acting as environmental sensor and a phosphor-relay system to translate the signal generated.45 The available literature has implicated these genes in cytokinin and osmo-perception as well as ABA-dependent abiotic plant stress responses.46,47 Expression of CRE1 in a SLN1 deficient yeast strain rescues the mutant phenotype if cytokinin is present.48 Previous work has shown that provision of osmotic support prevents ectopic lignification and necrosis induced by cellulose inhibition.7 CBI also induces starch increases in Arabidopsis seedlings, which can be suppressed by osmotic support (Wormit et al., under review). These results implicate osmosensing in CWI maintenance but do not clarify the specific function of turgor pressure in this context. Turgor pressure could function as indicator of plant CWI or similarly like in yeast complement the activity of the CWI maintenance pathway. In ahk1, ahk4/cre1and mca1seedlings the CBI-induced starch increase is detectable. However, the osmotic suppression is not detectable in ahk4/cre1and mca1seedlings (Wormit et al., under review). These observations suggest that CRE1 and MCA1 but not AHK1 are mediating the observed osmotic support effect. Since the CBI-induced starch increases are detectable in both cre1 and mca1 seedlings CWD perception itself is either occurring in parallel to turgor perception or is redundantly specified. More importantly the responses to CWD can be modulated by turgor pressure changes and CRE1 and MCA1 are required for thismechanism as shown by the observed effects on starch levels in the mutant seedlings.
Conclusions
The available data suggest both design similarities and functional conservation between the yeast and plant cell wall integrity monitoring and maintenance systems. These are illustrated by the color-coding in Figure 1A and B of signaling cascades in yeast (oval) and plant cells (rectangular). Despitethe CWI sensor proteins being well characterized in yeast our understanding of the corresponding plant proteins is limited. The strongest candidates in plants to perform this function are DAMP receptors and WAKs. DAMP receptors are plant specific and nothing similar has been observed in yeast. They could represent an additional level of detection enabling plants to deal with biotic stresses that yeast cells do not experience. Based on this knowledge, it is likely that WAKs represent the group of plant proteins most similar to CWI sensors in yeast based on their mode of action and apparent biological activities. Data from WAK2 implicates turgor pressure perception in the modulation of the CWD response in plants. Previous work in yeast has shown that the CWI and the HOG signaling/osmosensing pathway regulate the response to zymolase treatment jointly. The effects of zymolase and CBI on yeast and plant cell walls are similar (breakdown of the load bearing cell wall elements). The Arabidopsis protein CRE1 can functionally replace one of the two osmo sensors (SLN1) of the HOG pathway. Accordingly it is intriguing that osmotic support can neutralize the effects of CBI on starch levels in Arabidopsis wild type seedlings but not incre1 seedlings. Recent work has shown that the Arabidopsis MCA1 protein can complement a MID1 deficient yeast strain and mca1 seedlings are impaired in CBI induced lignin deposition. These observations suggest that in yeast and Arabidopsis, a similar matrix of signaling cascades may mediate the response to impairment of CWI and that protein activities are conserved to a certain degree between both species. From the current perspective this research area represents a novel, original approach to understand the mode of action of plant pathogen response mechanisms and environmental stress by placing the plant cell wall at the heart of initial perception, signaling and response to environmental and developmental stimuli.
Figure 1.
Schematic overview of signaling cascades implicated in CWI maintenance in a yeast (A, oval) and a plant (B, rectangular) cell. The cell wall is marked in blue, the plasma membrane in green and gene expression in red.
Acknowledgments
The authors would like to thank Joe McKenna for critical reading of the manuscript and helpful comments. Work in the Hamann lab is supported by the BBSRC Sustainable Bioenergy Center, the Porter Institute at Imperial College and the Gatsby Charitable Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17782
References
- 1.Szymanski DB, Cosgrove DJ. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol. 2009;19:R800–11. doi: 10.1016/j.cub.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Hématy K, Cherk C, Somerville S. Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol. 2009;12:406–13. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–91. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Peña JM, Garcia R, Nombela C, Arroyo J. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: a yeast dialogue between MAPK routes. Yeast. 2010;27:495–502. doi: 10.1002/yea.1792. [DOI] [PubMed] [Google Scholar]
- 5.Ringli C. Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol. 2010;153:1445–52. doi: 10.1104/pp.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seifert GJ, Blaukopf C. Irritable walls: the plant extracellular matrix and signaling. Plant Physiol. 2010;153:467–78. doi: 10.1104/pp.110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamann T, Bennett M, Mansfield J, Somerville C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. 2009;57:1015–26. doi: 10.1111/j.1365-313X.2008.03744.x. [DOI] [PubMed] [Google Scholar]
- 8.Zipfel C. Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol. 2009;12:414–20. doi: 10.1016/j.pbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, et al. Cell wall damage-induced lignin biosynthesis is regulated by a ROS- and jasmonic acid dependent process in Arabidopsis thaliana. Plant Physiol. 2011 doi: 10.1104/pp.111.175737. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C, Jung US, Garrett-Engele P, Roe T, Cyert MS, Levin DE. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol Cell Biol. 1998;18:1013–22. doi: 10.1128/mcb.18.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17:1385–94. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohmann S. Control of high osmolarity signalling in the yeast Saccharomyces cerevisiae. FEBS Lett. 2009;583:4025–9. doi: 10.1016/j.febslet.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 13.Lesage G, Sdicu AM, Menard P, Shapiro J, Hussein S, Bussey H. Analysis of beta-1,3-glucan assembly in Saccharomyces cerevisiae using a synthetic interaction network and altered sensitivity to caspofungin. Genetics. 2004;167:35–49. doi: 10.1534/genetics.167.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams KE, Cyert MS. The eukaryotic response regulator Skn7p regulates calcineurin signaling through stabilization of Crz1p. EMBO J. 2001;20:3473–83. doi: 10.1093/emboj/20.13.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodicio R, Heinisch JJ. Together we are strong–cell wall integrity sensors in yeasts. Yeast. 2010;27:531–40. doi: 10.1002/yea.1785. [DOI] [PubMed] [Google Scholar]
- 16.Lommel M, Bagnat M, Strahl S. Aberrant processing of the WSC family and Mid2p cell surface sensors results in cell death of Saccharomyces cerevisiae O-mannosylation mutants. Mol Cell Biol. 2004;24:46–57. doi: 10.1128/MCB.24.1.46-57.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponting CP, Hofmann K, Bork P. A latrophilin/CL-1-like GPS domain in polycystin-1. Curr Biol. 1999;9:R585–8. doi: 10.1016/S0960-9822(99)80379-0. [DOI] [PubMed] [Google Scholar]
- 18.Heinisch JJ, Dupres V, Alsteens D, Dufrene YF. Measurement of the mechanical behavior of yeast membrane sensors using single-molecule atomic force microscopy. Nat Protoc. 2010;5:670–7. doi: 10.1038/nprot.2010.19. [DOI] [PubMed] [Google Scholar]
- 19.Vay HA, Philip B, Levin DE. Mutational analysis of the cytoplasmic domain of the Wsc1 cell wall stress sensor. Microbiology. 2004;150:3281–8. doi: 10.1099/mic.0.27264-0. [DOI] [PubMed] [Google Scholar]
- 20.García R, Rodriguez-Pena JM, Bermejo C, Nombela C, Arroyo J. The high osmotic response and cell wall integrity pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in Saccharomyces cerevisiae. J Biol Chem. 2009;284:10901–11. doi: 10.1074/jbc.M808693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacoby T, Flanagan H, Faykin A, Seto AG, Mattison C, Ota I. Two protein-tyrosine phosphatases inactivate the osmotic stress response pathway in yeast by targeting the mitogen-activated protein kinase, Hog1. J Biol Chem. 1997;272:17749–55. doi: 10.1074/jbc.272.28.17749. [DOI] [PubMed] [Google Scholar]
- 22.Martín H, Flandez M, Nombela C, Molina M. Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol Microbiol. 2005;58:6–16. doi: 10.1111/j.1365-2958.2005.04822.x. [DOI] [PubMed] [Google Scholar]
- 23.Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M. Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J. 2003;34:351–62. doi: 10.1046/j.1365-313X.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- 24.Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–66. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Blanco C, Feng DX, Hu J, Sanchez-Vallet A, Deslandes L, Llorente F, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel JP, Raab TK, Somerville CR, Somerville SC. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 2004;40:968–78. doi: 10.1111/j.1365-313X.2004.02264.x. [DOI] [PubMed] [Google Scholar]
- 27.Vogel JP, Raab TK, Schiff C, Somerville SC. PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell. 2002;14:2095–106. doi: 10.1105/tpc.003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science. 2003;301:969–72. doi: 10.1126/science.1086716. [DOI] [PubMed] [Google Scholar]
- 29.Sanabria N, Goring D, Nurnberger T, Dubery I. Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytol. 2008;178:503–14. doi: 10.1111/j.1469-8137.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 30.De Lorenzo G, D'Ovidio R, Cervone F. The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol. 2001;39:313–35. doi: 10.1146/annurev.phyto.39.1.313. [DOI] [PubMed] [Google Scholar]
- 31.He ZH, Fujiki M, Kohorn BD. A cell wall-associated, receptor-like protein kinase. J Biol Chem. 1996;271:19789–93. doi: 10.1074/jbc.271.33.19789. [DOI] [PubMed] [Google Scholar]
- 32.Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA. 2010;107:9452–7. doi: 10.1073/pnas.1000675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner TA, Kohorn BD. Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell. 2001;13:303–18. doi: 10.1105/tpc.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decreux A, Messiaen J. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 2005;46:268–78. doi: 10.1093/pcp/pci026. [DOI] [PubMed] [Google Scholar]
- 35.Kohorn BD, Kobayashi M, Johansen S, Riese J, Huang LF, Koch K, et al. An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J. 2006;46:307–16. doi: 10.1111/j.1365-313X.2006.02695.x. [DOI] [PubMed] [Google Scholar]
- 36.Kohorn BD, Johansen S, Shishido A, Todorova T, Martinez R, Defeo E, et al. Pectin activation of MAP kinase and gene expression is WAK2 dependent. Plant J. 2009;60:974–82. doi: 10.1111/j.1365-313X.2009.04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol. 2007;17:922–31. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Guo H, Li L, Ye H, Yu X, Algreen A, Yin Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2009;106:7648–53. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–60. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010;152:1284–96. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA. 2007;104:3639–44. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desprez T, Vernhettes S, Fagard M, Refregier G, Desnos T, Aletti E, et al. Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiol. 2002;128:482–90. doi: 10.1104/pp.010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C. Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proc Natl Acad Sci USA. 2001;98:10079–84. doi: 10.1073/pnas.191361598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–92. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 45.Romir J, Harter K, Stehle T. Two-component systems in Arabidopsis thaliana–A structural view. Eur J Cell Biol. 2010;89:270–2. doi: 10.1016/j.ejcb.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Riefler M, Novak O, Strnad M, Schmulling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, et al. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:20623–8. doi: 10.1073/pnas.0706547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, et al. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409:1060–3. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]