Abstract
Cyst nematodes establish and maintain feeding sites (syncytia) in the roots of host plants by altering expression of host genes. Among these genes are members of the large gene family of class III peroxidases, which have reported functions in a variety of biological processes. In this study, we used Arabidopsis-Heterodera schachtii as a model system to functionally characterize peroxidase 53 (AtPRX53). Promoter assays showed that under non-infected conditions AtPRX53 is expressed mainly in the root, the hypocotyl and the base of the pistil. Under infected conditions, the AtPRX53 promoter showed upregulation at the nematode penetration sites and in their migration paths. Interestingly, strong GUS activity was observed in H. schachtii-induced syncytia during the early stage of infection and remained strong in the syncytia of third-stage juveniles. Also, AtPRX53 showed upregulation in response to wounding and jasmonic acid treatments. Manipulation of AtPRX53 expression through overexpression and knockout mutation affected both plant morphology and nematode susceptibility. While AtPRX53 overexpression lines exhibited short hypocotyls, aberrant flower development and reduced nematode susceptibility to H. schachtii, the atprx53 mutant showed long hypocotyls and a 3-carpel silique phenotype as well as a non significant increase of nematode susceptibility. Taken together these data, therefore, indicate diverse roles of AtPRX53 in the wound response, flower development and syncytium formation.
Keywords: Arabidopsis; class III peroxidase; cyst nematode,; GUS; qPCR
Introduction
Cyst nematodes are sedentary obligate biotrophic plant parasites that cause severe yield loss to crops worldwide.1 Infective second-stage juveniles (J2) penetrate roots and then migrate intracellularly in search of a suitable location to initiate a feeding site, called a syncytium. The formation of the syncytium is a tightly controlled process involving both the parasite and the host plant. While nematode effector proteins, which are produced in the esophageal gland cells and secreted into host plant cells through the nematode stylet, are critical for the establishment of the feeding sites and nematode parasitism,2-6 host factors also are pivotal in this process.7 Genome-wide expression profiling of the plant response to cyst nematodes resulted in the identification of genes that are regulated by cyst nematode infection.8-12 Despite considerable progress in the identification of host genes responsive to cyst nematode infection, little is known about the functional roles of most of these genes during parasitism. Examination of plant genes differentially expressed in response to cyst nematode infection revealed that members of the peroxidase gene family frequently are among the highly responsive genes,8,12 which suggested important roles during parasitism.
The peroxidase superfamily is divided into three distantly related structural classes.13 class I encompasses intracellular proteins and can be found in most living organisms but not in animals. These peroxidases function mainly in detoxifying excess H2O2.14 class II peroxidases are found exclusively in fungi and mostly act in degrading soil debris.15 class III peroxidases are found in land plants and contain N-terminal signal peptides for secretion to the cell wall or vacuoles.16-18 Genes coding for class III peroxidases have been duplicated extensively during evolution and thus form large gene families in all land plant species studied.16 Some of these enzymes have been shown to be involved in a wide range of physiological and developmental processes, which include cross-linking of cell wall components during cell wall formation and modification,19,20 lignification,21,22 suberization23 and auxin catabolism.24 Accumulated experimental evidence also implicates class III peroxidases in plant defense responses to pathogen/pest attacks, such as bacteria,25-27 fungi,28 viruses,29 insects30 and cyst nematodes.31 Two major functional roles have been attributed to class III peroxidases in response to pathogen attack. The first role is the cross-linking of cell wall components, which strengthens cell walls to impede pathogen invasion.32,33 A second role lies in the ability to generate reactive oxygen species (ROS) that produce adverse circumstances for pathogen survival26,34 and/or trigger downstream signaling pathways to activate additional defense mechanisms.35,36
The Arabidopsis (Arabidopsis thaliana) genome contains 73 genes predicted to encode class III peroxidases, of which only few have been functionally characterized.17,35,37 A further understanding of peroxidase functions, thus, is of prime interest. One member of the Arabidopsis class III peroxidase gene family, AtPRX53, previouly has been shown to be upregulated by Heterodera schachti infection.8,12 In the current study, we addressed the potential role of AtPRX53 during plant-nematode interactions. Using promoter activity assays we showed that AtPRX53 is strongly upregulated in H. schachtii-induced feeding sites during early infection. Gain- and loss-of-function mutations showed effects on plant morphology as well as on susceptibility to H. schachtii. Analysis of all data presented here suggests that AtPRX53 most likely is involved in cross-linking of cell wall compounds during H. schachtii-induced syncytium growth.
Results
AtPRX53 is upregulated in response to Heterodera schachtii infection, wounding and jasmonic acid treatment
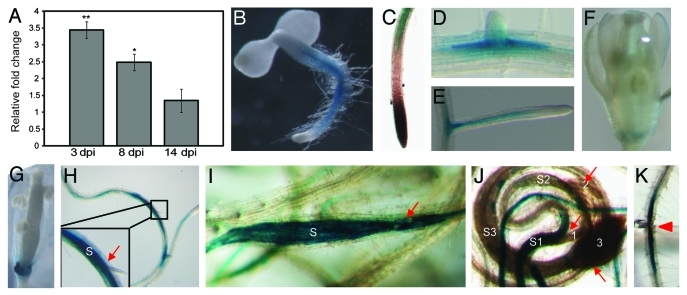
To begin investigating the function of AtPRX53 in the plant response to cyst nematode infection, we used quantitative real-time RT-PCR (qPCR) assays to assess the mRNA expression profile using gene-specific primers designed to discriminate between different members of the peroxidase gene family. Ten-day-old wild-type Arabidopsis seedlings (Col-0) were inoculated with H. schachtii, and root tissues were harvested from both infected and non-infected plants at 3, 8, and 14 d post inoculation (dpi) for RNA extraction. Data from three independent experiments showed significant upregulation of AtPRX53 in H. schachtii-infected roots at 3 and 8 dpi relative to non-infected roots (Fig. 1A), thus, confirming the 3 dpi observation of Puthoff et al.8 At 14 dpi, AtPRX53 mRNA abundance was decreased to non-infected control levels (Fig. 1A).
Figure 1.
Expression analysis of AtPRX53 in response to nematode infection. (A) Upregulation of AtPRX53 mRNA in wild-type Arabidopsis (Col-0) roots in response to Heterodera schachtii infection relative to the non-infected control. The expression levels of AtPRX53 were quantified by quantitative real-time RT-PCR (qPCR) in root tissues of wild-type plants. Infected and non-infected roots were collected at 3, 8 and 14 d post inoculation (dpi). Data are averages of three biologically independent experiments. Actin8 was used as internal control to normalize mRNA expression levels. Mean values significantly different from the non-infected control were determined by unadjusted paired t-tests. Asterisks indicate mean fold-changes significantly different from those of non-infected wild-type roots (p < 0.05). (B–K) Histochemical localization of GUS activity in transgenic Arabidopsis plants harboring the pAtPRX53:GUS construct. Three independent homozygous T3 lines expressing the pAtPRX53:GUS construct were generated, and GUS activity was histochemically analyzed in both non-infected and infected plants. (B) through (G), GUS staining in non-infected plants. Whole mount (B), close-up of main root tip (C), emerging lateral root (D and E), flower (F) and developing silique (G). (H) through (J), GUS staining in plants infected with Heterodera schachtii. Arrows point to nematodes and S indicates syncytium. Three days post inoculation (dpi) (H), 8 dpi (I) and 14 dpi (J), J3 nematode (1) with syncytium (S1); male (2) with syncytium (S2); mature female (3) with syncytium (S3). (K) GUS staining in root tissue wounded by stabbing with a needle. Arrowhead points to the site of wounding.
In order to identify where the AtPRX53 gene was expressed in the developing plant and infected roots, we generated transgenic Arabidopsis lines expressing the β-glucuronidase (GUS) reporter gene under the control of the AtPRX53 promoter. Three independent transgenic lines were analyzed and showed similar results. Under non-infected conditions, GUS activity was detected in the vascular tissues of the primary root but not in the meristem or the elongation zone of 3-d-old plants (Fig. 1B and 1C). GUS staining was also observed in the lateral roots 10-d-old plants (Fig. 1D and 1E). In shoots of non-infected plants, GUS activity was discovered in the hypocotyl (Fig. 1B), the base of the pistil (Fig. 1F), and the internode of the silique (Fig. 1G). Under infected conditions, the AtPRX53 promoter showed activity at the site of root penetration of nematodes and in their migration paths (data not shown). Strong GUS activity was detectable in H. schachtii-induced syncytia as early as 3 dpi (Fig. 1H), which was sustained also at 8 dpi (Fig. 1I). At 14 dpi, GUS activity remained strong in the syncytia of third-stage juveniles, but only weak or no GUS staining could be detected in the syncytia of fourth-stage females or males (Fig. 1J). These data implicated AtPRX53 in playing a role in the wound response and syncytium initiation and development.
Because we observed GUS activity at the sites of nematode penetration and migration, we tested whether the expression of AtPRX53 can also be activated in response to wounding alone. Roots of pAtPRX53:GUS seedlings were wounded, and 3 d post treatment GUS activity was histochemically assayed. GUS expression increased around wounding sites (Fig. 1K), which suggested that activation of AtPRX53 is part of the plant wound response associated with the early infection process.
Since jasmonic acid (JA) is well known as an essential signal in wound-induced gene expression, we assessed whether the AtPRX53 promoter contains JA-responsive cis-elements by scanning the AtPRX53 promoter region using the PLACE (Plant cis-acting elements) database.38 Two JA-responsive cis elements39 were identified (Table 1), potentially explaining the wound-responsiveness of the AtPRX53 promoter. We further investigated the mRNA expression level of AtPRX53 in response to JA treatment. In this experiment, 10-d-old Arabidopsis wild-type seedlings were treated with 100 µM methyl jasmonic acid (MeJA), and after 48 h, root tissues were collected for RNA extraction and qPCR analyses. Data obtained from three biological samples revealed a statistically significant 35-fold upregulation of AtPRX53 in MeJA-treated roots compared with the non-treated control. Also, our analysis revealed that the AtPRX53 promoter contains other stress-responsive elements including ethylene-40,41 and ABA-42 responsive cis-elements as well as two nematode boxes described by Escobar et al.43 (Table 1). Though these regulatory cis-elements are potentially interesting, their functionality was not pursued further in this study.
Table 1. Putative regulatory cis-elements present in the 986 bp promoter region upstream of the AtPRX53 translation start codon.
| Motif | Sequence | Annotation | Location (strand) | |
|---|---|---|---|---|
| ABRE |
ACGTG |
Abscisic acid response-like element |
454, 792 (-) |
|
| ELRECOREPCRP1 |
TTGACC |
Salicylic acid and elicitor-responsive element |
591 |
|
| ERELEE4 |
ATTTCAAA |
Ethylene-responsive element |
818 |
|
| MYB |
WAACCA |
Drought and abscisic acid-responsive element |
247 (-) |
|
| NEMATODE-box |
CAATTG |
Nematode-responsive box |
401, 685 |
|
| T/GBOXATPIN2 |
AACGTG |
Jasmonic acid-responsive element |
453, 792 (-) |
|
| W-box |
TTGAC |
Salicylic acid-responsive element |
591, 135 (-), 846 (-) |
|
| WRKY710S | TGAC | Defense-responsive element | 333, 399, 592, 135 (-), 846 (-), 915 (-) | |
Putative regulatory cis-elements were identified by the PLACE database.38 Location denotes the position of the first input nucleotide from the 5′ end of the promoter fragment. (-) denotes the antisense strand.
Morphology and nematode susceptibility as a function of altered AtPRX53 expression
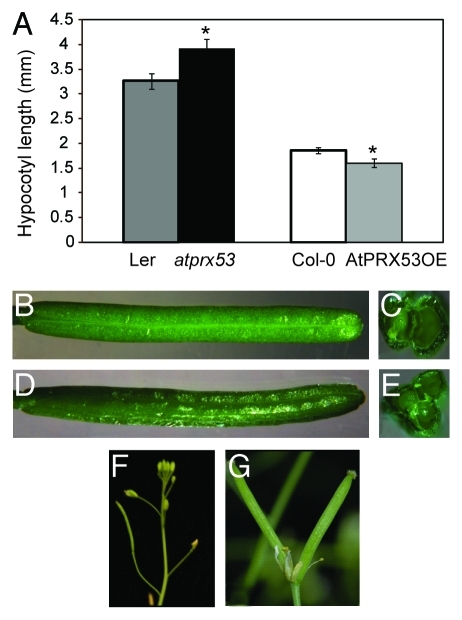
To study the phenotypic effects of altered AtPRX53 expression, we obtained a Ds transposon insertional mutant line (atprx53) from the Cold Spring Harbor Laboratory mutant collection.44,45 Sequence analysis revealed that the Ds transposon was inserted into the third exon (Fig. 2A). The mRNA expression level of AtPRX53 was quantified in wild-type plants (Ler) and the homozygous mutant line using qPCR. No AtPRX53 mRNA was detected after 40 cycles of amplification of cDNA from homozygous mutant plants (Fig. 2B). Morphological analyses of atprx53 mutant revealed a long hypocotyl phenotype relative to wild-type plants (Fig. 3A). We also discovered at least one 3-carpel silique in 68% of the atprx53 mutant plants assayed (Fig. 3B–E) while this phenotype was entirely absent from the wild type (Ler). In order to
Figure 2.
Characterization of the Arabidopsis atprx53 mutant. (A) Schematic representation of the atprx53 mutant allele used in this study showing the location of Ds transposon insertion. Black boxes indicate exons and lines between black boxes indicate introns. AtPRX53 encompasses four exons and three introns and the Ds transposon is located at the third exon, 550 bp downstream of the translation start codon. The translation start codon (ATG) and stop codon (TGA) are indicated. (B) AtPRX53 mRNA accumulation in the atprx53 mutant. AtPRX53 mRNA abundance was determined by qPCR using gene-specific primers. The PCR products were resolved on a syber safe-stained 2% agarose gel. No PCR products were detected after 40 cycles of amplification of cDNA from homozygous atprx53 mutants, whereas specific amplifications were detected after amplification of cDNA from wild-type plants (Ler). Arabidopsis Actin8 was used an internal positive control.
Figure 3.
Phenotype of Arabidopsis lines with altered AtPRX53 expression. (A) Hypocotyl length of the atprx53 mutant and a representative transgenic Arabidopsis AtPRX53 overexpression line (AtPRX53OE). Homozygous atprx53 mutant and T3 homozygous AtPRX53OE exhibited longer and shorter hypocotyls, respectively, than the corresponding wild type. Data are presented as means of 30 plants ± the standard error (SE). Asterisks indicate mean values significantly different from the wild type (p < 0.05). (B–G) Flower and silique phenotypes. Wild-type (Ler) silique longitudinal view (B) and cross section (C) with two carpels. atprx53 mutant silique longitudinal view (D) and cross section (E) with three carpels. Single wild-type (Col-0) silique (F) and AtPRX53OE double siliques (G).
scrutinize this phenotype, we overexpressed the AtPRX53 coding sequence in the atprx53 mutant. The 3-carpel silique phenotype was partially rescued in that only 14% of the transgenic T1 plants exhibited this alteration, thereby validating this silique phenotype as a consequence of the atprx53 mutant allele despite its low penetrance.
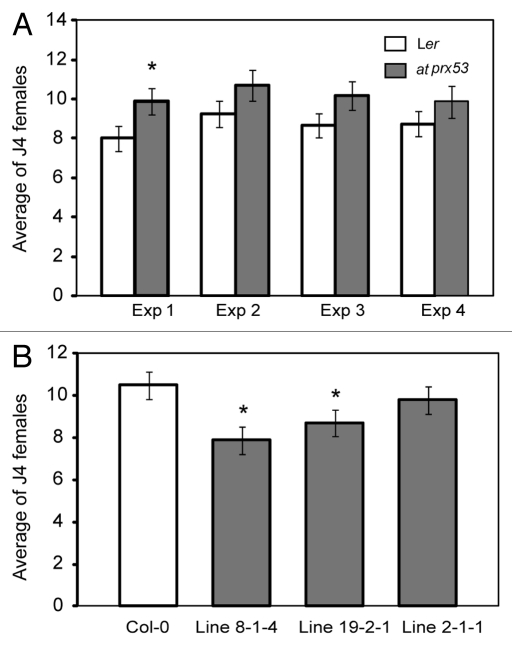
In order to evaluate the influence of AtPRX53 on nematode susceptibility, the atprx53 mutant along with the wild type were subjected to cyst nematode susceptibility assays. Ten-day-old plants were inoculated with H. schachtii J2 nematodes and the number of J4 females was determined three weeks after inoculation and used to quantify plant susceptibility. As shown in Figure 4A, a trend of increased susceptibility of the atprx53 mutant compared with the wild type was detected, although this increase was statistically non-significant. Because we found that reduced AtPRX53 expression can affect both plant morphology and nematode susceptibility, it was particularly interesting to see whether elevated AtPRX53 expression would produce opposite phenotypes. To investigate this, we generated transgenic lines overexpressing the AtPRX53 coding sequence in the Col-0 background under the control of the CaMV 35S promoter. Three independent homozygous T3 lines with confirmed AtPRX53 mRNA abundance increases were phenotypically investigated. In contrast to the atprx53 mutant, AtPRX53 overexpression lines exhibited significantly shorter hypocotyl lengths than the wild type (Fig. 3A). As was conducted for the atprx53 mutant, we assayed AtPRX53-overexpressing T3 lines along with wild-type plants for susceptibility to H. schachtii in four independent experiments. Compared with the wild type, AtPRX53 overexpression plants exhibited reduced susceptibility to H. schachtii (Fig. 4B). We also discovered altered silique morphology. We reproducibly observed a double silique phenotype in AtPRX53 overexpression lines albeit at a low frequency, i.e., ~5% of the tested lines showed at least one double silique (Fig. 3F and G).
Figure 4.
Nematode susceptibility assays of the atprx53 mutant and three AtPRX53OE lines. (A) The atprx53 mutant showed a consistent trend of increased susceptibility to Heterodera schachtii in four experiments (Exp) with Exp 1 showing statistically significant effects. (B) Two out of three AtPRX53OE lines showed significantly reduced susceptibility to H. schachtii. Arabidopsis plants were planted into 12-well plates containing modified Knop’s medium, and ten-day-old plants were inoculated with ~200 surface-sterilized H. schachtii second-stage juveniles per plant. Three weeks after inoculation, the number of fourth-stage (J4) females per plant was determined. Mean values significantly different from the corresponding wild type were determined by modified t-test (p < 0.05).
AtPRX53 is not involved in lignification or ROS-mediated defense responses
As has been reported, AtPRX53 may function in lignifications.21 We, therefore, tested the lignin deposition in the roots of the AtPRX53 overexpression lines as compared with the wild type at 3, 8 and 14 dpi with H. schachtii using phloroglucinol staining, which produces a reddish color in lignified cells. No clear differences between the tested lines could be observed (Figure S1). After nematode infection, lignin staining was visible around the nematode head in two genotypes but never in or around the syncytium, i.e., the location where we had observed strong AtPRX53 promoter activity (Fig. 1H and I). We, therefore, conclude that AtPRX53 has no observable function in lignification during cyst nematode infection.
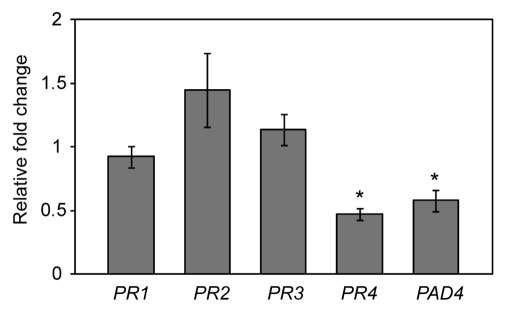
Peroxidases have been demonstrated to play a role in generating reactive oxygen species (ROS), which in turn function as signaling molecules in defense responses.35,46 If AtPRX53 had a function in ROS generation then this activity should be reflected in changes of mRNA abundances of pathogenesis-related (PR) genes. In particular, PR genes should be upregulated in AtPRX53 overexpression lines. To test this hypothesis, we quantified the expression level of seven PR genes (PR1, PR2, PR3, PR4, PR6, PDF1.2 and PAD4) in the roots of AtPRX53 overexpression lines and wild-type plants using qPCR. Data obtained from three independent experiments showed that the expression levels of PR1, PR2 and PR3 were similar to those in the wild type (Fig. 5), while PR6 and PDF1.2 were undetectable in roots. In contrast, PR4 and PAD4 showed significant downregulation in the roots of AtPRX53 overexpression plants compared with the wild-type plants. Absence of upregulation of PR genes in the tested AtPRX53 overexpression lines indicates that AtPRX53 does not function in ROS-mediated activation of defense response signaling in roots during cyst nematode parasitism. In addition, quantification of ROS in the AtPRX53 overexpression and wild-type plants using 3, 3′-diaminobenzidine (DAB) showed no significant differences (data not shown) supporting the above conclusion.
Figure 5.
Pathogenesis-related (PR) gene expression in a representative Arabidopsis line overexpressing AtPRX53 (AtPRX53OE). mRNA expression levels of PR genes were quantified by qPCR in root tissues of a representative AtPRX53OE line and wild-type plants 10 d after planting. Data are averages of three biologically independent experiments. Actin8 was used as internal control to normalize mRNA expression levels. Mean values significantly different from the wild type were determined by unadjusted paired t-tests. Asterisks indicate mean fold-changes significantly different from wild-type roots (p < 0.05).
Discussion
Secreted class III peroxidases represent a large plant protein family believed to be involved in a variety of physiological processes such as auxin catabolism, lignification, suberization, defense responses, and cell wall cross-linking.47 Functional characterization of individual isoforms is complicated because of genetic redundancies and low substrate specificities in vitro. In this study, we report on our work to determine the roles of AtPRX53 in plant development and cyst nematode parasitism.
Using promoter-GUS fusion assays, we demonstrated that the AtPRX53 promoter was active in the root vascular tissue, the sites of lateral root emergence as well as in the base of the flowers and maturing siliques. These spatial expression profiles are very similar to those obtained by Ostergaard et al.21 suggesting a specific function in these organs. Because our qPCR studies revealed a significant increase of AtPRX53 mRNA abundance early on during cyst nematode infection when penetration and migration through root tissues are associated with massive wounding of root tissues, we tested whether the expression of AtPRX53 can be induced by wounding. In this experiment, we showed that the AtPRX53 promoter in fact is wound-responsive. Wounding and pathogen responses share overlapping signaling pathways48-50 in which JA is a key factor.51 Therefore, we tested the AtPRX53 expression in response to JA application and found that AtPRX53 expression was highly upregulated after JA treatment. This result is consistent with the existence of two JA-responsive cis-elements in AtPRX53 promoter. In addition, AtPRX53 promoter contains two abscisic acid response-like elements (ABRE motif, Table 1). While we did not test the function of these cis elements, several pieces of evidence indicate that the expression of some peroxidase genes is controlled by abscisic acid.52,53 Whether nematodes-induce AtPRX53 expression is abscisic acid-dependent needs further investigation.
However, when assaying GUS activity following cyst nematode infection, we documented higher GUS activity not only at the site of penetration or the migration path, but rather in the developing syncytium at 3 and 8 d post H. schachtii inoculation. Once the syncytium had assumed its maximum size (14 dpi), promoter activity largely ceased, demonstrating a role for AtPRX53 only in the early stages of nematode infection when initiation and expansion of the syncytium are taking place. In other words, the role of AtPRX53 during cyst nematode infection most likely is part of a classical plant wound response but also is associated directly with the growing syncytium.
Interestingly, the atprx53 mutant showed a trend of non-significant increase of nematode susceptibility to cyst nematodes. In contrast, AtPRX53 overexpression lines exhibited a reduced nematode susceptibility phenotype. These data provide a convincing reason to claim an important role of AtPRX53 during plant-nematode interactions. Also, atprx53 mutant and AtPRX53 overexpression plants exhibited opposite morphological changes. While the atprx53 mutant showed long hypocotyls and three-carpel silique phenotypes, the AtPRX53 overexpression lines exhibited short hypocotyls as well as aberrant flower organ numbers in that two pistils originated from one flower. In agreement with Ostergaard et al.,21 we found that the AtPRX53 promoter was highly active in the base tissues of flowers corroborating our discovery of these morphological aberrations in the knockout and overexpression mutants. In other words, the three-carpel silique phenotype found in the atprx53 mutant is most likely associated with the absence of AtPRX53 activity, and the constitutive overexpression of AtPRX53 resulted in morphological changes in the same tissue, asserting a likely function of AtPRX53 in the formation of the Arabidopsis pistil.
AtPRX53, as an extracellular peroxidase, was first isolated from highly lignifying Arabidopsis cell suspension culture, suggesting a role of this gene in lignin biosynthesis.21,54 However, we did not detect any clear lignin deposition differences between Arabidopsis lines with increased AtPRX53 expression and the corresponding wild-type plants using phloroglucinol staining. Lignin deposition was only observed around the nematode head and to comparable degrees in all tested lines suggesting that AtPRX53 does not play a major role in lignification during the expansion of the nematode feeding site.
Another known function of peroxidases is generating ROS in SA-, JA- or ET-mediated defense response pathways.26 To test whether AtPRX53 functions in this manner, we quantified the expression of PR genes in AtPRX53 overexpression plants using qPCR. None of the PR genes showed upregulation in the transgenic plants overexpressing AtPRX53 relative to the wild type. In contrast, two PR genes, PR4 and PAD4, exhibited significant downregulated indicating that AtPRX53 likely is not involved in the generation of ROS and the activation of defense responses.
An additional possible role of AtPRX53 is mediating cell growth through several different mechanisms.55 First, peroxidases can activate cell elongation by generating ROS to cleave cell wall polymers, which loosens the cell wall, resulting in increased cell size.56-58 As a second possibility, peroxidases can induce cell growth also through IAA signaling pathways.46 I.e., Cosio et al.24 found that transgenic Arabidopsis constitutively expressing a zucchini peroxidase, which showed an auxin oxidase activity in vitro, exhibited longer hypocotyl lengths than wild-type plants. In both cases, increased peroxidase activity led to increased cell size. However, our data showed the exact opposite phenotypes of short hypocotyls in the AtPRX53 overexpression line and long hypocotyls in the mutant line, which argues against a role of AtPRX53 in mediating cell elongation through cell wall loosening or auxin catabolism.
A shortened or lengthened hypocotyl is a clear indicator of reduced or activated cell elongation, respectively, as no further cell divisions are taking place in this plant tissue.59 Our observation of reduced hypocotyl length in the AtPRX53 overexpression lines and increased length in the atprx53 mutant, therefore, suggested a strong negative effect of AtPRX53 on cell elongation. In fact, plant peroxidases have been reported to inhibit cell elongation by catalyzing cross linking of phenolic groups and proteins such as extensins20,60 or compounds like ferulic acid, monolignol and aliphatic components.19,61-63 The short and long hypocotyl phenotypes found in AtPRX53 overexpression and atprx53 mutant lines, respectively, provide strong support for the hypothesis that AtPRX53 could catalyze the cross linking of cell wall compounds to modulate, or potentially counteract, cell elongation. Such a role also makes sense with regard to the expression of AtPRX53 in the developing syncytium. After a competent cell is selected by the nematode, substantial cell wall changes and cell expansions occur as can be seen by the conspicuous swelling of Arabidopsis roots in the area of a syncytium. Cell wall cross linking during this phase appears to be a crucial component of controlled expansion of this novel plant organ. Once the syncytium is fully developed, the cell wall reorganization and extension discontinue and the expression of AtPRX53 is inhibited, which is what we observed in our studies where the AtPRX53 promoter was inactivated in the mature syncytium at 14 dpi. Consistent with this hypothesis, AtPRX53 is not expressed in the zone of root elongation but becomes active only in adjacent older root tissues, i.e., when cells cease growth and their cell walls need to harden.
Such a scenario could also explain the reduced susceptibility of AtPRX53 overexpression lines, which most likely is due to a reinforcement of cell walls, which could impede H. schachtii infection. The weak effect of AtPRX53 overexpression on nematode susceptibility could be due to the fact that early reinforcement of cell walls was not strong enough to inhibit H. schachtii invasion. The fact that the Arabidopsis genome contains a high number of genes coding for peroxidases with likely redundant functions could explain the only slight effect of the atprx53 mutant on nematode susceptibility. In this context, AtPrx54 which shares 73% amino acid identity with AtPRX53 in addition to several cis-regulatory elements64 showed a considerable upregulation (~5 fold) at 3 dpi with H. schachtii (data not shown). This common transcriptional regulation in response to H. schachtii infection reinforces a potential role of these peroxidases in cell wall cross linking.
Materials and methods
Plant materials and growth conditions
Arabidopsis [ecotype Columbia-0 (Col-0) and Landsberg erecta (Ler)] were used in this study. Seeds were surface-sterilized in 50% commercial bleach with 0.1% Tween20 for 5 min followed by five washes in sterile water. Plants were grown on Murashige and Skoog (MS) medium (PlantMedia, Catalog: 30630067-1) containing 2% sucrose solidified with 0.8% Phyto Agar (Research Products International Corp, Catalog: A20300-1000.0) or in Metro-Mix 200 soil mixture (Sun Gro Horticulture) in a growth chamber (16 h light/8 h dark) at 23°C.
Identification of atprx53 mutant
One Ds transposon insertional mutant (ET11708, atprx53) in the Ler background was obtained from the Cold Spring Harbor Laboratory mutant collection.44,45 Sequence analysis revealed that the Ds transposon element is inserted in exon 3,550 bp downstream of the translation start codon. The homozygosity of this mutant was verified using the primer pairs Forward/Ds3-4, Reverse/Ds5-4 and Foward/Reverse (Table S1) and the mRNA expression level was quantified by quantitative real-time RT-PCR (qPCR) using gene-specific primers for AtPRX53 (Table S1).
Plasmid construction and generation of transgenic Arabidopsis lines
For the AtPRX53 promoter:GUS (pAtPRX53:GUS) construct, a 986 bp fragment upstream of the translation start codon of the AtPRX53 gene was amplified from genomic DNA using primer pairs containing SalI and BamHI restriction sites in the forward and reverse primers, respectively (Table S1). PCR products were digested, gel purified and cloned into SalI/BamHI sites of the binary vector pBI101. For the overexpression construct, the coding sequence of AtPRX53 was amplified from Arabidopsis cDNA using gene-specific primers designed to create the BamHI and SacI restriction sites in AtPRX53 forward and reverse primers, respectively (Table S1). The amplified AtPRX53 fragment was then cloned into the respective sites in the binary vector pBI121. All constructs were confirmed by sequencing and introduced into Arabidopsis by Agrobacterium tumefaciens-mediated transformation following the floral dip method.65
Nematode susceptibility assay
Arabidopsis seeds of transgenic homozygous T3 lines, atprx53 mutant and wild-type controls (Col-0 or Ler) were surface sterilized and planted in a random block design in 12-well Falcon culture plates (BD Biosciences, Catalog: 353043) containing modified Knop's medium66 solidified with 0.8% Daishin agar (Brunschwig Chemie, Catalog: A20350-1000.0). Plants were grown at 26°C under 16 h light/8 h dark conditions. Ten-day-old seedlings were inoculated with ~200 surface-sterilized J2 Heterodera schachtii as previously described by Baum et al.67 Inoculated plants were maintained under the conditions described above. Three weeks post inoculation, the number of H. schachtii J4 females was counted using a dissecting microscope. Each plant line was replicated at least 15 times, and four independent experiments were performed. Average numbers of J4 females were calculated, and statistically significant differences between plant lines and the corresponding wild-type control were determined in a modified t-test using the statistical software package SAS.
Histochemical detection of GUS activity
The histochemical detection of GUS activity was performed with the substrate 5-bromo-4-chloro-3-indolylglucuronide (X-Gluc, Research Products International Corp, Catalog: B72200-1.0) according to Jefferson et al.68 At different time points post inoculation with H. schachtii, both infected and non-infected plants were stained with GUS solution at 37°C overnight. After GUS staining, plants were incubated in 70% ethanol to remove chlorophyll from the green tissues. Stained tissues were observed and photographed with a Zeiss Axiovert 100 microscope.
Wounding treatment
Wounding treatment was performed by repeatedly piercing whole Arabidopsis roots with a needle. Three days after wounding, whole roots were stained with GUS solution at 37°C overnight.
Quantitative real-time RT-PCR (qPCR)
Total RNA was extracted from approximately 100 mg Arabidopsis roots using the RNAqueous kit (Ambion, Catalog: AM1912) following the manufacturer’s instructions. Genomic DNA contamination was completely removed from the samples by DNase I (Invitrogen, Catalog: 18068-015) treatment. Gene-specific primers to Arabidopsis AtPRX53, PR1, PR2, PR3, PR4, PR6, PDF1.2, PAD4 and Actin8 were designed and used in qPCR (Table S1). Gene expression levels were quantified in three independent biological samples, each consisting of four technical replicates. PCR reactions were run in an ICycler (Bio-Rad). PCR program and quantification of the relative changes in gene expression were conducted as previously described.69
Hypocotyl length measurement
For hypocotyl length measurement, Arabidopsis seeds were planted in Petri dishes (150 x15 mm) containing modified Knop’s medium. Plates were incubated vertically in a growth chamber at 26°C under 16 h light/8 h dark condition. Seven days after planting, the hypocotyl length of at least 30 plants per line was measured in three independent experiments. Statistically significant differences between transgenic line and the corresponding wild-type control were determined by unadjusted unpaired t-test (p < 0.05).
Phloroglucinol histochemical staining of Arabidopsis roots
Arabidopsis roots, collected at 3, 8 and 14 d post inoculation, were fixed overnight in FAA solution (50% ethonal: formaldehyde: acetic acid = 18:1:1) at 4°C. After removing fixation solution, 1% phloroglucinol solution was added to the roots for 5 min. Stained tissues were observed and photographed with a Zeiss Axiovert 100 microscope within 15 min of phloroglucinol staining.70
Hydrogen peroxide staining of Arabidopsis roots
AtPRX53 overexpression and wild type roots were treated with 1 mg/ml 3,3′-Diaminobenzidine (DAB) (Acros Organics, Catalog: AC11209-0050) in 50 mM TRIS-acetate buffer, pH 5.0, at 25°C for 24 h in the dark.71 Stained roots were observed under a Zeiss Axiovert 100 microscope.
Accession Numbers
AtPRX53: At5g06720, PR1:At2g14610, PR2: At3g57260, PR3: At3g12500, PR4: At3g04720, PR6: At2g38900, PDF1.2: At5g44420, PAD4: At3g52430 and Actin8: At1g49240
Acknowledgments
This is a journal paper of the Iowa Agriculture and Home Economics Experiment Station, Ames, IA, supported by Hatch Act and State of Iowa funds. This work was funded by USDA National Research Initiative Competitive Grants Program Award 2008-35302-18824 and by grants from the Iowa Soybean Association. We thank T.R. Maier for technical assistance.
Note
Supplemental materials can be found at www.landesbioscience.com/journals/psb/article/17684/.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17684
References
- 1.Wrather JA, Anderson TR, Arsyad DM, Tan Y, Ploper LD, Porta-Puglia A, et al. Soybean disease loss estimates for the top ten soybean-producing countries in 1998. Can J Plant Pathol. 2001;23:115–21. doi: 10.1080/07060660109506918. [DOI] [PubMed] [Google Scholar]
- 2.Davis EL, Hussey R, Baum T, Bakker J, Schots A, Rosso M, et al. Nematode parasitism genes. Annu Rev Phytopathol. 2000;38:365–96. doi: 10.1146/annurev.phyto.38.1.365. [DOI] [PubMed] [Google Scholar]
- 3.Baum T, Hussey R, Davis E. Root-knot and cyst nematode parasitism genes: the molecular basis of plant parasitism. Genet Eng (N Y) 2007;28:17–43. doi: 10.1007/978-0-387-34504-8_2. [DOI] [PubMed] [Google Scholar]
- 4.Hewezi T, Howe P, Maier T, Hussey R, Mitchum M, Davis E, et al. Cellulose Binding Protein from the Parasitic Nematode Heterodera schachtii Interacts with Arabidopsis Pectin Methylesterase: Cooperative Cell Wall Modification during Parasitism. Plant Cell. 2008;20:3080–93. doi: 10.1105/tpc.108.063065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hewezi T, Howe PJ, Maier TR, Hussey RS, Mitchum MG, Davis EL, et al. Arabidopsis Spermidine Synthase Is Targeted by an Effector Protein of the Cyst Nematode Heterodera schachtii. Plant Physiol. 2010;152:968–84. doi: 10.1104/pp.109.150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel N, Hamamouch N, Li C, Hewezi T, Hussey RS, Baum TJ, et al. A nematode effector protein similar to annexins in host plants. J Exp Bot. 2010;61:235–48. doi: 10.1093/jxb/erp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheysen G, Mitchum MG. Molecular Insights in the Susceptible Plant Response to Nematode Infection. Plant Cell Monographs 2009:45-81. [Google Scholar]
- 8.Puthoff DP, Nettleton D, Rodermel S, Baum T. Arabidopsis gene expression changes during cyst nematode parasitism revealed by statistical analyses of microarray expression profiles. Plant J. 2003;33:911–21. doi: 10.1046/j.1365-313X.2003.01677.x. [DOI] [PubMed] [Google Scholar]
- 9.Klink VP, Alkharouf N, MacDonald M, Matthews B. Laser capture microdissection (LCM) and expression analyses of Glycine max (soybean) syncytium containing root regions formed by the plant pathogen Heterodera glycines (soybean cyst nematode) Plant Mol Biol. 2005;59:965–79. doi: 10.1007/s11103-005-2416-7. [DOI] [PubMed] [Google Scholar]
- 10.Klink VP, Overall C, Alkharouf N, MacDonald M, Matthews B. Laser capture microdissection (LCM) and comparative microarray expression analysis of syncytial cells isolated from incompatible and compatible soybean (Glycine max) roots infected by the soybean cyst nematode (Heterodera glycines) Planta. 2007;226:1389–409. doi: 10.1007/s00425-007-0578-z. [DOI] [PubMed] [Google Scholar]
- 11.Ithal N, Recknor J, Nettleton D, Maier T, Baum T, Mitchum M. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Interact. 2007;20:510–25. doi: 10.1094/MPMI-20-5-0510. [DOI] [PubMed] [Google Scholar]
- 12.Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil D, et al. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J. 2009;57:771–84. doi: 10.1111/j.1365-313X.2008.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welinder KG. Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol. 1992;2:388–93. doi: 10.1016/0959-440X(92)90230-5. [DOI] [Google Scholar]
- 14.Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, et al. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 2002;53:1305–19. doi: 10.1093/jexbot/53.372.1305. [DOI] [PubMed] [Google Scholar]
- 15.Martínez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, et al. Biodegradation of lignocellulosics: microbial chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol. 2005;8:195–204. [PubMed] [Google Scholar]
- 16.Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001;42:462–8. doi: 10.1093/pcp/pce061. [DOI] [PubMed] [Google Scholar]
- 17.Tognolli M, Penel C, Greppin H, Simon P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene. 2002;288:129–38. doi: 10.1016/S0378-1119(02)00465-1. [DOI] [PubMed] [Google Scholar]
- 18.Duroux L, Welinder KG. The peroxidase gene family in plants: A phylogenetic overview. J Mol Evol. 2003;57:397–407. doi: 10.1007/s00239-003-2489-3. [DOI] [PubMed] [Google Scholar]
- 19.Fry SC. Cross-linking of matrix polymers in the growing cell-walls of angiosperms. Annu Rev Plant Physiol Plant Mol Biol. 1986;37:165–86. doi: 10.1146/annurev.arplant.37.1.165. [DOI] [Google Scholar]
- 20.Brownleader MD, Ahmed N, Trevan M, Chaplin M, Dey P. Purification and Partial Characterization of Tomato Extensin Peroxidase. Plant Physiol. 1995;109:1115–23. doi: 10.1104/pp.109.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostergaard L, Teilum K, Mirza O, Mattsson O, Petersen M, Welinder KG, et al. Arabidopsis ATP A2 peroxidase. Expression and high-resolution structure of a plant peroxidase with implications for lignification. Plant Mol Biol. 2000;44:231–43. doi: 10.1023/A:1006442618860. [DOI] [PubMed] [Google Scholar]
- 22.Marjamaa K, Kukkola EM, Fagerstedt KV. The role of xylem class III peroxidases in lignification. J Exp Bot. 2009;60:367–76. doi: 10.1093/jxb/ern278. [DOI] [PubMed] [Google Scholar]
- 23.Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang XL, Sabatino A, et al. Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol. 1999;121:135–46. doi: 10.1104/pp.121.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosio C, Vuillemin L, De Meyer M, Kevers C, Penel C, Dunand C. An anionic class III peroxidase from zucchini may regulate hypocotyl elongation through its auxin oxidase activity. Planta. 2009;229:823–36. doi: 10.1007/s00425-008-0876-0. [DOI] [PubMed] [Google Scholar]
- 25.Delannoy E, Jalloul A, Assigbetse K, Marmey P, Giger JP, Lherminier J, et al. Activity of class III peroxidases in the defense of cotton to bacterial blight. Mol Plant Microbe Interact. 2003;16:1030–8. doi: 10.1094/MPMI.2003.16.11.1030. [DOI] [PubMed] [Google Scholar]
- 26.Almagro L, Ros LVG, Belchi-Navarro S, Bru R, Barcelo AR, Pedreno MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60:377–90. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 27.Lehtonen MT, Akita M, Kalkkinen N, Ahola-Iivarinen E, Ronnholm G, Somervuo P, et al. Quickly-released peroxidase of moss in defense against fungal invaders. New Phytol. 2009;183:432–43. doi: 10.1111/j.1469-8137.2009.02864.x. [DOI] [PubMed] [Google Scholar]
- 28.Chassot C, Nawrath C, Metraux JP. Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 2007;49:972–80. doi: 10.1111/j.1365-313X.2006.03017.x. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Vivancos P, Rubio M, Mesonero V, Periago PM, Barcelo AR, Martinez-Gomez P, et al. The apoplastic antioxidant system in Prunus: response to long-term plum pox virus infection. J Exp Bot. 2006;57:3813–24. doi: 10.1093/jxb/erl138. [DOI] [PubMed] [Google Scholar]
- 30.Little D, Gouhier-Darimont C, Bruessow F, Reymond P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007;143:784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonetti E, Veronico P, Melillo MT, Delibes A, Andres MF, Lopez-Brana I. Analysis of Class III Peroxidase Genes Expressed in Roots of Resistant and Susceptible Wheat Lines Infected by Heterodera avenae. Mol Plant Microbe Interact. 2009;22:1081–92. doi: 10.1094/MPMI-22-9-1081. [DOI] [PubMed] [Google Scholar]
- 32.Showalter AM. Structure and function of plant-cell wall proteins. Plant Cell. 1993;5:9–23. doi: 10.1105/tpc.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson PA, Galinha C, Pereira C, Fortunato A, Soares N, Amâncio S, et al. Rapid deposition of extensin during the elicitation of grapevine callus cultures is specifically catalyzed by a 40-kilodalton peroxidase. Plant Physiol. 2001;127:1065–76. doi: 10.1104/pp.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres MA, Jones JDG, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–8. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, et al. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006;47:851–63. doi: 10.1111/j.1365-313X.2006.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolwell GP, Daudi A. Reactive Oxygen Species in Plant-Pathogen Interactions. Reactive Oxygen Species in Plant Signaling 2009:113-33. [Google Scholar]
- 37.Passardi F, Tognolli M, De Meyer M, Penel C, Dunand C. Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta. 2006;223:965–74. doi: 10.1007/s00425-005-0153-4. [DOI] [PubMed] [Google Scholar]
- 38.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boter M, Ruiz-Rivero O, Abdeen A, Prat S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 2004;18:1577–91. doi: 10.1101/gad.297704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery J, Goldman S, Deikman J, Margossian L, Fischer RL. Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proc Natl Acad Sci USA. 1993;90:5939–43. doi: 10.1073/pnas.90.13.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itzhaki H, Maxson JM, Woodson WR. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-s-transferase (GSTI) gene. Proc Natl Acad Sci USA. 1994;91:8925–9. doi: 10.1073/pnas.91.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakashima K, Fujita Y, Katsura K, Maruyama K, Narusaka Y, Seki M, et al. Transcriptional regulation of ABI3-and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol Biol. 2006;60:51–68. doi: 10.1007/s11103-005-2418-5. [DOI] [PubMed] [Google Scholar]
- 43.Escobar C, De Meutter J, Aristizabal FA, Sanz-Alferez S, del Campo FF, Barthels N, et al. Isolation of the LEMM19 gene and promoter analysis during a compatible plant-nematode interaction. Mol Plant Microbe Interact. 1999;12:440–9. doi: 10.1094/MPMI.1999.12.5.440. [DOI] [PubMed] [Google Scholar]
- 44.Martienssen RA. Functional genomics: Probing plant gene function and expression with transposons. Proc Natl Acad Sci USA. 1998;95:2021–6. doi: 10.1073/pnas.95.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundaresan V, Springer P, Volpe T, Haward S, Jones JDG, Dean C, et al. Patterns of gene-action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 1995;9:1797–810. doi: 10.1101/gad.9.14.1797. [DOI] [PubMed] [Google Scholar]
- 46.Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003;21:829–37. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- 47.Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. J Exp Bot. 2009;60:391–408. doi: 10.1093/jxb/ern318. [DOI] [PubMed] [Google Scholar]
- 48.Maleck K, Dietrich R. Defense on multiple fronts: how do plants cope with diverse enemies? Trends Plant Sci. 1999;4:215–9. doi: 10.1016/S1360-1385(99)01415-6. [DOI] [PubMed] [Google Scholar]
- 49.Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–20. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheong YH, Chang H, Gupta R, Wang X, Zhu T, Luan S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002;129:661–77. doi: 10.1104/pp.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong X. JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1:316–23. doi: 10.1016/1369-5266(88)80053-0. [DOI] [PubMed] [Google Scholar]
- 52.Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001;125:1591–602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin CC, Kao CH. Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci. 2001;160:323–9. doi: 10.1016/S0168-9452(00)00396-4. [DOI] [PubMed] [Google Scholar]
- 54.Ostergaard L, Abelskov AK, Mattsson O, Welinder KG. Structure and organ specificity of an anionic peroxidase from Arabidopsis thaliana cell suspension culture. FEBS Lett. 1996;398:243–7. doi: 10.1016/S0014-5793(96)01244-6. [DOI] [PubMed] [Google Scholar]
- 55.Passardi F, Penel C, Dunand C. Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci. 2004;9:534–40. doi: 10.1016/j.tplants.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Cordoba-Pedregosa M, Gonzalez-Reyes J, Canadillas M, Navas P, Cordoba F. Role of Apoplastic and Cell-Wall Peroxidases on the Stimulation of Root Elongation by Ascorbate. Plant Physiol. 1996;112:1119–25. doi: 10.1104/pp.112.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fry SC. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J. 1998;332:507–15. doi: 10.1042/bj3320507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schweikert C, Liszkay A, Schopfer P. Scission of polysaccharides by peroxidase-generated hydroxyl radicals. Phytochemistry. 2000;53:565–70. doi: 10.1016/S0031-9422(99)00586-5. [DOI] [PubMed] [Google Scholar]
- 59.Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed N, Chaplin M, Trevan M, Dey P, Brownleader M. Purification and partial characterization of 'extensin peroxidase'. Biochem Soc Trans. 1995;23:154S. doi: 10.1042/bst023154s. [DOI] [PubMed] [Google Scholar]
- 61.Macadam JW, Nelson CJ, Sharp RE. Peroxidase-activity in the leaf elongation zone of tall fescue. 1. spatial-distribution of ionically bound peroxidase-activity in genotypes differing in length of the elongation zone. Plant Physiol. 1992;99:872–8. doi: 10.1104/pp.99.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warneck HM, Haug T, Seitz U. Activation of cell wall-associated peroxidase isoenzymes in pea epicotyls by a xyloglucan-derived nonasaccharide. J Exp Bot. 1996;47:1897–904. doi: 10.1093/jxb/47.12.1897. [DOI] [Google Scholar]
- 63.Wallace G, Fry SC. Action of diverse peroxidases and laccases on six cell wall-related phenolic compounds. Phytochemistry. 1999;52:769–73. doi: 10.1016/S0031-9422(99)00342-8. [DOI] [Google Scholar]
- 64.Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. J Exp Bot. 2009;60:391–408. doi: 10.1093/jxb/ern318. [DOI] [PubMed] [Google Scholar]
- 65.Clough SJ, Bent A. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 66.Sijmons P, Grundler F, Mende N, Burrows PR, Wyss U. Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J. 1991;1:10. doi: 10.1111/j.1365-313X.1991.00245.x. [DOI] [Google Scholar]
- 67.Baum TJ, Wubben MJE, Hardy KA, Su H, Rodermel SR. A screen for Arabidopsis thaliana mutants with altered susceptibility to Heterodera schachtii. J Nematol. 2000;32:166–73. [PMC free article] [PubMed] [Google Scholar]
- 68.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions - beta-glucuronidase as a sensitive and versatile gene fusion marker in higher-plants. EMBO J. 1987;6:3901–7. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 70.Newman LJ, Perazza DE, Juda L, Campbell MM. Involvement of the R2R3-MYB, AtMYB61, in the ectopic lignification and dark-photomorphogenic components of the det3 mutant phenotype. Plant J. 2004;37:239–50. doi: 10.1046/j.1365-313X.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- 71.Einset J, Nielsen E, Connolly E, Bones A, Sparstad T, Winge P, et al. Membrane-trafficking RabA4c involved in the effect of glycine betaine on recovery from chilling stress in Arabidopsis. Physiol Plant. 2007;130:511–8. doi: 10.1111/j.1399-3054.2007.00920.x. [DOI] [Google Scholar]