Abstract
The accumulation of osmolytes like glycinebetaine (GB) in cell is known to protect organisms against abiotic stresses via osmoregulation or osmoprotection. Transgenic plants engineered to produce GB accumulate very low concentration of GB, which might not be sufficient for osmoregulation. Therefore, other roles of GB like cellular macromolecule protection and ROS detoxification have been suggested as mechanisms responsible for abiotic stress tolerance in transgenic plants. In addition, GB influences expression of several endogenous genes in transgenic plants. The new insights gained about the mechanism of stress tolerance in GB accumulating transgenic plants are discussed.
Keywords: codA (choline oxidase A), glycinebetaine, H2O2, osmolyte, stress tolerance, transcriptomics, transgenics
Introduction
The accumulation of low molecular weight water-soluble compounds known as “compatible solutes” or “osmolytes” is the common strategy adopted by many organisms to combat the environmental stresses. The most common compatible solutes are betaines, sugars (mannitol, sorbitol, and trehalose), polyols, polyamines, and amino acid (proline). Their accumulation is favored under water-deficit or salt stress as they provide stress tolerance to cell without interfering cellular machinery.1 The tolerant or sensitive species show differential stress tolerance depending on the levels of accumulation of these compounds during abiotic stresses. Genes participating in the biosynthesis of different kinds of compatible solutes have been identified from varied sources. Genetic engineering with these endogenous or ectopic genes has therefore, been used successfully to synthesize compatible solutes in target organisms and improvement of stress tolerance.2,3 A correlation between accumulation of sugars like raffinose family oligosaccharides (RFO), trehalose, fructan, galactinol and sugar alcohols like mannitol, D-ononitol and abiotic stress tolerance has also been reported.4,5 Their biosynthetic genes have also been proven useful to improve abiotic stress tolerance in transgenic plants.4,6,7 Similar to sugars, accumulation of proline is a common physiological response in many plants under biotic and abiotic stresses.8 The main proline biosynthetic gene pyrroline-5-carboxylate synthase (P5CS) has been identified from different species and extensively used to enhance the levels of proline for abiotic stress tolerance in transgenic plants.9 Further, its stress-inducible expression has been shown as a better strategy than constitutive expression in order to minimize the undesirable effects in transgenic plants.10,11
Among the nitrogenous compounds, polyamines accumulate in a variety of plants in response to abiotic stresses like salt and drought.12 Three polyamines namely, putrescine (diamine), spermine (triamine) and spermidine (tetramine) are found to be involved in variety of physiological functions in all organisms. Genes involved in polyamines metabolism have been cloned and often used to alter polyamines levels in transgenic plants for conferring abiotic stress tolerance.13 However, it is not the higher accumulation of putrescine which increases plant stress tolerance; rather it is the conversion of putrescine into spermidine and spermine, polyamines responsible for stress tolerance.14 Another nitrogenous compound, glycinebetaine (GB) is a quaternary amine with zwitterionic nature and its natural accumulation is associated with abiotic stress tolerance in varied organisms. Like other compatible solutes, GB biosynthetic genes have also been widely used to improve abiotic stress tolerance in transgenic plants.15
Although compatible solutes fall in different bio-chemical groups, similar roles have been assigned to them in plant protection against stresses. However, a precise role of compatible solutes, including GB, in abiotic stress tolerance is largely unknown and two basic functions attributed to these solutes are osmotic adjustment and cellular compatibility. Osmotic adjustment occurs through concentration dependent effects on osmotic pressure to absorb more water from surroundings. In cellular compatibility mechanism, these compounds replace water in biochemical reactions thereby, maintaining normal metabolism during stress.16 One major issue with compatible solutes is their lower accumulation in transgenic plants as compared with their natural accumulators. At such low levels, compatible solutes might not contribute significantly to osmotic adjustment. Therefore, these compounds are also suggested to be involved in ROS scavenging, macromolecules (nucleic acids, proteins, lipids) protection, and act as reservoir of carbon and nitrogen source.2,16 In addition, new aspects of their functionality, especially GB, are emerging fast. Present review highlights the new emerging roles of GB in protecting plants against environmental stresses.
Glycinebetaine: roles, mechanism and emerging concepts
Glycinebetaine (GB) accumulates in a variety of organisms under abiotic stresses and has been studied in great details.1,16 Plants known to accumulate GB naturally have been reported to grow well under drought and saline environment.1,3 Accumulation of GB in transgenic apple expressing stress regulator gene, Osmyb4, was linked to enhanced drought and cold tolerance.17 Exogenous application of GB improves the growth and survival rate of plants under a variety of stresses18 and in food born bacteria Listeria monocytogenes.19 GB is synthesized either by oxidation of choline or by three known pathways.1 In plants, the enzyme choline monooxygenase (CMO) first converts choline into betaine aldehyde and then a NAD+ dependent enzyme, betaine aldehyde dehydrogenase (BADH) produces glycinebetaine. These enzymes are mainly found in chloroplast stroma and their activity is increased in response to salt stress. In E. coli, GB is synthesized by choline dehydrogenase enzyme (CDH) along with BADH. Whereas in soil bacterium, Arthrobacter globiformis, choline oxidaseA (codA) converts choline into GB and H2O2 in a single step.
Use of GB biosynthetic genes in transgenic plants
Major cereals like wheat, maize and barley do not accumulate significant amount of GB naturally. This could be due to the production of truncated transcripts for GB synthesizing enzyme (BADH), in these cereals.20 Among these, rice is the only cereal that does not accumulate GB naturally.21 Rice has two BADH and one CMO encoding genes, however, no GB accumulation occurs in rice under stress. The BADH transcripts are processed in an unusual manner in rice resulting in removal of translational initiation codon, loss of functional domains and premature stop codons.20 However, some correctly spliced BADH transcripts have also been reported from rice. Exactly similar observations were made for CMO transcripts in rice by same group.22 However, transgenic rice plants expressing functional BADH gene from barley could convert exogenously applied betaine aldehyde to GB at a level better than WT plants.23 Introduction of spinach CMO gene in rice also resulted in accumulation of detectable amount of GB.21 Therefore, rice produces highly reduced amount of functional BADH and CMO proteins resulting in undetectable amount of GB synthesis. Interestingly, BADH gene has been linked to fragrance in rice.24 Like rice many crop plants lack the ability to accumulate GB naturally during abiotic stress.15 Identification of genes of GB biosynthetic pathways has made it easy to engineer GB biosynthesis into non-accumulators by transgenic approach for improved stress tolerance. This approach has been successfuly used in diverse plant species, e.g., Arabidopsis,25-28 tobacco,29-31 Brassica,32,33 Persimmon,34 tomato,35,36 maize,37 rice,38-40 potato41 and wheat42 to improve their abiotic stress tolerance.
Among the different GB biosynthetic genes, choline oxidase (codA) from A. globiformis has been widely used for GB production in transgenic plants. This gene converts choline into GB in one step. Availability of endogenous choline, therfore, could limit the GB biosynthesis in transgenic plants.43 However, levels of endogenous choline were not changed significantly in transgenic Arabidopsis and rice plants expressing codA gene.25,38,39 Therefore, availability of choline does not affect the GB synthesis in these transgenic plants probably due to synergism in demand and supply of choline metabolism. However, a recent report indicates on limiting roles of choline availability on GB accumulation in GB deficient nearly isogenic lines of sorgham and maize.44 Therefore, this aspect needs to be further validated to conclude the effect of choline availability on GB levels.
Constitutive accumulation of compatible solutes like ployamines, proline and trehalose resulted in abnormal plant phenotype. Therefore, stress-inducible expression of genes encoding these solutes is often suggested.10,45 However, no such abnormality has been observed in transgenic plants accumulating GB constitutively. Su et al.40 have raised transgenic rice expressing cox gene from A. pascens under stress-inducible and constitutive promoters. GB accumulation following the salt stress was higher in lines with constitutive expression, suggesting that constitutive accumulation of GB is beneficial for stress tolerance without any phenotypic abnormality to plants. In some cases, localized accumulation of GB within the cell was found to affect the performence of transgenic plants under stress. GB synthesizing enzymes have been targeted to cytosol, mitochondria and chloroplast. In transgenic rice with chloroplast targeted GB accumulation, protection of photosynthetic machinery against salt and cold stress was better than in plants with cytosolic GB accumulation, even though GB accumulation was 5-fold higher in later plants.38 Transgenic tomato plants were raised with codA targeted to chloroplast, cytosol and to both cytosol and chloroplast, simultaneously.46 Plants with chloroplast targeted GB synthesis, even though accumulated least amount of GB, showed better seedling growth following chilling treatment. These results suggested that GB accumulation in chloroplast is a better strategy for engineering abiotic stress tolerance in plants.
GB acummulates at a high concentration (4–40 µmol g−1 FW) in naturally accumulating plants like spinach, sugar beet and acts as osmoregulator in abiotic stress conditions.1,15 However, GB synthesizing genes carrying transgenic plants produced much reduced amount of GB (0.05–5 µmol g−1 FW).1 Although GB at concentration of 0.035 μmole g−1 fresh weight, could impart cold and salinity stress tolerance in transgenic tobacco.30 Osmoregulation by GB at these concentrations is unlikely in transgenic plants rather protection of cellular macromolecules is the role played by GB in transgenic system. Several alternative modes of GB action (osmoprotection, protection of membarane and quaternary structure of enzymes, ROS detoxification) in abiotic stress tolerance in transgenic plants have also been reported (Table 1).
Table 1. Major roles of GB in transgenic plants under abiotic stresses*.
| Plant species transformed | Gene | Phenotype | Remark | Reference |
|---|---|---|---|---|
|
Arabidopsis thaliana |
codA |
Tolerance to various abiotic stresses |
Protection against damage of membrane, enzyme activity, photosynthesis |
25–28 |
|
Oryza sativa |
codA |
Tolerance to salt, cold and drought stress |
Protection against damage of membrane, enzyme activity, photosynthesis and yield loss; regulation of ROS detoxification and transcriptome changes |
38, 55 |
|
Lycopersicon esculentum |
codA |
Cold, salt and oxidative stress tolerance |
Protection of photosynthesis and reproductive organs; increased ROS detoxification |
46, 47 |
|
Nicotiana tabacum |
betA |
Tolerance to salt and drought |
Protection of photosynthesis |
30 |
|
BADH |
Tolerance to heat stress |
Protection of rubisco activity |
69 |
|
|
Triticum aestivum |
BADH |
Heat and drought tolerance |
Protection of photosynthesis |
42 |
| |
|
|
|
|
|
Zea mays |
betA |
Cold and drought tolerance |
Protection of photosynthesis and membrane integrity |
68 |
|
Diospyras kaki |
codA |
Salt tolerance |
Protection of photosynthesis |
34 |
|
Solanum tuberosum |
codA |
Tolerance to salt, drought and oxidative stress |
Protection of photosynthesis and membrane integrity |
41 |
| Gossypium hirsutum | betA | Drought tolerance | Protection of membrane integrity | 67 |
GB biosynthetic genes have been introduced in different transgenic plants by several researchers, however, for the sake of brevity, studies commented on role of GB, are listed here. Other studies are mentioned in text. Source of codA gene is Arthrobacter globiformis, while BADH genes are from spinach (ref. 69) and Artiplex (ref. 42). betA, E. coli gene encoding choline dehydrogenase.
GB and protection of reproductive organs during abiotic stress
Plant yield is severly comporised under abiotic stress due to limited growth of reproductive organs. New evidences indicate toward the protection of reproductive organs by GB.15 Indeed, improved plant growth in terms of biomass and yield was reported in transgenic tomato expressing codA gene from A. Globiformis.47 codA transgenic Arabidopsis plants produced about 22% more flowers and 28% more seeds than WT plants in unstressed conditions.35 These effects of GB were attributed to higher accumulation of GB in reproductive organs. Reproductive organs; flowers, siliques and inflorescence accumulated about 5-fold higher GB than leaves in plants experessing codA gene constitutively.35 Tomato plants expressing codA gene produced 10–30% more fruit than WT plants after chilling stress.35 All these effects were due to the protection of reproductive organs from stress by higher localized accumulation of GB.3
GB, protection of photosynthesis machionary and ROS detoxification during abiotic stress
Recently, Chen and Murata3 have proposed that in addition to other roles, GB could be involved in inhibiting ROS accumulation, protection of photosynthetic machinery, activation of some stress related genes and membrane protection. GB has also been implicated in protection of quaternary structure of proteins (thereby maintaining the enzyme activity) from damaging effects of environmental stresses.48 Many proteins are prone to aggregation under heat and salt stress thereby, losing their native structure and activity. The chaperone proteins (molecular chaperons) are known to prevent protein aggregation, disassemble protein aggregates, and help in protein refolding under stress. The low molecular weight compounds like osmolyte (chemical chaperons) have also been shown to stabilize protein native structure.49 A direct role of GB in chaperon-mediated protein disaggregation has been reported.50 GB could activate ClpB, a component of chaperon network and therefore, increased the efficiency of chaperone-mediated protein disaggregation under salt and heat stress in E. coli.50 During salt or drought stress, synthesis of proteins involved in PSII repair is affected leading to photoinhibition.51-53 GB antagonizes the inhibition of protein biosynthesis and thus enhances the PSII repair, which leads to increased stress tolerance.3
Reactive oxygen species (ROS) are continually produced in chloroplast and mitochondria as byproduct of metabolism. However, their production is enhanced under abiotic stresses which lead to photoinhibition of PSII in chloroplast. GB has been shown to protect the photosynthesis machinery by stabilizing the activity of repair proteins under high concentrations of NaCl.54 The role of GB in ROS detoxification is also evident by reduced accumulation of ROS in transgenic plants under water-deficit stress as compared with WT plants.55 Therefore, GB can provide tolerance to abiotic stresses even at low concentration by protecting photosynthesis under abiotic stress.15,51,53,54
Tolerance to abiotic stress is coupled with contribution of endogenous genes in transgenic plants
The proposed mechanisms for GB-mediated abiotic stress tolerance include stabilization of native structure of proteins and enzymes, osmoregulation, membrane integrity, protection of photosynthesis and detoxification of reactive oxygen radicals produced during stress (Table 1). Given the low levels of accumulation of GB in transgenic plants, these mechanisms may not explain observed stress tolerance in transgenic plants completely. Two osmolytes GB and proline have been shown to destabilize the double-helix DNA and lowers the melting temperature of DNA in vivo. This would make GB a candidate to regulate gene expression under abiotic stress by activating replication and transcription.56 In addition to GB, H2O2 is also produced as byproduct in codA expressing transgenic plants. Therefore, accumulation of H2O2 in Arabidopsis and tomato plants expressing codA gene was found to be higher than untransformed plants.35,57 H2O2 is a well-known regulator of gene expression during biotic and abiotic stress signaling.58 Transgenic rice carrying fungal glucose oxidase gene produced increased levels of H2O2 and showed tolerance to biotic stress.59 Similarly, exogenous application of H2O2 has also been shown to improve abiotic stress tolerance in tobacco.35 Although the amount of H2O2 produced in transgenic tomato was only 17–21% higher than wild-type plants under unstressed conditions, transcriptome changes were observed in transgenic plants.47
Other compatible solutes have also been shown to affect the expression of endogenous genes in transgenic plants carrying genes for their biosynthesis. Accumulation of putrescine in transgenic Arabidopsis plants, overexpressing ADC2 gene, resulted in altered expression of many genes, mainly those involved in GA metabolism.60 Similarly, stress-related genes were upregulated in transgenic Arabidopsis plants overexpressing spermidine synthase gene.61 In a relatively different approach, antisense plants for proline dehydrogenase gene resulted in higher proline accumulation and tolerance to abiotic stresses. cDNA microarray analysis revealed the up- or downregulation of some endogenous genes in transgenic plants.62 GB has also been shown to have such effects in plants. GB when applied exogenously resulted in change in transcript levels of WCOR410 and catalase gene in wheat and tomato plants, respectively.18,63 Expression of many stress-related genes changed in GB treated Arabidopsis plants.64 It was further revealed that RabAc4 (G-protein involved in membrane trafficking) is required for GB mediated chilling tolerance. codA expressing transgenic plants accumulate both GB and H2O2. In transgenic tomato carrying codA gene, expression of 30 genes was induced and that of 29 repressed.47 These reports indicate that effects of compatible solutes might be manifested through the induction of genes whose products are involved in stress tolerance and other plant responses.
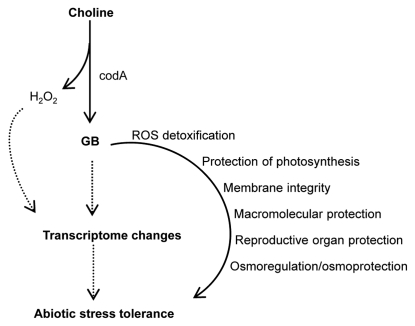
Recently, genome-wide transcriptome analysis in transgenic rice expressing codA gene showed altered expression of several transcripts even under unstressed conditions in relation to wild-type plants.55 About 50 genes known to be involved in one or other type of stress (both biotic and abiotic) were induced in transgenic plants. Genes involved in variety of cellular processes like transcription, signaling, membrane transport, metabolism and growth were also induced, supporting the idea of complex nature of genetic response to abiotic stress in plants.55,65 Upregulation of these genes might be responsible for observed stress tolerance in transgenic rice. However, the activation of these genes exclusively by GB or H2O2 alone could not be established, since both are capable of regulating gene expression. Wild-type plants when treated exogenously either with GB or H2O2 showed differential expression of some of the stress related genes whose expression levels were also altered in codA expressing rice.55 Furthermore, certain H2O2 marker genes like catalase, peroxidase and heat shock factors were induced in transgenic plants in unstressed condition.55,66 Therefore, transcriptomic changes, derived in parts by H2O2, might also contribute to the stress tolerance in codA-expressing transgenic plants along with other direct roles of glycinebetaine (Fig. 1).
Figure 1.
Model for mechanisms of abiotic stress tolerance in codA expressing plants. Dotted arrows indicate possible involvement of H2O2 or GB in transcriptome changes and subsequent stress tolerance. codA-mediated conversion of choline into GB, releases H2O2 as byproduct. The H2O2 might activate stress related transcripts in transgenic plants and enhance stress tolerance. Such gene regulation can also be result of GB accumulation. Given the limited accumulation of GB in transgenic plants, stress related transcriptome changes might contribute to the observed effects of GB on stress tolerance.
In conclusion, GB accumulation could contribute to osmoregulation in natural accumulators; however, osmoprotection seems to be responsible for tolerance to abiotic stresses in transgenic plants. Extensive work on GB has suggested its varied roles in plants. New evidences suggest the contribution of differentially expressing endogenous genes in GB mediated stress tolerance in plants. Further work would establish whether the transcriptome changes are direct targets of GB or are product of metabolic adjustment in transgenic plants.
Acknowledgments
Thanks are due to Professor A.K. Tyagi, National Institute of Plant Genome Research for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17801
References
- 1.Chen TH, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol. 2002;5:250–7. doi: 10.1016/S1369-5266(02)00255-8. [DOI] [PubMed] [Google Scholar]
- 2.Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K. Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol. 2006;17:113–22. doi: 10.1016/j.copbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Chen TH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13:499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, et al. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002;29:417–26. doi: 10.1046/j.0960-7412.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2005;24:23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- 6.Abebe T, Guenzi AC, Martin B, Cushman JC. Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol. 2003;131:1748–55. doi: 10.1104/pp.102.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami A, Sato Y, Yoshida M. Genetic engineering of rice capable of synthesizing fructans and enhancing chilling tolerance. J Exp Bot. 2008;59:793–802. doi: 10.1093/jxb/erm367. [DOI] [PubMed] [Google Scholar]
- 8.Siripornadulsil S, Traina S, Verma DP, Sayre RT. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell. 2002;14:2837–47. doi: 10.1105/tpc.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–9. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- 10.Su J, Wu R. Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci. 2004;166:941–8. doi: 10.1016/j.plantsci.2003.12.004. [DOI] [Google Scholar]
- 11.Molinari HBC, Marur CJ, Daros E, de Campos MKF, de Carvalho JFRP, Filho JCB, et al. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant. 2007;130:218–29. doi: 10.1111/j.1399-3054.2007.00909.x. [DOI] [Google Scholar]
- 12.Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34:35–45. doi: 10.1007/s00726-007-0501-8. [DOI] [PubMed] [Google Scholar]
- 13.Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, et al. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–49. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- 14.Capell T, Bassie L, Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA. 2004;101:9909–14. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TH, Murata N. Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ. 2011;34:1–20. doi: 10.1111/j.1365-3040.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- 16.Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. doi: 10.1016/0167-7799(96)80929-2. [DOI] [Google Scholar]
- 17.Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M. Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep. 2008;27:1677–86. doi: 10.1007/s00299-008-0587-9. [DOI] [PubMed] [Google Scholar]
- 18.Park EJ, Jeknic Z, Chen TH. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006;47:706–14. doi: 10.1093/pcp/pcj041. [DOI] [PubMed] [Google Scholar]
- 19.Dreux N, Albagnac C, Sleator RD, Hill C, Carlin F, Morris CE, et al. Glycine betaine improves Listeria monocytogenes tolerance to desiccation on parsley leaves independent of the osmolyte transporters BetL Gbu and OpuC. J Appl Microbiol. 2008;104:1221–7. doi: 10.1111/j.1365-2672.2007.03623.x. [DOI] [PubMed] [Google Scholar]
- 20.Niu X, Zheng W, Lu BR, Ren G, Huang W, Wang S, et al. An unusual post-transcriptional processing in two betaine aldehyde dehydrogenase (BADH) loci of cereal crops directed by short-direct repeats in response to stress conditions. Plant Physiol. 2007;143:1929–42. doi: 10.1104/pp.107.095752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirasawa K, Takabe T, Kishitani S. Accumulation of glycinebetaine in rice plants that overexpress choline monooxygenase from spinach and evaluation of their tolerance to abiotic stress. Ann Bot. 2006;98:565–71. doi: 10.1093/aob/mcl126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo D, Niu X, Wang Y, Zheng W, Chang L, Wang Q, et al. Functional defect at the rice choline monooxygenase locus from an unusual post-transcriptional processing is associated with the sequence elements of short-direct repeats. New Phytol. 2007;175:439–47. doi: 10.1111/j.1469-8137.2007.02124.x. [DOI] [PubMed] [Google Scholar]
- 23.Kishitani S, Takanami T, Suzuki M, Oikawa M, Yokoi S, Ishitani M, et al. Compatibility of glycinebetaine in rice plants: evaluation using transgenic rice plants with a gene for peroxisomal betaine aldehyde dehydrogenase from barley. Plant Cell Environ. 2000;23:107–14. doi: 10.1046/j.1365-3040.2000.00527.x. [DOI] [Google Scholar]
- 24.Fitzgerald TL, Waters DL, Henry RJ. Betaine aldehyde dehydrogenase in plants. Plant Biol (Stuttg) 2009;11:119–30. doi: 10.1111/j.1438-8677.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi H, Alia Mustardy L, Deshnium P, Ida M, Murata N. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997;12:133–42. doi: 10.1046/j.1365-313X.1997.12010133.x. [DOI] [PubMed] [Google Scholar]
- 26.Alia H, Sakamoto A, Murata N. Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J. 1998;16:155–61. doi: 10.1046/j.1365-313x.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto A, Valverde R. Alia, Chen TH, Murata N. Transformation of Arabidopsis with the codA gene for choline oxidase enhances freezing tolerance of plants. Plant J. 2000;22:449–53. doi: 10.1046/j.1365-313X.2000.00749.x. [DOI] [PubMed] [Google Scholar]
- 28.Sulpice R, Tsukaya H, Nonaka H, Mustardy L, Chen TH, Murata N. Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J. 2003;36:165–76. doi: 10.1046/j.1365-313X.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- 29.Lilius G, Holmberg N, Bülow L. Enhanced NaCl stress tolerance in transgenic tobacco expressing bacterial choline dehydrogenase. Biotechnology (N Y) 1996;14:177–80. doi: 10.1038/nbt0296-177. [DOI] [Google Scholar]
- 30.Holmström KO, Somersalo S, Mandal A, Palva TE, Welin B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot. 2000;51:177–85. doi: 10.1093/jexbot/51.343.177. [DOI] [PubMed] [Google Scholar]
- 31.Shen YG, Du BX, Zhang WK, Zhang JS, Chen SY. AhCMO, regulated by stresses in Atriplex hortensis, can improve drought tolerance in transgenic tobacco. Theor Appl Genet. 2002;105:815–21. doi: 10.1007/s00122-002-1006-1. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA, et al. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol. 2000;122:747–56. doi: 10.1104/pp.122.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad KVSK, Sharmila P, Kumar PA, Pardha Saradhi P. Transformation of Brassica juncea (L.) Czern with bacterial codA gene enhances its tolerance to salt stress. Mol Breed. 2000;6:489–99. doi: 10.1023/A:1026542109965. [DOI] [Google Scholar]
- 34.Gao M, Sakamoto A, Miura K, Murata N, Sugiura A, Tao R. Transformation of Japanese persimmon (Diospyros kaki Thunb.) with a bacterial gene for choline oxidase. Mol Breed. 2000;25:501–10. doi: 10.1023/A:1026513831290. [DOI] [Google Scholar]
- 35.Park EJ, Jeknic Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, et al. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J. 2004;40:474–87. doi: 10.1111/j.1365-313X.2004.02237.x. [DOI] [PubMed] [Google Scholar]
- 36.Goel D, Singh AK, Yadav V, Babbar SB, Murata N, Bansal KC. Transformation of tomato with a bacterial codA gene enhances tolerance to salt and water stresses. J Plant Physiol. 2011;168:1286–94. doi: 10.1016/j.jplph.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Quan R, Shang M, Zhang J. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004;166:141–9. doi: 10.1016/j.plantsci.2003.08.018. [DOI] [Google Scholar]
- 38.Sakamoto A. Alia, Murata N. Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol Biol. 1998;38:1011–9. doi: 10.1023/A:1006095015717. [DOI] [PubMed] [Google Scholar]
- 39.Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, et al. Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor Appl Genet. 2002;106:51–7. doi: 10.1007/s00122-002-1063-5. [DOI] [PubMed] [Google Scholar]
- 40.Su J, Hirji R, Zhang L, He C, Selvaraj G, Wu R. Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stress-protectant glycine betaine. J Exp Bot. 2006;57:1129–35. doi: 10.1093/jxb/erj133. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad R, Kim MD, Back KH, Kim HS, Lee HS, Kwon SY, et al. Stress-induced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. Plant Cell Rep. 2008;27:687–98. doi: 10.1007/s00299-007-0479-4. [DOI] [PubMed] [Google Scholar]
- 42.Wang GP, Li F, Zhang J, Zhao MR, Hui Z, Wang W. Over accumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica. 2010;48:30–41. doi: 10.1007/s11099-010-0006-7. [DOI] [Google Scholar]
- 43.Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson AD. The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J. 1998;16:487–96. doi: 10.1046/j.1365-313x.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 44.Peel GJ, Mickelbart MV, Rhodes D. Choline metabolism in glycinebetaine accumulating and non-accumulating near-isogenic lines of Zea mays and Sorghum bicolor. Phytochemistry. 2010;71:404–14. doi: 10.1016/j.phytochem.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Iordachescu M, Imai R. Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol. 2008;50:1223–9. doi: 10.1111/j.1744-7909.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 46.Park EJ, Jeknic Z, Pino MT, Murata N, Chen TH. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007;30:994–1005. doi: 10.1111/j.1365-3040.2007.01694.x. [DOI] [PubMed] [Google Scholar]
- 47.Park EJ, Jeknic Z, Chen TH, Murata N. The codA transgene for glycinebetaine synthesis increases the size of flowers and fruits in tomato. Plant Biotechnol J. 2007;5:422–30. doi: 10.1111/j.1467-7652.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto A, Murata N. The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 2002;25:163–71. doi: 10.1046/j.0016-8025.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 49.Diamant S, Eliahu N, Rosenthal D, Goloubinoff P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem. 2001;276:39586–91. doi: 10.1074/jbc.M103081200. [DOI] [PubMed] [Google Scholar]
- 50.Diamant S, Rosenthal D, Azem A, Eliahu N, Ben-Zvi AP, Goloubinoff P. Dicarboxylic amino acids and glycine-betaine regulate chaperone-mediated protein-disaggregation under stress. Mol Microbiol. 2003;49:401–10. doi: 10.1046/j.1365-2958.2003.03553.x. [DOI] [PubMed] [Google Scholar]
- 51.Al-Taweel K, Iwaki T, Yabuta Y, Shigeoka S, Murata N, Wadano A. A bacterial transgene for catalase protects translation of D1 protein during exposure of salt-stressed tobacco leaves to strong light. Plant Physiol. 2007;145:258–65. doi: 10.1104/pp.107.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allakhverdiev SI, Murata N. Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth Res. 2008;98:529–39. doi: 10.1007/s11120-008-9334-x. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13:178–82. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochem Biophys Acta 2007; 1767:414-421. [DOI] [PubMed]
- 55.Kathuria H, Giri J, Nataraja KN, Murata N, Udayakumar M, Tyagi AK. Glycinebetaine-induced water-stress tolerance in codA-expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotechnol J. 2009;7:512–26. doi: 10.1111/j.1467-7652.2009.00420.x. [DOI] [PubMed] [Google Scholar]
- 56.Rajendrakumar CS, Suryanarayana T, Reddy AR. DNA helix destabilization by proline and betaine: possible role in the salinity tolerance process. FEBS Lett. 1997;410:201–5. doi: 10.1016/S0014-5793(97)00588-7. [DOI] [PubMed] [Google Scholar]
- 57.Alia K, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen TH, et al. Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol. 1999;40:279–88. doi: 10.1023/A:1006121821883. [DOI] [PubMed] [Google Scholar]
- 58.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 59.Kachroo A, He Z, Patkar R, Zhu Q, Zhong J, Li D, et al. Induction of H2O2 in transgenic rice leads to cell death and enhanced resistance to both bacterial and fungal pathogens. Transgenic Res. 2003;12:577–86. doi: 10.1023/A:1025896513472. [DOI] [PubMed] [Google Scholar]
- 60.Alcázar R, Garcia-Martinez JL, Cuevas JC, Tiburcio AF, Altabella T. Overexpression of ADC2 in Arabidopsis induces dwarfism and late-flowering through GA deficiency. Plant J. 2005;43:425–36. doi: 10.1111/j.1365-313X.2005.02465.x. [DOI] [PubMed] [Google Scholar]
- 61.Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004;45:712–22. doi: 10.1093/pcp/pch083. [DOI] [PubMed] [Google Scholar]
- 62.Nanjo T, Fujita M, Seki M, Kato T, Tabata S, Shinozaki K. Toxicity of free proline revealed in an Arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase. Plant Cell Physiol. 2003;44:541–8. doi: 10.1093/pcp/pcg066. [DOI] [PubMed] [Google Scholar]
- 63.Allard F, Houde M, Krol M, Ivanov A, Huner NPA, Sarhan F. Betaine improves freezing tolerance in wheat. Plant Cell Physiol. 1998;39:1194–202. [Google Scholar]
- 64.Einset J, Nielsen E, Connolly EL, Bones A, Sparstad T, Winge P, et al. Membrane-trafficking RabA4c involved in the effect of glycine betaine on recovery from chilling stress in Arabidopsis. Physiol Plant. 2007;130:511–8. doi: 10.1111/j.1399-3054.2007.00920.x. [DOI] [Google Scholar]
- 65.Vij S, Tyagi AK. Emerging trends in the functional genomics of the abiotic stress response in crop plants. Plant Biotechnol J. 2007;5:361–80. doi: 10.1111/j.1467-7652.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 66.Miller G, Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot. 2006;98:279–88. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lv S, Yang A, Zhang K, Wang L, Zhang J. Increase of glycinebetaine synthesis improves drought tolerance in cotton. Mol Breed. 2007;20:233–48. doi: 10.1007/s11032-007-9086-x. [DOI] [Google Scholar]
- 68.Quan R, Shang M, Zhang H, Zhao Y, Zhang J. Engineering of enhanced glycinebetaine synthesis improves drought tolerance in maize. Plant Biotechnol J. 2004;2:477–86. doi: 10.1111/j.1467-7652.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- 69.Yang X, Liang Z, Lu C. Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol. 2005;138:2299–309. doi: 10.1104/pp.105.063164. [DOI] [PMC free article] [PubMed] [Google Scholar]