Abstract
Extracellular purine nucleotides appear capable of regulating plant development, defence and stress responses by acting in part as agonists of plasma membrane calcium channels. Factors stimulating ATP release include wounding, osmotic stress and elicitors. Here we show that exogenous abscisic acid and L-glutamate can also cause ATP accumulation around Arabidopsis thaliana roots. Release of ADP from root epidermis would trigger ionotropic receptor-like activity in the plasma membrane, resulting in transient elevation of cytosolic free calcium. Root epidermal protoplasts (expressing aequorin as a cytosolic free calcium reporter) can support an extracellular ADP-induced cytosolic calcium elevation in the presence of an extracellular reductant. This confirms that ADP could elicit calcium-based responses distinct to those of ATP, which have been shown previously to involve production of extracellular reactive oxygen species.
Keywords: abscisic acid, ADP, ATP, calcium, channel, glutamate, root
Extracellular purine nucleotides can regulate plant cell growth, stress responses, immunity and symbiotic events.1,2 Their activity at the plasma membrane elicits increased production of nitric oxide (NO), activation of NADPH oxidases to produce reactive oxygen species (ROS), and transient elevations of cytosolic free calcium ([Ca2+]cyt).3-9 Although there are no equivalents to animal ionotropic or metabotropic purinoreceptor genes apparent in higher plant genomes, plant responses to extracellular purine nucleotides are sensitive to antagonists of animal purinoceptors.1,10-12 Arabidopsis thaliana plasma membrane Ca2+ channel activity was recently found to be activated rapidly by extracellular ADP, consistent with the presence of an ionotropic receptor.13 Parallels with animal cells also extend to a conserved mechanism of ATP and ADP hydrolysis by extracellular apyrase,14 plus release of ATP by exocytosis15 and an ABC transporter.16
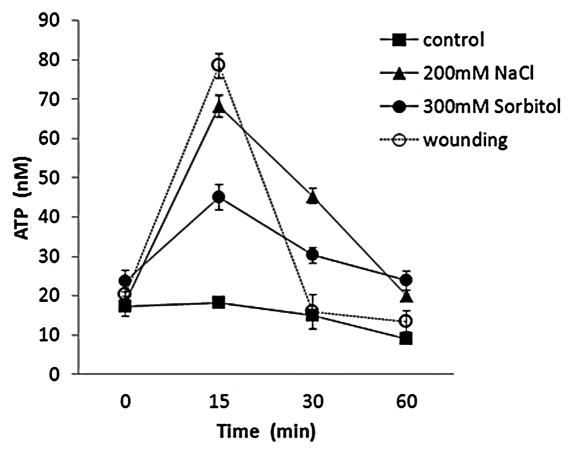
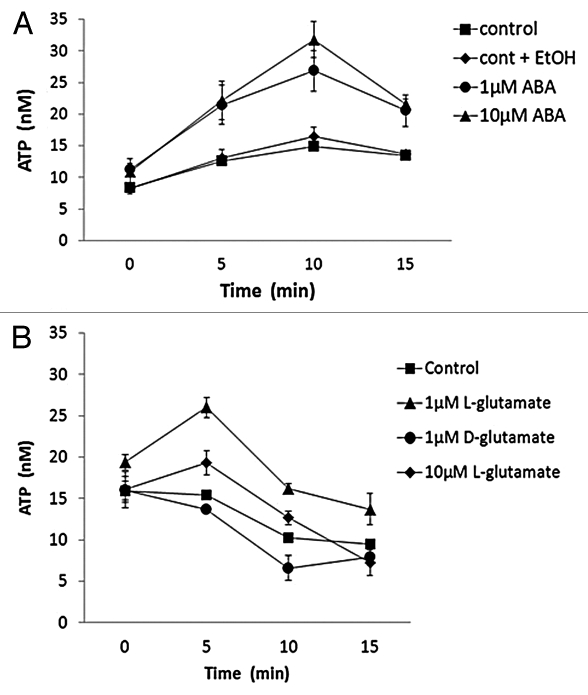
ATP is released at plant wound and growth points, in response to touch, elicitors and hyperosmotic stress imposed by salts.1,17,18 Here, wounding, hyperosmotic or sodicity stress resulted in distinct levels and time courses of ATP accumulation around excised roots of A. thaliana (Fig. 1). Recovery of ATP levels to control values was evident at the 30 min time point after wounding but remained significantly elevated in response to abiotic stresses (30 min time point; NaCl, p < 0.001; sorbitol, p < 0.036, Student’s t-test). The stress response hormone abscisic acid (ABA) at 1 and 10 μM also promoted ATP accumulation around excised roots (Fig. 2A), albeit to a lower level. Accumulation above the ethanol control was significant for both concentrations at 10 and 15 min. Application of 10 μM ABA inhibits A. thaliana primary root growth, a response involving a plasma membrane NADPH oxidase and plasma membrane proline-rich extensin-like receptor kinase (PERK4).19,20 PERK4 directs the activation of plasma membrane Ca2+ influx channels.20 As extracellular ATP activates root epidermal plasma membrane Ca2+ influx channels via activation of an NADPH oxidase, it becomes feasible that ABA inhibition involves ATP release and sensing upstream of NADPH oxidases and PERKs, resulting in an inhibitory Ca2+ influx. High [Ca2+]cyt can act as a “brake” to root hair elongation and perhaps this may also hold for elongation of primary roots.21 Production of ROS by NADPH oxidases also promotes root cell elongation by activating plasma membrane Ca2+ channels.22 Thus, the dose-dependent stimulation or inhibition of growth by extracellular ATP may be effected by the levels of ROS production and channel activation.
Figure 1.
Wounding and abiotic stress cause ATP accumulation around A. thaliana roots.A. thaliana (Col-0) was grown for seven days as described previously on MS medium with 1% (w/v) sucrose.13 Eight roots of similar lengths were placed in the well of a 96-well plate containing sterile liquid MS/1% (w/v) sucrose, with or without test substances. ATP content of samples was determined with a luciferin/luciferase assay (Molecular Probes), using a Fluostar Optima plate reading luminometer. Wounding was inflicted by moving a gloved finger up and down the roots to damage root hairs prior to immersion in assay medium. Results are mean ± SEM from 3 independent trials.
Figure 2.
ABA and L-glutamate cause ATP accumulation around A. thaliana roots. Roots were exposed to (A) ABA with 0.025% (v/v) ethanol (EtOH) as the solvent carrier control and (B) L- or D-glutamate. Conditions as in Figure 1. Results are mean ± SEM from 3 independent trials.
Extracellular glutamate can also elevate root [Ca2+]cyt by activating plasma membrane glutamate receptor-like (GLR) channels.23,24 As shown in Figure 2B, 1 μM L-glutamate caused transient ATP accumulation but equimolar D-glutamate did not, indicating stereospecificity of the response. Exposure to 10 μM L-glutamate was less effective than 1 μM, suggesting a biphasic response. At these concentrations, glutamate is unlikely to be transported into roots by the low affinity amino acid permease AAP1 or by H+-coupled symport25,26 and so should be acting extracellularly. L-glutamate at 10 μM and above inhibits A. thaliana primary root growth,27 and it will be interesting to see how this relates, if at all, to ATP functions. Perception of extracellular glutamate at the epidermis upstream of ATP release could be mediated by GLR2.1, 3.3, 3.5 and 5 as the most highly expressed of the gene family in this cell file. Across the meristematic region GLR2.4, 3.3, 3.6,5 are the most highly expressed while across the elongation zone, GLR 1.4, 2.1, 3.3, 3.5 and 3.6 predominate.28
Once purine nucleotides are released from roots, they can activate plasma membrane Ca2+-permeable channels directly (ADP)13 or via production of extracellular ROS (ATP).11 Accordingly, ATP activation of root epidermal plasma membrane Ca2+-permeable channels can be prevented by applying dithiothreitol (DTT) as a reductant.11 Here, ADP-induced [Ca2+]cyt elevation of A. thaliana root epidermal protoplasts (measured as in ref. 13) was unaffected by incubation in DTT (1 mM) (0.1 mM ADP; 0.45 ± 0.04 μM [Ca2+]cyt, n = 6: ADP + DTT; 0.52 ± 0.03 μM [Ca2+]cyt, n = 9), confirming that extracellular ROS are not involved in ADP activation of Ca2+ influx.
Addition of purine nucleotides to A. thaliana root epidermis causes transient changes in net Ca2+ and K+ fluxes downstream of the initial sensing event.13 ADP promotes net Ca2+ influx but ATP and the weakly hydrolysable analog adenosine 5′-(α,β-methylene)triphosphate (αßATP) only promote influx when applied up to 100 μM, above which Ca2+ efflux is promoted.13 Under control conditions used in ref. 13, there is a net K+ efflux from both elongation zone (-235 ± 16 nmol.m−2.s−1 ; n = 33) and mature epidermis (-44 ± 3 nmol.m−2.s-1; n = 25) detected by extracellular vibrating K+-selective microelectrodes. From the data presented in ref. 13, this indicates that in the elongation zone, ADP, ATP and αßATP up to approximately 10 μM would decrease net K+ efflux and promote Ca2+ influx. Above 10 μM, K+ efflux would be promoted. At the mature epidermis ADP up to 10 μM and ATP/αßATP up to approximately 100 μM would decrease K+ efflux while promoting Ca2+ influx. Above these levels, K+ efflux would be promoted. At present the contribution of underlying cells and the walls are unknown. If the origin were the epidermal plasma membrane, the results indicate involvement of K+ efflux pathways and it will be interesting to test whether the epidermal K+ efflux channel AtGORK is involved.29 How the cation fluxes are generated and what their consequences are now need to be elucidated.
Acknowledgments
Financial support was from the Leverhulme Trust and the Alexander James Keith Fund.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17014
References
- 1.Tanaka K, Gilroy S, Jones AM, Stacey G. Extracellular ATP signaling in plants. Trends Cell Biol. 2010;20:601–8. doi: 10.1016/j.tcb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chivasa S, Murphy AM, Hamilton JM, Lindsey K, Carr JP, Slabas AR. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009;60:436–48. doi: 10.1111/j.1365-313X.2009.03968.x. [DOI] [PubMed] [Google Scholar]
- 3.Foresi NP, Laxalt AM, Tonón CV, Casalongué CA, Lamattina L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol. 2007;145:589–92. doi: 10.1104/pp.107.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SJ, Wu JY. Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J Exp Bot. 2008;59:4007–16. doi: 10.1093/jxb/ern242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichler SA, Torres J, Rivera AL, Cintolesi VA, Clark G, Roux SJ. Intersection of two signaling pathways: extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J Exp Bot. 2009;60:2129–38. doi: 10.1093/jxb/erp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark G, Wu M, Wat N, Onyirimba J, Pham T, Herz N, et al. Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygens species. Plant Mol Biol. 2010;74:423–35. doi: 10.1007/s11103-010-9683-7. [DOI] [PubMed] [Google Scholar]
- 7.Sueldo DJ, Foresi NP, Casalongué CA, Lamattina L, Laxalt AM. Phosphatidic acid formation is required for extracellular ATP-mediated nitric oxide production in suspension-cultured tomato cells. New Phytol. 2010;185:909–16. doi: 10.1111/j.1469-8137.2009.03165.x. [DOI] [PubMed] [Google Scholar]
- 8.Terrile MC, Tonón C, Iglesias MJ, Lamattina L, Casalongué CA. Extracellular ATP and nitric oxide signaling pathways regulate redox-dependent responses associated to root hair growth in etiolated Arabidopsis seedlings. Plant Signal Behav. 2010;5:698–701. doi: 10.4161/psb.5.6.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonón C, Terrile MC, Iglesias MJ, Lamattina L, Casalongué CA. Extracellular ATP, nitric oxide and superoxide act co-ordinately to regulate hypocotyl growth in etiolated Arabidopsis seedlings. J Plant Physiol. 2010;167:540–6. doi: 10.1016/j.jplph.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Demidchik V, Nichols C, Dark AM, Olynyk M, Glover BJ, Davies JM. Is ATP a signaling agent in plants? Plant Physiol. 2003;133:456–61. doi: 10.1104/pp.103.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, et al. Plant extracellular ATP signaling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 2009;58:903–13. doi: 10.1111/j.1365-313X.2009.03830.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Swanson SJ, Gilroy S, Stacey G. Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol. 2010;154:705–19. doi: 10.1104/pp.110.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demidchik V, Shang ZL, Shin R, Colaço R, Laohavisit A, Shabala S, et al. Receptor-like activity evoked by extracellular ADP in Arabidopsis thaliana root epidermal plasma membrane. Plant Physiol. 2011;156:1375–85. doi: 10.1104/pp.111.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riewe D, Grosman L, Fernie AR, Wucke C, Geigenberger P. The potato-specific apyrase is apoplastically localized and has influence on gene expression, growth and development. Plant Physiol. 2008;146:1579–98. doi: 10.1104/pp.108.115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Sivaguru M, Stacey G. Extracellular ATP in plants: Visualisation, localization and analysis of physiological significance in growth and signaling. Plant Physiol. 2006;142:984–92. doi: 10.1104/pp.106.085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ. A role for ectoapyrase in xenobiotic resistance. Plant Cell. 2000;12:519–33. doi: 10.1105/tpc.12.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S-H, Yang SH, Kim T-J, Han J-S, Suh J-W. Hypertonic stress increased extracellular ATP levels and the expression of stress-responsive genes in Arabidopsis thaliana seedlings. Biosci Biotechnol Biochem. 2009;73:1252–6. doi: 10.1271/bbb.80660. [DOI] [PubMed] [Google Scholar]
- 18.Weerasinghe RR, Swanson SJ, Okada SF, Garrett MB, Kim SY, Stacey G, et al. Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett. 2009;583:2521–6. doi: 10.1016/j.febslet.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak JM, Mori IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signalling in Arabidopsis. EMBO J. 2003;22:2623–33. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai L, Zhang G, Zhou Y, Zhang Z, Wang W, Du Y, et al. Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca2+ signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J. 2009;60:314–27. doi: 10.1111/j.1365-313X.2009.03956.x. [DOI] [PubMed] [Google Scholar]
- 21.Monshausen GB, Messerli MA, Gilroy S. Imaging of the yellow cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147:1690–8. doi: 10.1104/pp.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 23.Qi Z, Stephens NR, Spalding EP. Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 2006;142:963–71. doi: 10.1104/pp.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demidchik V, Essah PA, Tester M. Glutamate activates cation currents in the plasma membrane of Arabidopsis root cells. Planta. 2004;219:167–75. doi: 10.1007/s00425-004-1207-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y-H, Foster J, Chen J, Voll LM, Weber APM, Tegeder M. AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J. 2007;50:305–19. doi: 10.1111/j.1365-313X.2007.03045.x. [DOI] [PubMed] [Google Scholar]
- 26.Svennerstam H, Ganeteg U, Bellini C. Näsholm. Comprehensive screening of Arabidopsis mutants suggests the lysine histidine transporter 1 to be involved in plant uptake of amino acids. Plant Physiol. 2007;143:1853–60. doi: 10.1104/pp.106.092205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walch-Liu P, Liu L-H, Remans T, Tester M, Forde BG. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:1045–57. doi: 10.1093/pcp/pcj075. [DOI] [PubMed] [Google Scholar]
- 28.Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, et al. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–5. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- 29.Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, et al. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced death. J Cell Sci. 2010;123:1468–79. doi: 10.1242/jcs.064352. [DOI] [PubMed] [Google Scholar]