Abstract
We review recent results about the functioning of aquatic carnivorous traps from the genus Utricularia. The use of high speed cameras has helped to elucidate the mechanism at the origin of the ultra fast capture process of Utricularia, at a millisecond time scale. As water is pumped out of the trap, pressure decreases inside the trap and elastic energy is stored due to the change of shape of the trap body. This energy is suddenly released when the trap is fired: the trap door undergoes an elastical instability: buckling, which allows its fast and passive opening and closure. This mechanism is used by Utricularia both to catch preys touching its trigger hairs and to fire spontaneously at regular time intervals. The results leading to this interpretation are reviewed and discussed and suggestions for further work are briefly presented.
Keywords: aquatic carnivorous plant, biophysics, bladderwort, continuous pumping, fast plant motion, functioning, instability, mechanics, resetting, spontaneous firings
Among the carnivorous plants, several strategies exist to catch animal preys: either passive as for Nepenthes, either active as for Dionea. To take preys by surprise, active traps have to be quick. Dionea closes its leaves in about a tenth of a second, but it is not the fastest: as was showed recently, aquatic species from the genus Utricularia have traps that are able to suck preys in less than one millisecond.1,2 Recorded speeds reach around 1 m/s, with accelerations of a few hundreds of g. To understand what is at the root of such a fast suction, physicists and biologists recently studied traps of different aquatic species of Utricularia with a fast camera up to 15.000 frames per second.1,2 The evolution of traps over long periods using a time lapse technique also brought to light the interesting behavior of spontaneous firings. This short review is aimed at explaining the current views on the biophysical aspects of the functioning of Utricularia traps explaining these different characteristics, complementing the very recent review by L. Adamec3 focusing on the biological and ecophysiological aspects of this fascinating plant. Short explicative movies by the authors of the present review are also available at www.youtube.com/user/PMarmottant under the titles “Hungry carnivorous plants” and “The ultra-fast trap of an aquatic carnivorous plant.”
Trap setting: the deflation process
The body of an Utricularia trap (Fig. 1) is a millimeter sized bladder made of two layers of cells, and is closed by a door articulated around hinges. Specific glands pump water out of the trap, decreasing its volume.4,5 Due to the geometry of the trap body, this transformation is smooth and continuous,2,6 and entails a progressive lowering of the inner pressure. Since the fluid pressure inside the trap (pint) is lower than in the surrounding water (pext), it is often referred as “negative pressure,” even if pint remains positive. In the following, we define the “pressure difference” ∆p = pext − pint. In set conditions, the trap is deflated and has a positive ∆p (Fig. 2a-b). The value of ∆p has been measured by intrusive methods, using a glass capillary containing a gas bubble directly inserted in the trap.7,8 Typical values were close to ∆p ≈ 15 kPa. However, nobody was able to reproduce these experiments since, due to leaks introduced by the insertion of the capillary. As discussed in ref. 9, these leaks can be significant and lead to underestimate the value of ∆p. The evolution of pressure in time can also be inferred from measurements on trap thickness: the higher the value of ∆p, the more deflated the trap is. Different methods to access the trap thickness were used: position sensors,8,10 image analysis,9 or tracer dilution for the trap volume.7 All show that the deflation rate decreases with time. After typically one to several hours, the deflation saturates or varies only very slowly.2,9,10 Following the most recent ideas (often referred to as “continuous pumping” and “water recirculation”), water is still probably continuously pumped out of the trap during this steady-state, but the outwards flow is compensated by an inwards flow either due to trap wall permeability or to the fact that the trap door is not closed perfectly watertight.6,9 As stressed in ref. 3, there is a lack of experimental data to firmly validate this hypothesis. Also, the pumping rate could be affected by the change of pressure inside the trap.
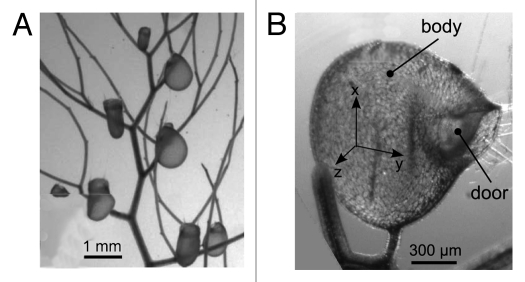
Figure 1.
(A) View of a composed leaf of Utricularia inflata with eight visible traps. (B) A single trap of Utricularia inflata. The trigger hairs are situated on the door but are not visible on this picture.
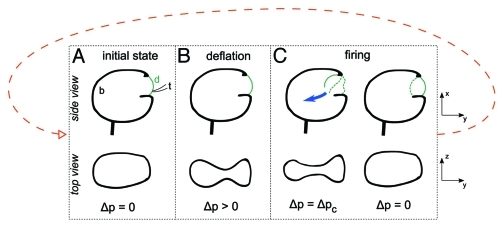
Figure 2.
Simplified scheme of the different stages of trap setting and firing. In the initial state (A), the trap is fully inflated and no pressure difference exist between the interior and exterior of the trap body. Pumping of water deflates the trap (mainly in the z direction) and creates a pressure difference ∆p (B). In the buckling scenario, when ∆p exceeds a critical value ∆pc , the trap door undergoes a buckling instability enabling its opening and closure (C). Dashed lines indicate earlier positions of the door. As soon as the door is closed, the cycle starts again. On the first panel, the trap body (B), door (d) and trigger hairs (t) are indicated. For clarity, the trigger hairs are not drawn on the other panels.
Trap firing: a mechanical instability?
When the trap is set, any slight touch on one of the four trigger hairs inserted on the door fires the trap: recent studies using fast cameras showed that the door opens in less than 1 ms.2 The close-by liquid is sucked in as the trap body inflates and the pressure difference ∆p goes back to zero. The opening of the door is associated with an inversion of its curvature (outwards to inwards, see left part of Figure 2C), which was previously thought to be a simple consequence of the aspiration.5,11 However, high speed recordings of the opening process show that this curvature inversion occurs prior to anything else,2 suggesting that it is the key of the trap functioning.
In physics, fast curvature inversions are well-known in a process called buckling: a curved membrane is able to withstand a pressure difference ∆p without changing much until a critical value ∆pc at which the curvature suddenly changes sign.12 Numerical simulations showed that this process was compatible with the opening of the door of Utricularia, using realistic geometrical and elastical parameters.2,6 This reminds of the closure mechanism of Dionea which was shown to be based on the same elastical instability.13 However, in that case, the driving force for buckling was an active change of the geometry of the wall cells. For Utricularia, no need for such an energy consuming process: due to the inwards pressure force, the door is already metastable and any tiny perturbation is able to trigger the curvature inversion. This process also allows a fast and passive closing of the door: as soon as the trap has inflated, no more pressure force acts on the door wall, and its inwards curvature becomes unstable: it thus spontaneously goes back to its outwards curvature, closing the door again (right part of Figure 2C).
The origin of the perturbation leading to door buckling is the prey action on the trigger hairs. Do they act as a cantilever, creating a small deformation on the door sufficient to trigger buckling? Another explanation cannot be excluded: as for Dionea, touching a hair could trigger a biochemical reaction. In the case of Utricularia, it has to be assumed that turgor pressure is lowered by this reaction. Then, the door loses its rigidity, lowering the critical buckling pressure ∆pc. The latter thus goes below the actual pressure difference ∆p, triggering buckling. In both cases, ∆p has to be very close to the critical value for buckling ∆pc, ensuring that the trap is always triggered, but also that the response is as fast as possible. Moreover, the deformation of the trap body due to the underpressure ∆p has to be important, so that the volume of fluid sucked in during aspiration is maximum. The geometries and elasticities of both the trap door and the trap body are indeed optimized to achieve these functions.2,9
Spontaneous firings
It is only recently that an unexpected behavior of Utricularia traps was reported: traps don’t only fire when triggered by an animal prey but also fire spontaneously, without any apparent cause.1,2 A systematic study of these spontaneous firings on Utricularia inflata and Utricularia australis revealed that they follow characteristic temporal patterns and are capable of going on for weeks on a single trap.9 During this period of observation, some traps fired more than two hundred times. On a single excised leaf, many traps fire with a well-defined frequency, of the order of 0.2 to 5 firings per hour, with fluctuations of variable importance (Fig. 3A and3B). As the authors showed, these spontaneous firings and their characteristics can be explained using the hypothesis of trap door buckling. Briefly, if a trap door has a value of the critical pressure for buckling ∆pc low enough so that it can actually be reached during the deflation phase (where the pressure difference ∆p increases), the door then spontaneously inverts its curvature and opens when ∆p = ∆pc: the trap spontaneously fires and the pressure difference is reset to zero. Deflation starts again due to continuous pumping and ∆pc will be reached again after some time, giving rise to spontaneous firings regularly spaced in time. The trap accomplishes “mechanical oscillations,” running through all the steps of Figure 2 continuously.
Figure 3.
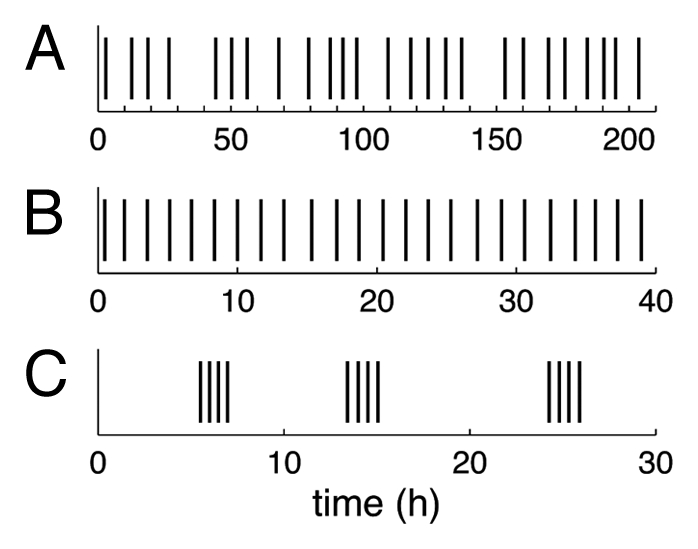
Spontaneous firings recorded on different Utricularia traps. Vertical bars mark the times at which a spontaneous firings occur. Traps (A) and (B) show regular firings with more fluctuations for the first trap, the second having a very well defined firing frequency. Trap (C) fires by groups of four firings (“bursts”) separated by a variable amount of time. Data taken from reference 9.
The existence of spontaneous firings is thus a strong evidence of the buckling hypothesis, but it does not mean that the functioning of Utricularia traps is purely mechanical. In fact, some traps present spontaneous firings organized in bursts9 (see Figure 3C), which could be the sign of a biochemical response: even if the opening of the door is spontaneous, the simple motion of water during aspiration is able to act on the hairs via fluid friction. The duration of the burst would then be linked to the duration of the induced response.9
Interestingly, most of the observed traps presented spontaneous firings, suggesting that this is not a malfunctioning. On the contrary, such regular water aspirations could help diversify the nutrients intake, by capturing detritus and phytoplankton for example. Several studies14-16 show the presence of such organisms in traps and discuss their role. See also the more detailed review in ref. 3.
Conclusions and perspectives
Utricularia species seem to have developed very well designed mechanical traps using a subtle balance between a passive physical instability and an active response to detect preys. This mechanism, where energy is slowly stored in the elastic trap walls due to continuous pumping and swiftly released when the trap is fired, enables multiple openings and closures without any morphological change, contrary to Dionea where tissue growth is required to reset the trap, leading it to stop working after a few resettings. This could be one reason of the success of this genus, represented worldwide with more than 200 species.17
Work remains to be done to fully understand these processes. No recent study was able to directly access the actual inner pressure of the trap though it is the principal ingredient of its functioning. Resolving this challenge would allow to get statistics of the underpressure in the trap when it spontaneously fires and to confront directly the model developed in reference 9. Also, if there is evidence of a biochemical response of the trigger hairs, it has never been clearly detected and quantified, which would probably help to understand the burst phenomenon. Other questions remain, and the interested reader should also consult the “inspirations for further research” listed in reference 3.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17804
References
- 1.Marmottant P, Vincent O, Quilliet C. Study of the ultrafast trap of an aquatic carnivorous plant. Book of Abstracts, Plant Biomechanics Conference 2009. [Google Scholar]
- 2.Vincent O, Weißkopf C, Poppinga S, Masselter T, Speck T, Joyeux M, et al. Ultra-fast underwater suction traps. Proc Biol Sci. 2011;278:2909–14. doi: 10.1098/rspb.2010.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamec L. The smallest but fastest: Ecophysiological characteristics of traps of aquatic carnivorous Utricularia. Plant Signal Behav. 2011;6:640–6. doi: 10.4161/psb.6.5.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd FE. The carnivorous plants. Chronica Botanica Company, 1942. [Google Scholar]
- 5.Juniper BE, Robins RJ, Joel DM. The carnivorous plants. Academic Press, 1989. [Google Scholar]
- 6.Joyeux M, Vincent O, Marmottant P. Mechanical model of the ultra-fast underwater trap of Utricularia. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;83:021911. doi: 10.1103/PhysRevE.83.021911. [DOI] [PubMed] [Google Scholar]
- 7.Sydenham PH, Findlay GP. The rapid movement of the bladder of Utricularia Sp. Aust J Biol Sci. 1973;26:1115–26. [Google Scholar]
- 8.Sasago A, Sibaoka T. Water Extrusion in the Trap Bladders of Utricularia vulgaris I. A Possible Pathway of Water across the Bladder Wall. Bot Mat Tokyo. 1985;98:55–66. doi: 10.1007/BF02488906. [DOI] [Google Scholar]
- 9.Vincent O, Roditchev I, Marmottant P. Spontaneous Firings of Carnivorous Aquatic Utricularia Traps: Temporal Patterns and Mechanical Oscillations. PLoS ONE. 2011;6:e20205. doi: 10.1371/journal.pone.0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamec L. The comparison of mechanically stimulated and spontaneous firings in traps of aquatic carnivorous Utricularia species. Aquat Bot. 2011;94:44–9. doi: 10.1016/j.aquabot.2010.09.004. [DOI] [Google Scholar]
- 11.Lloyd FE. The mechanism of the water tight door of the Utricularia trap. Plant Physiol. 1929;4:87–102. doi: 10.1104/pp.4.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landau LD, Lifshitz EM. Theory of Elasticity. Elsevier, 1986. [Google Scholar]
- 13.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–5. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 14.Gordon E, Pacheco S. Prey composition in the carnivorous plants Utricularia inflata and U. gibba (Lentibulariaceae) from Paria Peninsula, Venezuela. Rev Biol Trop. 2007;55:795–803. doi: 10.15517/rbt.v55i3-4.5956. [DOI] [PubMed] [Google Scholar]
- 15.Alkhalaf IA, Hübener T, Porembski S. Prey spectra of aquatic Utricularia species (Lentibulariaceae) in northeastern Germany: The role of planktonic algae. Flora. 2009;204:700–8. [Google Scholar]
- 16.Sirová D, Borovec J, Černá B, Rejmánková E, Adamec L, Vrba J. Microbial community development in the traps of aquatic Utricularia species. Aquat Bot. 2009;90:129–36. doi: 10.1016/j.aquabot.2008.07.007. [DOI] [Google Scholar]
- 17.Taylor P. The genus Utricularia: a taxonomic monograph. Kew: Royal Botanic Gardens, 1989. [Google Scholar]