Abstract
Quantitative analysis of mitochondrial FA β-oxidation (FAO) has drawn increasing interest for defining lipid-induced metabolic dysfunctions, such as in obesity-induced insulin resistance, and evaluating pharmacologic strategies to improve β-oxidation function. The aim was to develop a new assay to quantify β-oxidation function in intact mitochondria and with a low amount of cell material. Cell membranes of primary human fibroblasts were permeabilized with digitonin prior to a load with FFA substrate. Following 120 min of incubation, the various generated acylcarnitines were extracted from both cells and incubation medium by protein precipitation/desalting and subjected to solid-phase extraction. A panel of 30 acylcarnitines per well was quantified by MS/MS and normalized to citrate synthase activity to analyze mitochondrial metabolite flux. Pretreatment with bezafibrate and etomoxir revealed stimulating and inhibiting regulatory effects on β-oxidation function, respectively. In addition to the advantage of a much shorter assay time due to in situ permeabilization compared with whole-cell incubation systems, the method allows the detection of multiple acylcarnitines from an only limited amount of intact cells, particularly relevant to the use of primary cells. This novel approach facilitates highly sensitive, simple, and fast monitoring of pharmacological effects on FAO.
Keywords: β-oxidation function, acylcarnitines, tandem mass spectrometry, mitochondria, digitonin, drug discovery, mode of action, obesity, insulin resistance, fatty acid β-oxidation
The mitochondrial FA β-oxidation (FAO) pathway is a major contributor to cellular energy production and homeostasis, particularly once glycogen stores are depleted owing to fasting (1). Substantial amounts of energy are provided by FAO for heart, skeletal muscle, and liver function. In monogenetic deficiencies of FAO or the carnitine shuttle system, accumulation of acyl-CoA intermediates and depletion of energy and free carnitine occur, specifically at times of increased energy demands, including fever and impaired food supply (2). Altered FA catabolism is considered key in the development of obesity-induced insulin resistance, because cellular overload with long-chain FAs is thought to exert a disastrous effect on mitochondrial function (3, 4). Cytoprotective mechanisms against FA lipotoxicity include channelling of FAs into triglyceride pools and enhancement of FA degradation via mitochondrial β-oxidation (4).
Thus, quantitative analysis of FAO has become increasingly important in assessing lipid-induced metabolic impairment and evaluating potential pharmacologic strategies designed to improve β-oxidation flux (4). The most commonly applied method to assess functional FAO in cellular models has been the analysis of total FAO flux following incubation of intact cells with 14C- or 3H-labeled FAs and detection of 14CO2 or 3H2O release (5–8). Analyses of specific FAO steps and disturbances within were subsequently developed following incubation of radiolabeled, stable-isotope-labeled, or unlabeled precursors. [U-14C]hexadecanoate was used to measure acylcarnitine and acyl-CoA intermediates by radio-HPLC in mitochondrial preparations (9) and permeabilized human fibroblasts or peripheral blood cells (10, 11). The development of MS/MS technology allowed the sensitive and specific quantitative profiling of accumulating acylcarnitines following 72 or 96 h incubation of intact cells with stable isotopes or unlabeled substrates (12–16). A faster approach of MS/MS-based acylcarnitine profiling was reported, applying incubation with stable isotopically labeled palmitate to cell homogenates containing intact mitochondria (17).
However, for the purpose of “mode of action” prediction in drug discovery a method is missing that allows maintaining cell organelle integrity to study β-oxidation function in a low amount of cell material and avoiding the need of laborious cell homogenization techniques. We therefore aimed to develop a cost- and time-efficient ESI-MS/MS-based targeted metabolite approach to quantitatively track the metabolites of each enzymatic step through the mitochondrial β-oxidation machinery in permeabilized cells by utilizing unlabeled FA substrate. The new assay allows highly sensitive monitoring of pharmacological intervention on β-oxidation in human fibroblasts and other cells such as adipocytes.
MATERIALS AND METHODS
Materials
DMEM (1 g/l d-glucose), PBS, FBS Gold, stable glutamine, and penicillin/streptomycin were purchased from PAA Laboratories GmbH (Pasching, Austria). l-carnitine, BSA (essentially FA-free), digitonin, ATP, ADP, CoA, cytochrome C, palmitic acid, DMSO, bezafibrate, etomoxir, acetyl-CoA, oxaloacetate, and DTNB were all obtained from Sigma-Aldrich (Steinheim, Germany). l-[2H3]carnitine (D3-C0), l-[2H3]butyrylcarnitine (D3-C4), l-[2H3]octanoylcarnitine (D3-C8), and l-[2H3]palmitoylcarnitine (D3-C16) internal standards (Cambridge Isotope Laboratories) were obtained from Promochem (Wesel, Germany). Solid-phase extraction columns (silica-based, 100 mg, Bond Elut-C18) were purchased from Varian (Middelburg, The Netherlands). Acetonitrile, methanol, and formic acid of HPLC quality were from Merck (Darmstadt, Germany). Butanolic hydrochloric acid (HCl) was prepared by saturating dry HPLC grade 1-butanol with HCl gas. Microtiter plates (96-well; Nunc) were obtained from Labor Schubert (Munich, Germany).
Cell lines and culture
A primary human fibroblast cell line was derived from skin of a healthy child with normal metabolic newborn screening test results who presented to the hospital for elective surgery for inguinal hernia repair, after informed consent was obtained. Cells were routinely grown as monolayers in T75 culture flasks (Sarstedt; Nümbrecht, Germany) using DMEM with 1 g/l glucose supplemented with 10% FBS and 2 mM stable glutamine at 37°C in a humidified 5% CO2 atmosphere. Passage numbers ranged from P7 to P14; in this interval, human fibroblasts retained a normal morphology. Murine 3T3-L1 preadipocytes were cultured and differentiated as described in the supplemental data.
Preparation of FA substrate solution and incubation media
FA stock solution was prepared in chloroform. In this work, palmitic acid was used as FA substrate. For each experiment, palmitic acid stock solution (20 mM, 100 µl) was transferred into a glass tube followed by evaporation to dryness under a stream of nitrogen. FA substrate solution was produced by adding assay buffer (17) containing 110 mM KCl, 1 mM EGTA, 10 mM HEPES buffer, 5 mM MgCl2, 10 mM K3PO4 (pH 7.2) and FA-free BSA in a molar ratio of 5:1 of the dried FA bound to BSA (200 µM FA, BSA 0.04 mM). The FA substrate was then incubated at 37°C in a water bath for 30 min and subsequently subjected to sonification three times for 10 s (amplitude 10%).
Additionally, the FA substrate solution was supplemented with appropriate cofactors such as 1 mM ADP, 5 mM ATP, 0.1 mM CoA, 0.2 mg/ml cytochrome C, and 0.4 mM l-carnitine (final concentrations), considering potential losses through membrane permeabilization. Except as otherwise indicated, final concentrations of the FA and the FA-free BSA in the supplemented FA substrate solution (“FA incubation medium”) were 100 µM and 0.04 mM, respectively. The control incubation medium was assay buffer containing all cofactors including 0.4 mM l-carnitine and 0.04 mM FA-free BSA.
Digitonin purification procedure and preparation of permeabilization buffer
Commercially available digitonin (Sigma-Aldrich; 50–80% purity grade) was dissolved in 100% ethanol at 75°C followed by precipitation on ice for 20 min and subsequent separation by centrifugation (10 min, 2,000 g, 4°C) (18). This procedure was repeated twice, and the resulting digitonin pellets from each purification cycle were collected. Additionally, supernatants from each cycle were combined and heated to 75°C followed by precipitation and subsequent centrifugation. Purified digitonin was then vacuum dried (ILMVAC Speed-Vac; Ilmvac GmbH, Ilmenau, Germany) and redissolved in 100% DMSO at 50 mg/ml. For cell permeabilization, digitonin stock solution was added to the assay buffer in a final concentration of 20 µg/ml (0.04% DMSO), defined as a result of digitonin titration experiments (0, 10, 20, 50, 100, and 200 µg/ml purified digitonin at 105 cells per well of a 12-well plate, corresponding to 105 cells/3.8 cm2).
Incubation conditions
After reaching 90% confluency, primary human fibroblasts were washed twice with 5 ml PBS and harvested following trypsinization. The cell pellet of one T75 flask was resuspended in 13 ml DMEM supplemented with 10% FBS and 2 mM stable glutamine. Following gentle mixing of the cell suspension, 1 ml of cells was seeded immediately into each well of a 12-well culture plate (approximately 105 cells/well, corresponding to an average protein concentration of approximately 50 µg protein/well). The plate was briefly mixed for equal distribution of the cells within the wells, and cells were incubated at 37°C in humidified 5% CO2/95% air. Twenty-four hours later, at 85% to 90% confluency, the cells were washed once with 0.5 ml PBS and incubated with 0.3 ml permeabilization buffer at 37°C for 5 min. Following permeabilization, cells were incubated with 0.3 ml FA incubation medium at 37°C for 2 h.
MS/MS sample preparation
Following incubation, both incubation medium and cell lysate were combined and subjected to the extraction of acylcarnitines. The incubation medium (260 µl) was transferred into a 10 ml polypropylene tube. Cells were precipitated in 1.7 ml acetonitrile-methanol (4:1, v/v) per well, and the cell lysate was then transferred into the same sample tube, resulting in a volume ratio of 6.5:1 (acetonitrile-methanol to sample). Forty microliter internal standard mixture containing 300 pmol D3-C0, 120 pmol D3-C4, 60 pmol D3-C8, and 120 pmol D3-C16 were added to each sample. After vigorous mixing on an orbital shaker (New Brunswick Scientific; Edison, NJ) at 160 rpm for 30 min at room temperature, samples were centrifuged at 4,000 g for 20 min at room temperature. One milliliter of cell-free supernatant was then added to solid-phase extraction columns (1 ml capacity), which were washed with 1 ml of 100% methanol prior to use. Bound acylcarnitines were stepwise eluted with 2 × 1 ml methanol (100%) and 2 × 1 ml methanol-H2O 1:2. The volume of 4 ml eluate was collected in silanized glass tubes and vacuum concentrated (ILMVAC Speed-Vac) before being subjected to derivatization and quantification.
ESI-MS/MS analysis
The following devices were used: Savant Model SC210 A SpeedVac Plus centrifugal evaporator (Life Sciences; Frankfurt, Germany); Titramax 1000 orbital shaker (Heidolph; Kelheim, Germany). Analyses were performed on an API365 ESI-MS/MS system equipped with a TurboIon spray device and a Series 200 lp HPLC pump (PE Sciex; Toronto, Canada), and a CTC PAL Autosampler (CTC Analytics; Zwingen, Switzerland). A restriction capillary between the HPLC pump and the autosampler adjusts the pressure to 200 psi. The Analyst 1.4 software (AB SCIEX; Framingham, MA) was used for instrument control and data acquisition. Data processing and calculation were performed with the ChemoView Software (AB SCIEX). Acylcarnitines and free carnitine were analyzed as described previously (19, 20). Free carnitine was quantified relative to D3-C0, short-chain acylcarnitines (C2 to C5) were quantified relative to D3-C4, medium-chain acylcarnitines (C6 to C12) relative to D3-C8, and long-chain acylcarnitines (C14 to C18) relative to D3-C16. MS/MS data in pmol were expressed relative to citrate synthase (CS) activity (nkat/well), resulting in normalized amounts of each metabolite (mmol/kat).
Citrate synthase assay
Cells of a separate well of a 12-well plate were lysed by incubation with 200 µl assay buffer containing 1% Triton X-100 at 37°C for 30 min. CS activity was assayed using 0.2 mM acetyl-CoA, 0.4 mM oxaloacetate, 0.2 mM DTNB in 50 mM Tris-HCl (pH 8.0), 100 mM KCl, and 1 mM EDTA, as described previously (21). CS from porcine heart was used as a positive control (Sigma-Aldrich). CS activity was calculated as the mean of three independent assays per metabolite experiment and expressed as nkat/well.
Data processing
Processing, normalization to CS activity, visualization, and exploratory analysis of MS/MS data were carried out in the data analysis and statistics language R (22). All processing steps were performed in a fully automated processing script in order to minimize the possibility of data handling errors. This analysis pipeline guarantees full reproducibility of all processing steps, also in future analyses, and finally serves as a comprehensive documentation of the entire procedure. Each measured metabolite was checked against limits of detection (LODs) and limits of quantification (LOQs) (determined in blank-sample experiments, described below). Because the data cover several orders of magnitude, some of the data visualization was done on a logarithmic scale.
Statistical analysis
Data were expressed as mean ± SEM of at least three independent experiments performed in triplicates each. All statistics of the presented data were performed in GraphPad Prism 4 (GraphPad Software; La Jolla, CA) using one-way ANOVA and Tukey's post hoc tests to assess differences. One sample t-test was used to test for differences between pharmacological treatments and the untreated control. For all tests, P < 0.05 was considered significant.
RESULTS AND DISCUSSION
Characterization of the assay
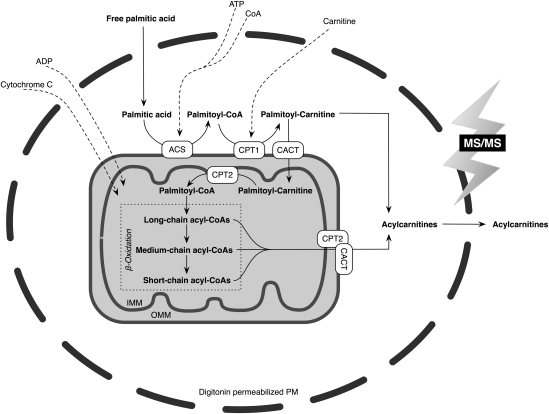
Table 1 shows the simultaneous quantification of a panel of 30 acylcarnitines achieved after a substrate load with palmitic acid. As a critical assay step, in situ permeabilization of cell membranes with digitonin allowed the study of an intact organelle environment in a much shorter incubation and assay turnaround time compared with whole-cell incubation assays (12–16). An assay scheme including the analyzed metabolic pathway is provided in Fig. 1. Digitonin titration experiments in primary human fibroblasts analyzing lactate dehydrogenase and CS as markers for the cytosolic and mitochondrial compartments, respectively, determined a concentration of 20 µg/ml purified digitonin at 105 cells per well (3.8 cm2) to be optimal to render the plasma membrane permeable while keeping the mitochondrial membrane intact. Similar concentration-dependent effects of digitonin on various organelle membranes in fibroblasts, including the mitochondrial membrane, have been reported previously (23).
TABLE 1.
Simultaneous quantification of 30 acylcarnitines of palmitate oxidation.
| Acylcarnitine | m/z | Mean pmol | SEM pmol | LOD pmol | LOQ pmol | |
| High abundance | C2:0 | 260.2 | 2097.70 | 66.61 | 27.24 | 57.14 |
| C12:0 | 400.3 | 462.82 | 41.83 | 3.48 | 9.91 | |
| C16:0 | 456.4 | 381.65 | 7.42 | 0.86 | 2.30 | |
| C6:0 | 316.3 | 137.08 | 6.12 | 10.61 | 26.26 | |
| C10:0 | 372.3 | 136.51 | 8.28 | 2.90 | 7.73 | |
| C8:0 | 344.3 | 113.34 | 6.38 | 6.50 | 17.50 | |
| C14:0 | 428.4 | 43.58 | 0.82 | 0.41 | 1.13 | |
| C16:0-OH | 472.4 | 11.31 | 0.39 | 0.60 | 1.25 | |
| C16:1 | 454.4 | 11.09 | 0.21 | 0.12 | 0.34 | |
| C14:1 | 426.4 | 3.96 | 0.13 | 0.19 | 0.60 | |
| C14:0-OH | 444.4 | 3.50 | 0.12 | 0.24 | 0.65 | |
| C18:1 | 482.4 | 1.67 | 0.06 | 0.07 | 0.19 | |
| Medium abundance | C4:0 | 288.2 | 79.94 | 3.25 | 16.68 | 38.30 |
| C10:1 | 370.3 | 23.82 | 2.60 | 4.66 | 12.43 | |
| C5:0-DC | 388.3 | 8.78 | 0.81 | 1.58 | 4.30 | |
| C18:0 | 484.4 | 3.05 | 0.11 | 0.36 | 0.94 | |
| C16:1-OH | 470.4 | 1.31 | 0.06 | 0.17 | 0.48 | |
| C12:1 | 398.3 | 0.93 | 0.07 | 0.12 | 0.31 | |
| C18:2 | 480.4 | 0.63 | 0.04 | 0.07 | 0.19 | |
| C18:1-OH | 498.4 | 0.23 | 0.02 | 0.05 | 0.14 | |
| Low abundance | C6:0-DC | 402.3 | 34.31 | 4.14 | 17.64 | 41.57 |
| C3:0 | 274.2 | 20.63 | 1.92 | 15.31 | 37.54 | |
| C4:0-DC | 374.3 | 4.41 | 0.38 | 2.90 | 7.34 | |
| C5:1 | 300.2 | 3.71 | 0.37 | 2.33 | 4.68 | |
| C8:1 | 342.3 | 3.20 | 0.33 | 1.63 | 4.18 | |
| C12:0-DC | 486.4 | 0.33 | 0.02 | 0.22 | 0.50 | |
| C18:0-OH | 500.4 | 0.13 | 0.01 | 0.05 | 0.14 | |
| C16:2 | 452.4 | 0.07 | 0.01 | 0.05 | 0.14 | |
| C16:2-DC | 476.5 | 0.05 | 0.01 | 0.02 | 0.10 | |
| C16:1-DC | 540.4 | 0.03 | 0.01 | 0.02 | 0.10 | |
| Below detection limit | C3:0-DC | 360.2 | 11.02 | 1.16 | 12.48 | 32.14 |
| C5:0 | 302.2 | 9.68 | 0.48 | 20.11 | 45.34 | |
| C6:0-OH | 332.3 | 7.71 | 1.19 | 7.99 | 23.21 | |
| C4:0-OH | 304.2 | 2.84 | 0.35 | 3.48 | 9.22 | |
| C5:0-OH | 318.2 | 2.77 | 0.23 | 4.85 | 12.70 | |
| C10:2 | 368.3 | 0.51 | 0.08 | 0.79 | 1.99 | |
| C14:2 | 424.3 | 0.25 | 0.03 | 0.29 | 0.77 | |
| C12:2 | 396.3 | 0.13 | 0.01 | 0.19 | 0.46 | |
| C18:2-OH | 496.4 | 0.10 | 0.01 | 0.31 | 0.74 | |
| C18:1-DC | 568.5 | 0.04 | 0.01 | 0.05 | 0.12 | |
| C16:0-DC | 542.4 | 0.02 | 0.004 | 0.12 | 0.38 | |
| C18:0-DC | 570.5 | 0.01 | 0.004 | 0.07 | 0.19 | |
| C18:2-DC | 568.5 | 0.01 | 0.002 | 0.05 | 0.17 |
Metabolites were categorized according to their abundance (pmol) following determination by ESI-MS/MS, prior to normalization to citrate synthase activity (n = 11 samples of within-run analyses).
Fig. 1.
Schematic representation of the in situ permeabilization assay for metabolite profiling of palmitate β-oxidation in intact mitochondria. ACS, acyl-CoA synthetase; CACT, carnitine acylcarnitine translocase (officially named as SLC25A20); CPT1, carnitine palmitoyltransferase 1; CPT2, carnitine palmitoyltransferase 2; IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane; PM, plasma membrane.
In addition to the advantage of a shorter assay time, the introduction of the permeabilization step and the surplus of FA substrate allowed MS/MS-based quantitation of acylcarnitines by utilization of only very low amounts of cell material. In comparison, the amount of protein required for comparable whole-cell incubation systems is at least twice to three times as high (13, 14, 16). Thus, the method is applicable even when availability of cell material is sparse, e.g., in the investigation of primary cells such as human adipocytes.
To increase the low aqueous solubility of the long-chain FA substrates in concentrations >10 µM (24), complexing of the FA with a carrier is required. Although albumin is frequently used, methyl-β-cyclodextrin (MBCD) is an alternative vehicle for the solubilization of FAs (25, 26). We found that MBCD applied as described previously (25) led to an improved reconstitution of defined acylcarnitine standards in solution compared with albumin. However, the quantity of acylcarnitines produced during FAO was higher when palmitate was complexed with albumin compared with MBCD, particularly for acylcarnitines of long- and medium-chain length (data not shown). This implies a more-efficient presentation of FAs to the mitochondrial membrane by albumin in the context of the cell permeabilization assay.
Acylcarnitines arise from intramitochondrial CoA esters generated on each step of the catabolic pathways of FAs and amino acids, provided that saturating amounts of l-carnitine are present to drive the equilibrium toward acylcarnitine ester formation via the carnitine acyltransferase system (9). Through the reverse action of the carnitine-acylcarnitine translocase, these acylcarnitines can exit the mitochondria (27). In our assay conditions, we detected approximately two thirds of the acylcarnitines produced in the particulate fraction and approximately one third in the incubation medium (data not shown). Therefore, the method was developed to allow quantification from both compartments in a combined sample to increase signal intensity. The distribution of accumulating metabolites in cells and media using this approach differs markedly from whole-cell incubation systems in which only 1% to 6% of produced acylcarnitine species were retained intracellularly following loading of normal fibroblasts with l-carnitine and palmitic acid (15). This difference in distribution between compartments appears to be the result of considerably longer incubation times of 72 to 96 h necessary in intact cells as compared with only 2 h in our permeabilized cell preparation.
Because of the necessity to measure acylcarnitines in both the particulate fraction and the incubation medium, a specific extraction procedure was devised comprising protein precipitation by organic solvents and desalting. Various combinations of organic solvents were tested for extraction of acylcarnitines of diverse chain-lengths and polarities. A solution of acetonitrile-methanol (4:1, v/v) added to the sample in a volume ratio of 6.5:1 was determined to be most efficient. This is similar to the procedure described by Minkler, Ingalls, and Hoppel, who found acetonitrile-methanol (4:1, v/v) added to the sample in a high ratio of 10:1 to be most advantageous for desalting (28).
In a subsequent purification step, cation exchange solid-phase extraction using silica gel was applied; this has previously been shown to be effective to sufficiently isolate acylcarnitines from urine and plasma (29–32). The relatively apolar ratio of 6.5 parts acetonitrile-methanol (4:1, v/v) to 1 part sample volume resulted in sufficient retention (96.0% to 99.5%) of acylcarnitines to the silica-gel columns. In particular, with regard to the retention of long-chain acylcarnitines, the use of acetonitrile-methanol in a ratio of 4:1 (v/v) was superior to the volume ratio of 3:1 suggested by Vernez, Wenk, and Krähenbühl (29). We found the elution of acylcarnitines from the column to be most efficient at four times the column volume (1 ml), containing two steps (1 ml) with methanol 100% followed by two steps (1 ml) with methanol-H2O 1:2. Methanol 100% disrupted the ion-exchange interaction between the silica-gel column and predominantly the long-chain acylcarnitines, leading to their elution in the first two fractions, whereas the subsequent use of a more-polar solvent resulted in elution of mainly short-chain acylcarnitines in the following two fractions. The medium-chain acylcarnitines were recovered in all four fractions in almost equal amounts.
The acylcarnitine species subsequently quantified by ESI-MS/MS were normalized to CS activity, a stable indicator of mitochondrial mass (33). This was preferred to cellular protein concentration in order to avoid artifacts due to possible variations in cell size and thus varying protein-mitochondria relation.
Validation of the assay
The recovery of the acylcarnitines by the extraction method was estimated by adding deuterated internal standards either at the beginning or at the end of the procedure (set at 100%), and by calculating the differences. The extraction efficiencies for D3-C0, D3-C4, D3-C8, and D3-C16 were all ≥75%.
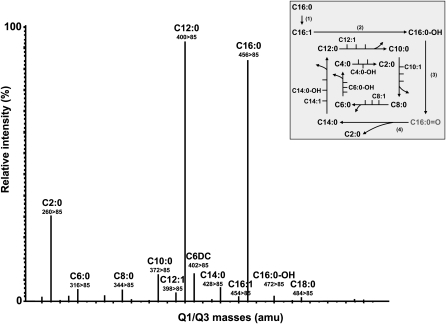
The LOD and the LOQ were determined by treating blank samples (without cells; n = 10) in the same way as samples after cell incubation and defined as LOD = mean + 3 SD and LOQ = mean + 10 SD (34). Abundance of acylcarnitine species after palmitic acid load by MS/MS and their LODs and LOQs are presented in Table 1. Out of 43 theoretically measurable acylcarnitines, 13 were below the detection limit, including dicarboxylic (DC) long-chain and hydroxylic (OH) species and unsaturated acylcarnitines with two double bonds. Metabolites were defined as “low-abundant” when they were detected above the LOD (>1.0 to 4.5 times LOD) but were below the LOQ, again comprising various DC, OH, and unsaturated acylcarnitines (n = 10). Acylcarnitines directly derived from enzymatic conversions within each palmitate β-oxidation cycle (Fig. 2, insert) were detected in considerably higher amounts (“medium-abundant” and “high-abundant”; n = 20, Table 1). Saturated long-, medium-, and short-chain acylcarnitines generated after each completed cycle of palmitate oxidation and long-chain mono-unsaturated and OH species of the first two cycles were detected in highest amounts and categorized as “high-abundant” (>10 times LOD, >5 times LOQ). “Medium-abundant” metabolites were quantified in amounts clearly above detection limits (>4.5 to 10 times LOD, >1.5 to 5 times LOQ), comprising C18 species and more-distal FAO acylcarnitine products (medium- and short-chain species) (Table 1; Fig. 2). In the presence of excess palmitic acid, the C18 species are derived from elongation and desaturation reactions resulting in stearic acid (C18:0) and oleic acid (C18:1) and subsequent β-oxidation of these FAs.
Fig. 2.
Multiple reaction monitoring (MRM) transitions of acylcarnitines derivatized as their butyl esters following loading with palmitic acid in the in situ permeabilization metabolite assay. Primary human fibroblasts were loaded with palmitic acid (100 µM, 120 min). The signals represent molecular ions of acylcarnitine butyl ester derivatives detected by applying ESI-MS/MS in the MRM mode. Quadrupole Q1/Q3 transitions were monitored by the acylcarnitine-specific daughter ion of 85 amu. The inserted scheme shows the metabolites of mitochondrial palmitic acid β-oxidation, as detected by the in situ permeabilization ESI-MS/MS method. Four enzymatic reactions are involved in each β-oxidation cycle, resulting in consecutive dehydrogenation (1), hydration (2), dehydrogenation (3), and thiolytic cleavage (4) reactions, to generate acetyl-CoA (C2:0) and a new acyl-CoA of two less carbon atoms than the original one. C16:0 = O indicated in gray is not part of the analyzed acylcarnitine panel but is displayed in order to indicate one complete β-oxidation cycle.
The inter-assay variation [coefficient of variation (CV); n = 6] was determined in the fibroblast cell line during the course of 2 weeks and was calculated at 34%, 36%, and 40% for high-abundant long-, medium-, and short-chain species, respectively, whereas for medium-abundant acylcarnitines, corresponding CVs were 37%, 43%, and 42%. The average intra-assay variation (n = 11) for high-abundant acylcarnitines was 9%, 20%, and 10% for long-, medium-, and short-chain species, respectively, whereas for medium-abundant acylcarnitines, corresponding CVs were 19%, 28%, and 21%. Although the method comprises a series of steps throughout the protocol, potentially all that cause variability, particularly factors relating to cell culture conditions must be controlled. For example, cell confluence and the ratio of accessible cell membranes to digitonin concentration might have an impact on the substrate concentration available at the mitochondrial site. Thus, the procedure requires that standardization of cell culture conditions and appropriate controls be carried along within each single experiment.
Substrate concentrations and time course
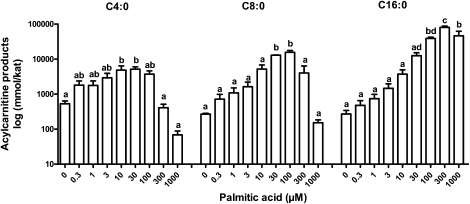
To define an appropriate substrate concentration, various palmitate concentrations were evaluated (Fig. 3). With increasing chain length, a saturating plateau of the produced acylcarnitines was reached at increasingly higher palmitate concentrations and was found to be 30 µM for C4:0-carnitine, 100 µM for C8:0-carnitine, and 300 µM for C16:0-carnitine (Fig. 3). At concentrations of 300 µM and greater, the production of medium- and short-chain metabolites was significantly impaired, possibly due to substrate inhibition (35). Time experiments to define metabolite saturation revealed a near-plateau situation after an incubation time of 60 min for medium- and short-chain acylcarnitines and after 90 min for long-chain acylcarnitines, whereas a plateau was reached at 120 min for all chain length species (data not shown). Therefore, saturating conditions of 120 min incubation with 100 µM palmitate were chosen for all loading experiments. A typical profile of multiple reaction monitoring transitions of acylcarnitines is shown in Fig. 2.
Fig. 3.
Concentration profile of various acylcarnitine products of palmitate oxidation, analyzed by the in situ permeabilization metabolite assay. Primary human fibroblasts were loaded with eight different concentrations of palmitic acid or incubated with control medium containing no palmitic acid (0 µM) for 120 min. Representative acylcarnitine products of short- (C4:0), medium- (C8:0), and long-chain (C16:0) species are depicted. Three independent experiments were performed in triplicates each. Results are presented as mean ± SEM. For each metabolite, values not sharing a common letter are significantly different (P < 0.05).
Application potential of the assay
In addition to utilization in fibroblasts, the in situ permeabilization assay is also applicable to a wide range of various cell cultures, in both adherent and suspension cells, which have been shown to be amenable to permeabilization (36, 37). In our own experience, those include differentiated 3T3-L1 adipocytes (see supplementary Fig. I) and primary human adipocytes, an intestinal epithelial cell line (Mode-K), and Epstein-Barr virus-transformed lymphoblasts (data not shown). In addition to palmitic acid, other substrates were successfully utilized, including oleic acid (C18:1) to study mitochondrial unsaturated FAO and 2-oxoisocaproic acid to study branched-chain amino acid catabolism (data not shown).
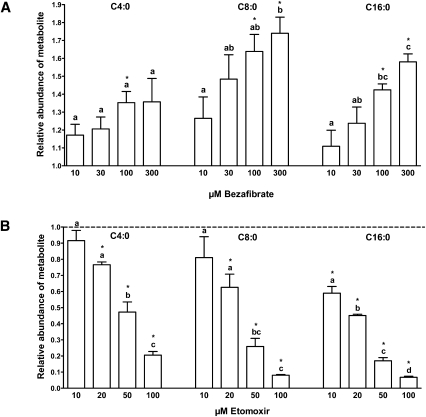
To investigate the potential of detecting drug-induced effects on FAO for the purpose of “mode of action” screening, cells were exposed to pharmacological interventions resulting in known stimulating or inhibiting effects on FAO. Bezafibrate, broadly used as a hypolipidemic drug, is a peroxisomal proliferator-activated receptor (PPAR) agonist known to increase palmitate oxidation in human fibroblasts as measured, e.g., by the 3H2O release method (38). In our assay, bezafibrate led to increases in metabolite abundance as compared with controls, irrespective of acylcarnitine chain length (Fig. 4A). However, concentration-dependent effects of bezafibrate on palmitate oxidation were strongest in acylcarnitines of long-chain species and weaker or negative in medium- and short-chain acylcarnitines in human fibroblasts. This is in accordance with previous results on the drug's PPAR-agonistic stimulation of enzymes predominantly involved in long-chain FAO, such as the carnitine palmitoyl-transferases (CPTs) and very-long-chain acyl-CoA dehydrogenase, whereas upregulating effects on the expression of several other FAO enzymes, including medium-chain acyl-CoA dehydrogenase, were less pronounced (38, 39).
Fig. 4.
Pharmacological treatment with the PPAR agonist bezafibrate and the CPT1 inhibitor etomoxir followed by palmitate loading. Representative acylcarnitine products of short- (C4:0), medium- (C8:0), and long-chain (C16:0) species are depicted. Three independent experiments were performed in triplicates each. Results are presented as mean ± SEM. Statistically significant differences of pharmacological treatments relative to the untreated control are indicated by an asterisk (P < 0.05). For each metabolite, values not sharing a common letter indicate statistically significant differences in metabolite abundance between concentrations (P < 0.05). A: Stimulation of FA β-oxidation following pretreatment with the PPAR agonist bezafibrate and subsequent palmitate loading (100 µM, 120 min). Bezafibrate was dissolved in DMSO; the final concentration of DMSO per well was 0.05%. Primary human fibroblasts (105 cells/well) were incubated with increasing bezafibrate concentrations (10, 30, 100, 300 µM) for 48 h (38) in DMEM (1 g/l glucose) and 0.25% FA-free BSA. Results are expressed as fold increase in metabolite abundance compared with the untreated control (0.05% DMSO). B: Inhibition of FA β-oxidation following pretreatment with the CPT1 inhibitor etomoxir and subsequent palmitate loading (100 µM, 120 min). Etomoxir was dissolved in H2O. Prior to permeabilization, fibroblasts (105 cells/well) were pretreated with different etomoxir concentrations (10, 20, 50, 100 µM) for 30 min (42) in DMEM (1 g/l glucose) and 0.25% FA-free BSA. Results are expressed as fold decrease in metabolite abundance compared with the untreated control (H2O; indicated by the dotted line).
To investigate opposite regulatory effects on FAO, etomoxir was applied. Etomoxir has been shown to irreversibly inhibit the activity of carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme of FAO (Fig. 1), in primary human fibroblasts (40). In our experiments, pretreatment of cells with etomoxir resulted in a concentration-dependent reduction of the abundance of FAO metabolites directly derived from enzymatic conversions within the palmitate β-oxidation (Fig. 4B). In addition, this example shows that the method allows quantitating differential drug effects on acylcarnitines of various chain lengths. A strong inhibition of long-chain acylcarnitine production was evident even in low concentrations of etomoxir, whereas this effect subsequently diminished toward medium- and short-chain acylcarnitine generation. The metabolite pattern thus clearly reflects the pharmacological function of etomoxir of blocking mitochondrial long-chain FAO regulated by CPT1, but not primarily affecting oxidation of medium- or short-chain FAs, which can pass the mitochondrial membranes directly (41).
In conclusion, the in situ assay described in this work allows highly sensitive and in particular fast monitoring of FAO function of intact organelles of various cell types, resulting in comprehensive high content information on FAO pathways. Compared with previous methods, this ESI-MS/MS-based assay specifically avoids the requirements of large amounts of cells, extensive cell homogenization techniques, long incubation times, and high costs due to radioactive substrates. The method enables quantitative investigations of the regulatory effects of pharmacological and potentially other agents, nutritive or chemopreventive, on the catabolism of FAs and thus has the potential to facilitate the development of new therapeutic strategies for states of β-oxidation dysfunctions, such as in obesity and insulin resistance.
Supplementary Material
Acknowledgments
The authors thank Sandra Sonnenschein for technical support.
Footnotes
Abbreviations:
- CPT1
- carnitine palmitoyltransferase 1
- CS
- citrate synthase
- CV
- coefficient of variation
- DC
- dicarboxylic
- D3-C0
- l-[2H3]carnitine
- D3-C4
- l-[2H3]butyrylcarnitine
- D3-C8
- l-[2H3]octanoylcarnitine
- D3-C16
- l-[2H3]palmitoylcarnitine
- FAO
- FA β-oxidation
- HCl
- hydrochloric acid
- LOD
- limit of detection
- LOQ
- limit of quantification
- MBCD
- methyl-β-cyclodextrin
- MRM
- multiple reaction monitoring
- OH
- hydroxylic
- PPAR
- peroxisomal proliferator-activated receptor
This work was supported by German Federal Ministry of Education and Research Grant 0315088 (R.E., S.C.S., B.F.) and by a grant of the “Hochschul-und Wissenschaftsprogramm” of the Ludwig-Maximilians-Universität München (N.C.S.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Eaton S., Bartlett K., Pourfarzam M. 1996. Mammalian mitochondrial beta-oxidation. Biochem. J. 320: 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matern D., Cuthbert C. D., Tortorelli S., Rinaldo P.2008. Inherited abnormalities in mitochondrial fatty acid oxidation. In Walker's Pediatric Gastrointestinal Disease. R. E. Kleinman, O. J. Goulet, G. M. Vergani, et al., editors. BC Decker, Inc., Hamilton, Ontario. 1287–1307.
- 3.Muoio D. M., Newgard C. B. 2008. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 193–205 [DOI] [PubMed] [Google Scholar]
- 4.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., et al. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7: 45–56 [DOI] [PubMed] [Google Scholar]
- 5.Kølvraa S., Gregersen N., Christensen E., Hobolth N. 1982. In vitro fibroblast studies in a patient with C6-C10-dicarboxylic aciduria: evidence for a defect in general acyl-CoA dehydrogenase. Clin. Chim. Acta. 126: 53–67 [DOI] [PubMed] [Google Scholar]
- 6.Saudubray J. M., Coude F. X., Demaugre F., Johnson C., Gibson K. M., Nyhan W. L. 1982. Oxidation of fatty acids in cultured fibroblasts: a model system for the detection and study of defects in oxidation. Pediatr. Res. 16: 877–881 [DOI] [PubMed] [Google Scholar]
- 7.Rhead W. J., Amendt B. A., Fritchman K. S., Felts S. J. 1983. Dicarboxylic aciduria: deficient [1-14C]octanoate oxidation and medium-chain acyl-CoA dehydrogenase in fibroblasts. Science. 221: 73–75 [DOI] [PubMed] [Google Scholar]
- 8.Manning N. J., Olpin S. E., Pollitt R. J., Webley J. 1990. A comparison of [9,10-3H]palmitic and [9,10-3H]myristic acids for the detection of defects of fatty acid oxidation in intact cultured fibroblasts. J. Inherit. Metab. Dis. 13: 58–68 [DOI] [PubMed] [Google Scholar]
- 9.Kler R. S., Jackson S., Bartlett K., Bindoff L. A., Eaton S., Pourfarzam M., Frerman F. E., Goodman S. I., Watmough N. J., Turnbull D. M. 1991. Quantitation of acyl-CoA and acylcarnitine esters accumulated during abnormal mitochondrial fatty acid oxidation. J. Biol. Chem. 266: 22932–22938 [PubMed] [Google Scholar]
- 10.Schaefer J., Pourfarzam M., Bartlett K., Jackson S., Turnbull D. M. 1995. Fatty acid oxidation in peripheral blood cells: characterization and use for the diagnosis of defects of fatty acid oxidation. Pediatr. Res. 37: 354–360 [DOI] [PubMed] [Google Scholar]
- 11.Pourfarzam M., Schaefer J., Turnbull D. M., Bartlett K. 1994. Analysis of fatty acid oxidation intermediates in cultured fibroblasts to detect mitochondrial oxidation disorders. Clin. Chem. 40: 2267–2275 [PubMed] [Google Scholar]
- 12.Law L. K., Tang N. L., Hui J., Ho C. S., Ruiter J., Fok T. F., Wanders R. J., Lam C. W. 2007. A novel functional assay for simultaneous determination of total fatty acid beta-oxidation flux and acylcarnitine profiling in human skin fibroblasts using (2)H(31)-palmitate by isotope ratio mass spectrometry and electrospray tandem mass spectrometry. Clin. Chim. Acta. 382: 25–30 [DOI] [PubMed] [Google Scholar]
- 13.Roe D. S., Yang B. Z., Vianey-Saban C., Struys E., Sweetman L., Roe C. R. 2006. Differentiation of long-chain fatty acid oxidation disorders using alternative precursors and acylcarnitine profiling in fibroblasts. Mol. Genet. Metab. 87: 40–47 [DOI] [PubMed] [Google Scholar]
- 14.Okun J. G., Kölker S., Schulze A., Kohlmüller D., Olgemöller K., Lindner M., Hoffmann G. F., Wanders R. J., Mayatepek E. 2002. A method for quantitative acylcarnitine profiling in human skin fibroblasts using unlabelled palmitic acid: diagnosis of fatty acid oxidation disorders and differentiation between biochemical phenotypes of MCAD deficiency. Biochim. Biophys. Acta. 1584: 91–98 [DOI] [PubMed] [Google Scholar]
- 15.Shen J. J., Matern D., Millington D. S., Hillman S., Feezor M. D., Bennett M. J., Qumsiyeh M., Kahler S. G., Chen Y. T., Van Hove J. L. 2000. Acylcarnitines in fibroblasts of patients with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and other fatty acid oxidation disorders. J. Inherit. Metab. Dis. 23: 27–44 [DOI] [PubMed] [Google Scholar]
- 16.Nada M. A., Chace D. H., Sprecher H., Roe C. R. 1995. Investigation of beta-oxidation intermediates in normal and MCAD-deficient human fibroblasts using tandem mass spectrometry. Biochem. Mol. Med. 54: 59–66 [DOI] [PubMed] [Google Scholar]
- 17.Tyni T., Pourfarzam M., Turnbull D. M. 2002. Analysis of mitochondrial fatty acid oxidation intermediates by tandem mass spectrometry from intact mitochondria prepared from homogenates of cultured fibroblasts, skeletal muscle cells, and fresh muscle. Pediatr. Res. 52: 64–70 [DOI] [PubMed] [Google Scholar]
- 18.Janski A. M., Cornell N. W. 1980. Subcellular distribution of enzymes determined by rapid digitonin fractionation of isolated hepatocytes. Biochem. J. 186: 423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fingerhut R., Röschinger W., Muntau A. C., Dame T., Kreischer J., Arnecke R., Superti-Furga A., Troxler H., Liebl B., Olgemöller B., et al. 2001. Hepatic carnitine palmitoyltransferase I deficiency: acylcarnitine profiles in blood spots are highly specific. Clin. Chem. 47: 1763–1768 [PubMed] [Google Scholar]
- 20.Fingerhut R., Ensenauer R., Röschinger W., Arnecke R., Olgemöller B., Roscher A. A. 2009. Stability of acylcarnitines and free carnitine in dried blood samples: implications for retrospective diagnosis of inborn errors of metabolism and neonatal screening for carnitine transporter deficiency. Anal. Chem. 81: 3571–3575 [DOI] [PubMed] [Google Scholar]
- 21.Srere P. A., Brazil H., Gonen L. 1963. The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem. Scand. 17: S129–S134 [Google Scholar]
- 22.Team R. D. C.2011. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- 23.Wanders R. J., Kos M., Roest B., Meijer A. J., Schrakamp G., Heymans H. S., Tegelaers W. H., van den Bosch H., Schutgens R. B., Tager J. M. 1984. Activity of peroxisomal enzymes and intracellular distribution of catalase in Zellweger syndrome. Biochem. Biophys. Res. Commun. 123: 1054–1061 [DOI] [PubMed] [Google Scholar]
- 24.Richieri G. V., Ogata R. T., Kleinfeld A. M. 1992. A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. J. Biol. Chem. 267: 23495–23501 [PubMed] [Google Scholar]
- 25.Ulloth J. E., Casiano C. A., De Leon M. 2003. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J. Neurochem. 84: 655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunaldi K., Huang N., Hamilton J. A. 2010. Fatty acids are rapidly delivered to and extracted from membranes by methyl-beta-cyclodextrin. J. Lipid Res. 51: 120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pande S. V. 1975. A mitochondrial carnitine acylcarnitine translocase system. Proc. Natl. Acad. Sci. USA. 72: 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minkler P. E., Ingalls S. T., Hoppel C. L. 2005. Strategy for the isolation, derivatization, chromatographic separation, and detection of carnitine and acylcarnitines. Anal. Chem. 77: 1448–1457 [DOI] [PubMed] [Google Scholar]
- 29.Vernez L., Wenk M., Krähenbühl S. 2004. Determination of carnitine and acylcarnitines in plasma by high-performance liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun. Mass Spectrom. 18: 1233–1238 [DOI] [PubMed] [Google Scholar]
- 30.Poorthuis B. J., Jille-Vlckova T., Onkenhout W. 1993. Determination of acylcarnitines in urine of patients with inborn errors of metabolism using high-performance liquid chromatography after derivatization with 4’-bromophenacylbromide. Clin. Chim. Acta. 216: 53–61 [DOI] [PubMed] [Google Scholar]
- 31.Minkler P. E., Hoppel C. L. 1993. Quantification of carnitine and specific acylcarnitines by high-performance liquid chromatography: application to normal human urine and urine from patients with methylmalonic aciduria, isovaleric acidemia or medium-chain acyl-CoA dehydrogenase deficiency. J. Chromatogr. 613: 203–221 [DOI] [PubMed] [Google Scholar]
- 32.Minkler P. E., Hoppel C. L. 1993. Quantification of free carnitine, individual short- and medium-chain acylcarnitines, and total carnitine in plasma by high-performance liquid chromatography. Anal. Biochem. 212: 510–518 [DOI] [PubMed] [Google Scholar]
- 33.Patti M. E., Corvera S. 2010. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 31: 364–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long G. L., Winefordner J. D. 1983. Limit of detection: a closer look at the IUPAC definition. Anal. Chem. 55: 712A–724A [Google Scholar]
- 35.Passeron S., Savageau M. A., Harary I. 1968. Optimal conditions for palmitate oxidation by rat heart homogenates. Arch. Biochem. Biophys. 128: 124–128 [DOI] [PubMed] [Google Scholar]
- 36.King M. A., Eddaoudi A., Davies D. C. 2007. A comparison of three flow cytometry methods for evaluating mitochondrial damage during staurosporine-induced apoptosis in Jurkat cells. Cytometry A. 71: 668–674 [DOI] [PubMed] [Google Scholar]
- 37.Selbert S., Fischer P., Pongratz D., Stewart M., Noegel A. A. 1995. Expression and localization of annexin VII (synexin) in muscle cells. J. Cell Sci. 108: 85–95 [DOI] [PubMed] [Google Scholar]
- 38.Djouadi F., Aubey F., Schlemmer D., Ruiter J. P., Wanders R. J., Strauss A. W., Bastin J. 2005. Bezafibrate increases very-long-chain acyl-CoA dehydrogenase protein and mRNA expression in deficient fibroblasts and is a potential therapy for fatty acid oxidation disorders. Hum. Mol. Genet. 14: 2695–2703 [DOI] [PubMed] [Google Scholar]
- 39.Bonnefont J. P., Bastin J., Behin A., Djouadi F. 2009. Bezafibrate for an inborn mitochondrial beta-oxidation defect. N. Engl. J. Med. 360: 838–840 [DOI] [PubMed] [Google Scholar]
- 40.Caspary F., Elliott G., Nave B. T., Verzaal P., Rohrbach M., Das P. K., Nagelkerken L., Nieland J. D. 2005. A new therapeutic approach to treat psoriasis by inhibition of fatty acid oxidation by Etomoxir. Br. J. Dermatol. 153: 937–944 [DOI] [PubMed] [Google Scholar]
- 41.Aas M. 1971. Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim. Biophys. Acta. 231: 32–47 [DOI] [PubMed] [Google Scholar]
- 42.Sandhir R., Khan M., Chahal A., Singh I. 1998. Localization of nervonic acid beta-oxidation in human and rodent peroxisomes: impaired oxidation in Zellweger syndrome and X-linked adrenoleukodystrophy. J. Lipid Res. 39: 2161–2171 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.