Abstract
Eicosanoids are key mediators and regulators of inflammation and oxidative stress often used as biomarkers for diseases and pathological conditions such as cardiovascular and pulmonary diseases and cancer. Analytically, comprehensive and robust quantification of different eicosanoid species in a multi-method approach is problematic because most of these compounds are relatively unstable and may differ in their chemical properties. Here we describe a novel ultra-performance liquid chromatography-selected reaction monitoring mass spectroscopy (UPLC-SRM/MS) method for simultaneous quantification of key urinary eicosanoids, including the prostaglandins (PG) tetranor PGE-M, 8-iso-, and 2,3-dinor-8-iso-PGF2α; the thromboxanes (TXs) 11-dehydro- and 2,3-dinor-TXB2; leukotriene E4; and 12-hydroxyeicosatetraenoic acid. In contrast to previous methods, which used time-consuming and complex solid phase extraction, we prepared samples with a simple liquid/liquid extraction procedure. Because collision-induced dissociation produced characteristic product ions for all analytes, no derivatization step for SRM/MS analysis was necessary. Analytes were separated with a short UPLC reversed-phase column (1.7 µm particles), allowing shorter run times than conventional HPLC columns. The method was validated and applied to human urine samples showing excellent precision, accuracy, detection limits, and robustness. In summary, the developed method allows robust and sensitive profiling of urinary eicosanoid species, making it a useful and valuable tool for biomarker profiling in clinical/toxicological studies.
Keywords: ultraperformance liquid chromatography-selected reaction monitoring mass spectroscopy, eicosanoids, urine, biomarkers, LC-MS/MS
Eicosanoids are signaling molecules produced and secreted from many cells types under normal and pathophysiological conditions. In humans these metabolites are excreted to body fluids, such as plasma and urine (1). Precursors are 20-carbon PUFAs such as arachidonic acid (C20:4) esterified to phospholipids of cell membranes (2). Fatty acids (FAs) are mobilized from phospholipids by phospholipase A2 before they are available for further metabolism to eicosanoids (3). Therefore, FA membrane composition (i.e., the PUFA content) is cell type specific and therefore is crucial for cellular functions and influenced by nutrition (4–8).
Eicosanoid species have various and diverse physiological effects. However, the n-6 fatty acid arachidonic acid (C20:4) is a well known eicosanoid precursor giving rise to mainly proinflammatory eicosanoids, including prostaglandins (PGs) (two series), thromboxanes (TXs) (two series), LTs (four series), and hydroxyeicosatetraenoic acid (HETE) derivates (2, 9). Concentrations are elevated in various diseases and pathophysiological conditions, including cardiovascular disease (CVD), type-1/type-2 diabetes, obesity, bowel and pulmonary diseases, neurodegenerative diseases, multiple sclerosis, cystic fibrosis, and several forms of cancer (1).
Key urinary eicosanoid species produced from arachidonic acid related to proinflammatory states or oxidative stress are the following: A) tetranor PGE-M, a stable metabolite of PGE2 (present in plasma, relatively instable) synthesized by cyclooxygenase (COX)-1 and COX-2 (9, 10). Urinary levels are elevated in cancer patients (e.g., patients with lung cancer) (11). B) 8-iso-PGF2α (also named 8-epi-PGF2α, 8-isoprostane, or iPF2α-III) is probably the most popular eicosanoid molecule used as biomarker for oxidative stress because it has been validated (12). It is generated nonenzymatically by free radical-mediated oxidation of arachidonic acid (10). It is also present in plasma but is relatively unstable (13). Increased levels can be found in patients with neurodegenerative diseases or type-1/type-2 diabetes (14–16). C) 2,3-dinor-8-iso-PGF2α, a β-oxidation product of 8-iso-PGF2 (17). D) 2,3-dinor-TXB2 and E) 11-dehydro-TXB2, stable urinary metabolites of TXA2 produced by COX-1 (10, 18). Levels are elevated in diabetes patients and assumed to reflect platelet activation (16, 19, 20). F) LTE4, a metabolite of LTD4 formed from LTC4; leukotriene synthesis is regulated by 5-lipoxygenase (10, 21). LTE4 is used as biomarker to predict asthma and is believed to reflect the whole body LT status (22, 23). G) 12-HETE generated by 12-lipoxygenase (10). Its concentrations are increased in female diabetes patients and hypertension patients (24, 25). Similar to 11-dehydro-TXB2, it is assumed that increased 12-HETE levels are associated with platelet activation (26).
Urinary eicosanoids are commonly determined by GC-MS or LC-MS/MS, often requiring a complex and time-consuming sample extraction and derivatization. Although there are existing methods for profiling of single urinary eicosanoid classes/species, methods for quantification of urinary eicosanoid species generated from different pathways are rare.
The aim of this study was to develop a simple, robust, and sensitive method for quantification of urinary eicosanoid species generated from different pathways (particularly for the metabolites described above) by LC-MS/MS applicable in clinical studies. The novel method was validated in human urine and has been applied to a series of smoker urine samples because it is known that cigarette smoking and nicotine consumption lead to elevated urinary levels of eicosanoids with proinflammatory effects (27).
MATERIALS AND METHODS
Chemicals, solutions, and study samples
Acetic acid (≥ 99%), ammonium hydroxide (28% in water), creatinine (anhydrous), formic acid (≥ 95%), hydrochloric acid (∼37%), picric acid (1% in water), sodium hydroxide (≥ 97%, pellets), 0.1% formic acid in water (LC-MS grade), and 0.1% formic acid in acetonitrile (LC-MS grade) were purchased from Sigma-Aldrich (Munich, Germany). Chloroform (picograde), ethyl acetate (optigrade), methanol (optigrade), and water (optigrade) were obtained from LGC Standards (Wesel, Germany). Tetranor PGE-M, 2,3-dinor-8-iso-PGF2α, 8-iso-PGF2α, 2,3-dinor-TXB2, 11-dehydro-TXB2, LTE4, 12(S)-HETE, D6-tetranor-PGE-M, D4-8-iso-PGF2α, D4-11-dehydro-TXB2, D5-LTE4, and D8-12(S)-HETE were purchased from Biomol (Hamburg, Germany) with purities higher than 97%. All substances were stored at −40°C. Working solutions of the desired concentrations were prepared by dilution in methanol. Urine samples were obtained from a dietary controlled trial with healthy subjects. Informed consent and approval from the ethics committee of the Bavarian State Board of Physicians was obtained.
Sample preparation
Unless otherwise indicated, aliquots of 3 ml urine were used for analysis. Before extraction, 20 µl of acetic acid and 30 µl of an internal standard (IS) mixture, containing 6 ng D6-tetranor-PGE-M, 6 ng D4-8-iso-PGF2α, 6 ng D4-11-dehydro-TXB2, 1.5 ng D5-LTE4, and 1.5 ng D8-12(S)-HETE, were added to each sample.
We applied a modified liquid-liquid extraction (LLE) technique described by Bligh and Dyer (28). Accordingly, 11.25 ml B and D solution (methanol:chloroform 2:1 v/v) was added to each sample. After mixing the components vigorously, the sample was left at room temperature for 1 h. Next, 3.75 ml chloroform and 3.75 ml water were added, and the sample was mixed for some seconds and centrifuged for 10 min at 2500 rpm. The recovered chloroform phase was evaporated to dryness in a SpeedVac centrifuge (Thermo Scientific, Dreieich, Germany). The residue was dissolved in 100 µl methanol.
For testing purposes, different solid phase extraction (SPE) approaches were performed. For C18 reversed-phase (RP) SPE, a Bond Elut C18 (500 mg, 3 ml) cartridge was used (Agilent, Waldbronn, Germany). The sorbent was rinsed with 5 ml methanol and 5 ml water. After applying the sample, the sorbent was rinsed again with 5 ml water. Washing was performed with 3 ml 5% methanol in water. After drying the sorbent under vacuum, the sample was eluted with 4 ml methanol and evaporated to dryness in a SpeedVac centrifuge. The residue was dissolved in 100 µl methanol.
For polymeric SPE (I), an Oasis® HLB cartridge (500 mg, 6 ml) was used (Waters, Eschborn, Germany). Extraction was carried out as described by Neale and Dean (29). After rinsing the sorbent with 5 ml methanol, 5 ml acetonitrile, and 5 ml water, the sample was applied. The sorbent was washed with 3 ml 5% acetonitrile in water. After drying the sorbent under vacuum, elution was performed with 4 ml acetonitrile. The sample was evaporated to dryness in a SpeedVac centrifuge and dissolved in 100 µl methanol.
For polymeric SPE (II), a Strata × 33 u cartridge (200 mg, 6 ml) was used (Phenomenex, Aschaffenburg, Germany), and extraction was performed according to the protocol from Dumlao and colleges (30). After the sorbent was rinsed with 5 ml methanol and 5 ml water, the sample was loaded. Next, it was washed with 3 ml 10% methanol in water. After drying the sorbent under vacuum, elution was performed with 4 ml methanol. The sample was evaporated to dryness in a SpeedVac centrifuge and dissolved in 100 µl methanol.
For polymeric RP/weak anion exchange SPE, an Easy cartridge (200 mg, 6 ml) was used (Macherey-Nagel, Düren, Germany); for polymeric RP/strong anion exchange SPE, an Oasis® MAX cartridge (500 mg, 6 ml) was used (Waters, Eschborn, Germany). Extraction was performed for both SPE cartridges as described by Dahl and colleges (31). The sorbents were rinsed with 5 ml methanol containing 2% formic acid and 5 ml water. After applying the sample, washing was performed with 5 ml water, 3 ml 25% methanol in water, and 3 ml acetonitrile. The sorbent was dried under vacuum, and elution was performed with 2 × 2 ml methanol. The sample was evaporated to dryness in a SpeedVac centrifuge and dissolved in 100 µl methanol.
Ultraperformance liquid chromatography-selected reaction monitoring mass spectroscopy analysis
The analysis was carried out on a triple quadrupole mass spectrometer API 5000 (AB Sciex, Darmstadt, Germany) LC-MS/MS system, equipped with a 1200 series binary pump (G1312B), a degasser (G1379B), and a column oven (G1316B) (Agilent, Waldbronn, Germany) connected to an HTC Pal autosampler (CTC Analytics, Zwingen, Switzerland). A Turbo V ion spray source operating in negative electrospray ionization (ESI) mode was used for detection (AB Sciex, Darmstadt, Germany). High-purity nitrogen was generated by a nitrogen generator NGM 22-LC/MS (cmc Instruments, Eschborn, Germany).
Chromatographic separation was performed on a Waters (Eschborn, Germany) Acquity ultraperformance liquid chromatography (UPLC) BEH C18 column (2.1 × 50 mm) with a 1.7 µm particle size. The column was maintained at 30°C, and the injection volume was set to 5 µl. Eluent A consisted of 0.1% formic acid in water; eluent B was 0.1% formic acid in acetonitrile. Gradient elution was performed with 5% B for 1 min, a linear increase to 53% B until 9.5 min, a linear increase to 76% B until 11 min, a step to 100% B until 11.1 min, hold for 1 min at 100% B, and reequilibration from 12.1 min to 14 min with 5% B. The flow rate was set to 600 µl/min. The turbo ion spray source settings were as follows: ion spray voltage = −4 kV, heater temperature = 600°C, source gas 1 = 20 psi, source gas 2 = 5 psi, CAD gas = 5 psi, and curtain gas = 40 psi. Analytes were monitored in the multiple reaction monitoring mode. The MS program was separated into five periods as shown in Table 1. Quadrupoles were working at unit resolution.
TABLE 1.
MS parameters and RTs (turbo spray IS-voltage −4 kV; source temperature 600°C)
| MW | MRM | IS MRM | Dwell time | DP | CE | RT | MS period | |
| g/mol | m/z | m/z | ms | V | V | min | ||
| Tetranor PGE-M | 328.4 | 327.0→309.0 | 332.8→315.2 | 100 | −85 | −18 | 4.5 | 1 |
| 2,3-Dinor-8-iso-PGF2α | 326.4 | 325.0→237.3 | [D4]8-iso-PGF2α | 150 | −130 | −18 | 6.8 | 2 |
| 8-Iso-PGF2α | 354.5 | 353.1→193.0 | 357.2 → 197.0 | 100 | −150 | −36 | 7.7 | 3 |
| 2,3-Dinor-TXB2 | 342.4 | 341.1→123.2 | [D4]11-dh-TXB2 | 100 | −15 | −22 | 6.8 | 2 |
| 11-Dehydro-TXB2 | 368.5 | 367.1→305.1 | 371.1→309.1 | 100 | −110 | −22 | 8.3 | 3 |
| LTE4 | 439.6 | 438.0→333.1 | 443.2→338.1 | 150 | −115 | −28 | 9.6 | 4 |
| 12-HETE | 320.5 | 319.0→179.0 | 327.1→184.0 | 100 | −135 | −22 | 11.9 | 5 |
CE, collision energy; DP, declustering potential; MRM, multiple reaction monitoring; RT, retention time.
Calibration and quantification
Calibration was achieved by spiking 3 ml aliquots of urine with different levels of eicosanoid standards. A seven-point calibration was performed by adding increasing amounts of each standard (see Table 3) and IS as described in the sample preparation section. Calibration curves were calculated by linear regression without weighting. Data analysis was performed with Analyst software 1.5.1 (AB Sciex, Darmstadt, Germany).
TABLE 3.
Calibration data
| LOD | LOQ | Calibration Range | IS Added | Slope | Correlation Coefficient | |
| ng/ml urine | ng/ml urine | ng/ml urine | ng/ml urine | |||
| Tetranor PGE-M | 0.05 | 0.15 | 0.2–20 | 2.0 | 0.567 | 0.999 |
| 2,3-Dinor-8-iso-PGF2α | 0.05 | 0.15 | 0.2–20 | D4-8-iso-PGF2α | 0.739 | 0.997 |
| 8-Iso-PGF2 | 0.02 | 0.06 | 0.025–2.5 | 2.0 | 0.663 | 0.999 |
| 2,3-Dinor-TXB2 | 0.05 | 0.15 | 0.25–40 | D4-11-dh-TXB2 | 0.0383 | 0.999 |
| 11-Dehydro-TXB2 | 0.06 | 0.18 | 0.2–20 | 2.0 | 0.436 | 0.997 |
| LTE4 | 0.002 | 0.006 | 0.0125–1.25 | 0.5 | 1.664 | 0.998 |
| 12-HETE | 0.002 | 0.006 | 0.025–2.5 | 0.5 | 7.012 | 0.996 |
Calibration lines were generated by plotting the ratios of the areas analyte/IS against the spiked concentrations (ng/ml). LOD, limit of detection; LOQ, limit of quantification.
Creatinine analysis
For urine flow normalization, creatinine was determined. Urine (20 µl) was diluted with 1 ml water. The sample was centrifuged at 600 rpm, and the supernatant was transferred to a 96 well plate. After adding 200 µl reaction solution (0.25 M NaOH:0.1% picric acid v/v), the plate was incubated at 36°C for 45 min. Absorption at 492 nm was analyzed with a Genios microplate reader (Tecan, Crailsheim, Germany). Each plate comprised a calibration set and solvent blanks.
Statistical analysis
The level of significance between the groups (smokers and nonsmokers) was assessed using an independent-samples Mann-Whitney U test.
RESULTS
Eicosanoid fragmentation
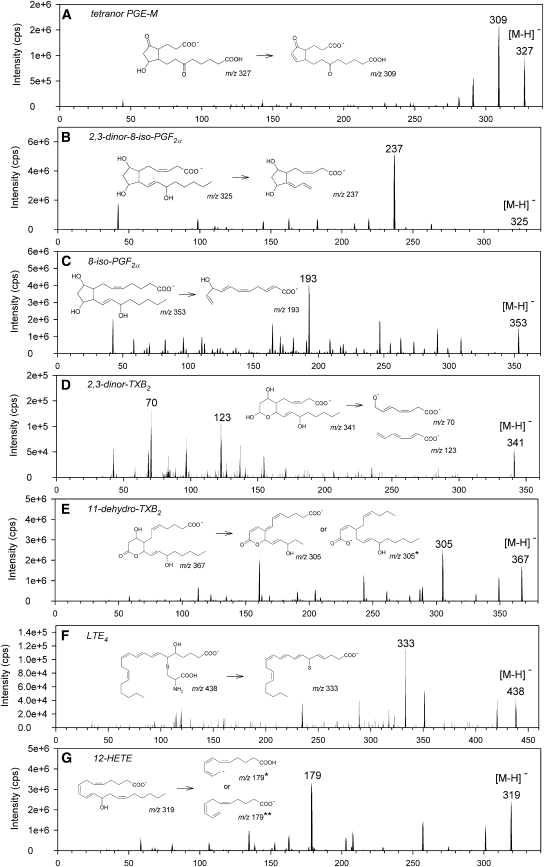
To analyze eicosanoids, we applied ESI in the negative ion mode and acquired product ion spectra. Although LTE4 could also be ionized in the positive mode, the negative ion mode was more advantageous for all other analytes (data not shown). The obtained fragmentation patterns and proposed fragmentation pathways are shown in Fig. 1. All analytes displayed [M-H]− ions.
Fig. 1.
Product ion spectra and proposed fragmentation pathways. A: Tetranor PGE-M; B: 2,3-dinor-8-iso-PGF2α; C: 8-iso-PGF2α; D: 2,3-dinor-TXB2; E: 11-dehydro-TXB2 (*described by Murphy et al. [43]). F: LTE4; G: 12-HETE (*described by Murphy et al. [43]; **described by Suzuki et al. [24]).
The most intensive product ion for tetranor PGE-M was m/z 309, resulting from loss of one water molecule (Fig. 1A). 2,3-Dinor-8-iso-PGF2α showed primarily one product ion at m/z 237 due to loss of a C5 side chain and water (Fig. 1B). 8-Iso-PGF2α forms a prominent ion at m/z 193, obtained supposedly by a complex fragmentation due to break-up of the cyclopentane ring and dispatch of a C9 side chain, water, and two protons (Fig. 1C). For 2,3-dinor-TXB2, the most prominent ion was at m/z 70, which might be a two times negatively charged product ion (m/z 140/2) generated through fracture of the tetrahydropyran ring and loss of two protons (Fig. 1D). However, for quantification, m/z 123 instead of m/z 70 was used because the appropriate mass transition showed less matrix interferences (data not shown). 11-Dehydro-TXB2 displayed a product ion at m/z 305 that might result from loss of butane and water plus two protons (Fig. 1E). LTE4 showed an ion at m/z 333 being formed by fragmentation of the amino-carboxyl moiety from the sulfur atom and dispatch of water (Fig. 1F). 12-HETE's major product ion was at m/z 179 and was probably formed through loss of a C9 side chain (Fig. 1G), which is in agreement with previous studies (24). In conclusion, we could find mass transitions for all tested compounds suitable for development of a quantitative selected reaction monitoring (SRM)/MS method because collision-induced dissociation produced characteristic product ions for all analytes (Table 1).
Eicosanoid chromatography
A proper chromatographic separation is crucial for eicosanoid analysis because these compounds often occur in isobaric forms. In addition, urine contains many interfering matrix components, leading to ion suppression and misquantification. Previous studies have shown that RP HPLC is an adequate technique for separation of most eicosanoid species (30, 32, 33). Based on these data, we established chromatographic separation using a C18 RP analytical column. In contrast to previously published methods, we chose a short UPLC column containing very small particles (50 × 2.1 mm; 1.7 µm) allowing shorter runtimes (14 min including reequilibration compared with >20 min [HPLC column; 100 × 3.0 mm; 3.5 µm]).
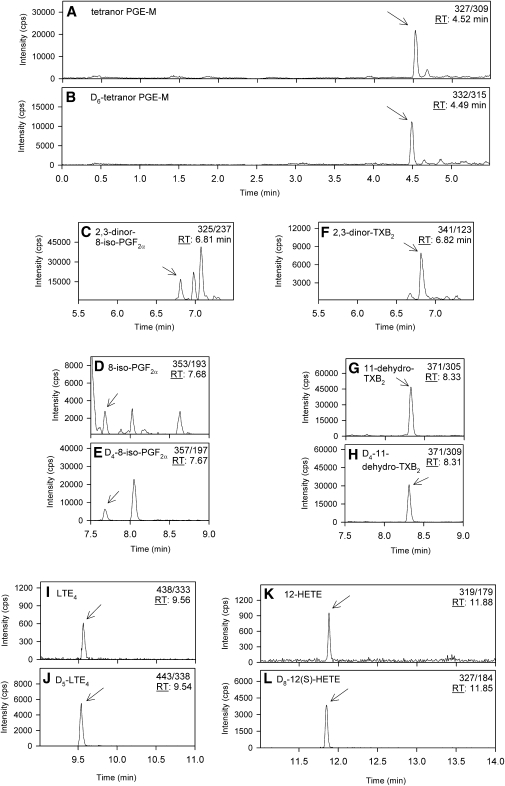
All peaks were nicely shaped, sharp, and separated from interfering matrix components (Fig. 2A–L) . We could also achieve a coelution of analyte and internal standard (IS), except for 2,3-dinor-8-iso-PGF2α and 2,3-dinor-TXB2 (no stable isotope-labeled substances available). This is important to compensate for matrix effects and varying ionization efficiencies during gradient elution. Gradient elution was performed with a mixture of acetonitrile and water, including 0.1% formic acid, which improved ionization efficiency (data not shown). Because several mass transitions are required and the peaks were sharp and narrow and therefore nicely separated, the MS program was split into five periods to gain more and shorter scan cycles per peak: 0–5.5 min (tetranor PGE-M), 5.5–7.5 min (2,3-dinor-8-iso-PGF2α; 2,3-dinor-TXB2), 7.5–9.0 min (8-iso-PGF2α; 11-dehydro-TXB2), 9.0–11.0 min (LTE4), and 11.0–14.0 min (12-HETE) (Fig. 2; Table 1).
Fig. 2.
Multiple reaction monitoring chromatograms of a human urine sample and the corresponding internal standards. A: Tetranor PGE-M (1.70 ng/ml). B: D6-Tetranor PGE-M. C: 2,3-Dinor-8-iso-PGF2α (1.21 ng/ml). D: 8-Iso-PGF2α (211.60 pg/ml). E: D4-8-iso-PGF2α. F: 2,3-Dinor-TXB2 (4.66 ng/ml). G: 11-Dehydro-TXB2 (1.59 ng/ml). H: D4-11-dehydro-TXB2. I: LTE4 (109.38 pg/ml). J: D5-LTE4. K: 12-HETE (206.90 pg/ml). L: D8-12(S)-HETE.
Sample extraction and matrix effects
To extract all analytes of interest with one common sample preparation approach, different extraction procedures were tested. Several working groups have described sample preparation approaches with SPE containing sorbents bound to carbon chain RP phases (24, 34, 35) or polymeric RP phases (29, 31, 36–39). Therefore, we tested sample preparation using C18 RP SPE and polymeric RP SPE. We also evaluated mixed-mode SPE containing polymeric RP phases combined with weak or strong anion exchange functions because most compounds have a negative charge at basic pH. Tests were carried out by adding an eiconsanoid standard mixture to a human urine pool and comparing the obtained signal intensities and signal to noise (S/N) ratios (Table 2).
TABLE 2.
Sample preparation tests using LLE or SPE
| Urine (pH∼8) |
Urine (pH∼4) |
Urine (pH∼8) |
Urine (pH∼4) |
|||||
| Signal Int. (cps) | SD | Signal Int. (cps) | SD | S/N | SD | S/N | SD | |
| Tetranor PGE-M | ||||||||
| LLE | — | — | 2,202 | 190 | — | — | 55.5 | 19.7 |
| SPE: C18 RP | — | — | 4,399 | 334 | — | — | 24.0 | 4.3 |
| SPE: pol. RP (I) | — | — | 3,311 | 196 | — | — | 26.1 | 3.6 |
| SPE: pol. RP (II) | — | — | 4,610 | 865 | — | — | 19.1 | 3.7 |
| SPE: pol. RP -A- | — | — | 1,569 | 73 | — | — | 24.2 | 11.4 |
| SPE: pol. RP -A | — | — | 5,017 | 96 | — | — | 40.5 | 12.2 |
| 2,3-Dinor-8-iso-PGF2α | ||||||||
| LLE | 519 | 46 | 7,147 | 96 | 53.0 | 8.4 | 167.7 | 60.7 |
| SPE: C18 RP | 6,949 | 148 | 6,731 | 80 | 161.9 | 22.9 | 132.6 | 13.6 |
| SPE: pol. RP (I) | 4,890 | 202 | 5,421 | 148 | 261.1 | 34.9 | 95.3 | 6.9 |
| SPE: pol. RP (II) | 8,990 | 328 | 7,059 | 482 | 152.5 | 52.3 | 165.6 | 7.6 |
| SPE: pol. RP -A- | 4,481 | 83 | 4,011 | 312 | 245.5 | 87.3 | 137.4 | 17.3 |
| SPE: pol. RP -A | — | — | 24,177 | 1,718 | — | — | 378.2 | 65.5 |
| 8-iso-PGF2α | ||||||||
| LLE | 336 | 115 | 1,307 | 371 | 21.0 | 18.6 | 54.2 | 9.4 |
| SPE: C18 RP | 1,480 | 98 | 1,396 | 8 | 36.1 | 2.5 | 29.5 | 6.4 |
| SPE: pol. RP (I) | 452 | 27 | 492 | 130 | 51.9 | 2.8 | 46.1 | 1.6 |
| SPE: pol. RP (II) | 1,645 | 164 | 1,243 | 313 | 39.2 | 14.8 | 32.8 | 5.5 |
| SPE: pol. RP -A- | 623 | 154 | 283 | 11 | 42.0 | 3.9 | 26.0 | 9.3 |
| SPE: pol. RP -A | — | — | 2,378 | 289 | — | — | 90.1 | 37.3 |
| 2,3-dinor-TXB2 | ||||||||
| LLE | 151 | 5 | 1,216 | 76 | 29.6 | 7.8 | 92.7 | 8.5 |
| SPE: C18 RP | 1,029 | 168 | 1,311 | 59 | 87.8 | 23.1 | 43.0 | 56.8 |
| SPE: pol. RP (I) | 554 | 117 | 519 | 132 | 63.7 | 4.7 | 47.4 | 0.2 |
| SPE: pol. RP (II) | 1,890 | 228 | 1,159 | 80 | 113.0 | 24.7 | 97.8 | 29.6 |
| SPE: pol. RP -A- | 678 | 57 | 456 | 39 | 77.9 | 17.3 | 39.0 | 6.5 |
| SPE: pol. RP -A | — | — | 2,161 | 272 | — | — | 95.0 | 40.1 |
| 11-dehydro-TXB2 | ||||||||
| LLE | 6,684 | 14 | 19,994 | 21 | 286.8 | 89.6 | 360.1 | 130.7 |
| SPE: C18 RP | 15,048 | 591 | 14,911 | 55 | 173.2 | 36.7 | 181.0 | 18.5 |
| SPE: pol. RP (I) | 2,884 | 227 | 5,971 | 641 | 147.5 | 20.3 | 327.5 | 1.0 |
| SPE: pol. RP (II) | 15,445 | 100 | 14,899 | 733 | 232.7 | 42.6 | 205.0 | 11.2 |
| SPE: pol. RP -A- | 4,283 | 105 | 2,104 | 81 | 212.7 | 84.6 | 59.2 | 15.1 |
| SPE: pol. RP -A | — | — | 15,412 | 1,898 | — | — | 311.0 | 138.7 |
| LTE4 | ||||||||
| LLE | 196 | 23 | 1,562 | 88 | 54.5 | 11.2 | 201.5 | 122.5 |
| SPE: C18 RP | 2,006 | 150 | 1,762 | 45 | 303.7 | 62.2 | 287.1 | 67.3 |
| SPE: pol. RP (I) | — | — | 79 | 8 | — | — | 30.7 | 5.2 |
| SPE: pol. RP (II) | 764 | 5 | 1,161 | 88 | 92.5 | 1.6 | 125.7 | 1.0 |
| SPE: pol. RP -A- | 294 | 16 | 704 | 16 | 82.3 | 3.1 | 121.8 | 10.1 |
| SPE: pol. RP -A | — | — | 1,480 | 92 | — | — | 178.4 | 113.1 |
| 12-HETE | ||||||||
| LLE | 4,119 | 228 | 4,158 | 76 | 461.9 | 118.3 | 475.5 | 17.7 |
| SPE: C18 RP | 4,399 | 194 | 3,456 | 2 | 439.1 | 201.0 | 383.7 | 20.4 |
| SPE: pol. RP (I) | — | — | — | — | — | — | — | — |
| SPE: pol. RP (II) | 4,394 | 64 | 3,580 | 13 | 271.8 | 191.1 | 416.3 | 96.7 |
| SPE: pol. RP -A- | 941 | 27 | 134 | 36 | 106.1 | 19.1 | 12.6 | 2.5 |
| SPE: pol. RP -A | — | — | 2,904 | 120 | — | — | 242.3 | 43.3 |
Displayed are mean signal intensities and S/N ratios of a urine pool spiked with 0 .5 ng/ml tetranor PGE-M, 1.0 ng/ml 2,3-dinor-8-iso-PGF2α, 0.15 ng/ml 8-iso-PGF2α, 2.0 ng/ml 2,3-dinor-TXB2, 1.0 ng/ml 11-dehydro-TXB2, 0.15 ng/ml LTE4, and 0.15 ng/ml 12(S)-HETE. SPE cartridges and applied extraction protocols are described in the Materials and Methods section. S/N was calculated with the Analyst 1.5.1 software. Underlined are the highest S/N ratios. A-, weak anion exchange function; A–, strong anion exchange function; L/L-E, liquid/liquid extraction; pol., polymeric; RP, reversed-phase; S/N, signal/noise.
We could extract all eicosanoid species (except for 12-HETE) with each tested SPE cartridge. For most eicosanoid species, an extraction at acidic pH 4 led to improved results compared with pH 8. C18 RP SPE was ideal for LTE4 and 12-HETE extraction, and polymeric RP SPE was very well suited for 2,3-dinor-TXB2 and 11-dehydro-TXB2 extraction (acidic pH, S/N). Sample preparation with mixed mode SPE containing a strong anion exchange function worked very well for most analytes; it was optimal for extraction of both PGF2α species and tetranor PGE-M (acidic pH, S/N). By contrast, mixed mode SPE containing a weak anion exchange function was not so ideal for eicosanoid extraction compared with all other tested SPEs.
Because of its easy practical viability and to save costs, we thought that a LLE procedure using chloroform according to Bligh and Dyer typically used for polar lipids like glycerophospholipids might be a suitable alternative to sample preparation with SPE (28, 40). To our knowledge, this has not been tested for urinary eicosanoid sample preparation. We found that LLE allowed extraction of all analytes, but the extraction efficiency depended on the pH of the urine matrix. The number of the extracted species increased with decreasing urinary pH (optimum at pH 4) (Table 2). Although the signal intensities for some analytes were found to be higher when using SPE, over the complete range of analytes generally the best S/N ratios were achieved with LLE (optimal for tetranor PGE-M, 11-dehydro-TXB2, and 12-HETE). Therefore, for our further investigations, samples were prepared with LLE after urine samples were acidified (pH 4).
Calibration and quantification
For quantification and to compensate for variations in sample preparation and ionization efficiency, stable isotope labeled eicosanoids were added as IS before extraction. Calibration lines were generated by adding different concentrations of eicosanoids to a human urine pool (Table 3). The ratio between analyte and IS was used for quantification. For 2,3-dinor-8-iso-PGF2α, the IS D4-8-iso-PGF2α, and for 2,3-dinor-TXB2, the IS D4-11-dehydro-TXB2 was used because stable isotope-labeled substances were not available for these compounds. Evaluation of all possible analyte-IS pairs showed the best accuracies and precisions for these analyte-IS allocations. The obtained calibration curves were linear in the tested calibration range, and the correlation coefficients (R2) were > 0.99 for all analytes. We could achieve excellent limits of detection (LOD) and limits of quantification (LOQ) (Table 3). LOD was defined as a signal to noise ratio of 3. The LOQ was calculated as the triple fold of the LOD.
Method validation and sample stability
Method accuracy was calculated by using three spiked urine samples covering the entire calibration range. Accuracies between 95 and 113% were found for all tested compounds (Table 4). Precisions were determined in three unspiked urine matrices at three different levels. Intra- and interday coefficients of variation were below 12% for all analytes (Table 4). Recoveries were calculated as the difference between area ratios of prespiked samples (eicosanoid standards were added before LLE) and postspiked samples (eicosanoid standards were added to the recovered chloroform phase). Internal standards were added before LLE to both samples. Observed recoveries ranged between 25% (tetranor-PGE-M) and 100% (11-dehydro TXB2) (Table 4). Matrix effects were investigated using three individual urine samples by comparing the peak areas between urine samples and controls (water) at two levels. The percentage of area ratios difference for three different urine matrices compared with nonmatrix samples ranged from −13.9% to 10.7%, demonstrating that no significant matrix effect was present (Table 5).
TABLE 4.
Accuracies and intraday and interday precisions and recoveries
| Spiked | Accuracy | Concentration | Intraday (n = 5) |
Interday (n = 6) |
Spiked | Recovery | |||
| ng/ml urine | % | ng/ml urine | ng/ml ± SD | CV (%) | ng/ml ± SD | CV (%) | ng/ml urine | % | |
| Tetranor PGE-M | 0.30 | 100.5 | 0.10 | 0.10 ± 0.01 | 5.85 | 0.10 ± 0.01 | 7.66 | 0.50 | 28.3 |
| 2.00 | 109.4 | 2.30 | 2.32 ± 0.10 | 4.52 | 2.42 ± 0.17 | 6.90 | 5.00 | 29.1 | |
| 10.0 | 103.5 | 10.8 | 10.7 ± 0.23 | 2.14 | 10.5 ± 0.38 | 3.60 | 20.00 | 25.0 | |
| 2,3-Dinor-8-iso-PGF2α | 0.15 | 97.0 | 0.26 | 0.26 ± 0.01 | 4.29 | 0.28 ± 0.02 | 7.81 | 0.25 | 36.8 |
| 10.0 | 96.9 | 10.0 | 9.97 ± 0.32 | 3.24 | 10.0 ± 0.91 | 9.11 | 2.50 | 32.8 | |
| 15.0 | 94.9 | 13.5 | 13.6 ± 0.71 | 5.24 | 14.8 ± 0.67 | 4.55 | 10.00 | 30.5 | |
| 8-Iso-PGF2α | 0.03 | 98.3 | 0.032 | 0.032 ± 0.003 | 9.07 | 0.032 ± 0.004 | 11.5 | 0.05 | 61.3 |
| 2.00 | 112.8 | 2.40 | 2.41 ± 0.11 | 4.37 | 2.48 ± 0.17 | 6.74 | 0.50 | 57.9 | |
| 10.0 | 110.1 | 11.0 | 11.1 ± 0.67 | 6.04 | 11.7 ± 0.40 | 3.38 | 2.00 | 58.7 | |
| 2,3-Dinor-TXB2 | 0.15 | 101.0 | 0.22 | 0.22 ± 0.01 | 5.03 | 0.22 ± 0.02 | 7.65 | 0.25 | 50.7 |
| 10.0 | 104.1 | 10.0 | 10.2 ± 0.42 | 4.13 | 11.1 ± 0.48 | 4.33 | 2.50 | 34.8 | |
| 15.0 | 98.76 | 15.0 | 15.3 ± 1.07 | 6.95 | 15.2 ± 0.41 | 2.70 | 10.00 | 43.7 | |
| 11-Dehydro-TXB2 | 0.03 | 97.3 | 2.50 | 2.43 ± 0.08 | 3.49 | 2.43 ± 0.11 | 4.59 | 0.05 | 100.0 |
| 2.00 | 112.8 | 4.70 | 4.65 ± 0.16 | 3.42 | 4.74 ± 0.37 | 7.90 | 0.50 | 96.1 | |
| 10.0 | 110.5 | 13.5 | 13.4 ± 0.35 | 2.60 | 13.5 ± 0.53 | 3.87 | 2.00 | 90.1 | |
| LTE4 | 0.015 | 102.0 | 0.02 | 0.021 ± 0.001 | 2.93 | 0.022 ± 0.002 | 8.49 | 0.025 | 56.8 |
| 1.00 | 108.5 | 1.10 | 1.08 ± 0.04 | 4.12 | 1.13 ± 0.10 | 8.67 | 0.25 | 53.5 | |
| 5.00 | 104.9 | 5.30 | 5.23 ± 0.06 | 1.08 | 5.47 ± 0.18 | 3.29 | 1.00 | 59.9 | |
| 12-HETE | 0.015 | 112.9 | 0.025 | 0.025 ± 0.003 | 11.2 | 0.026 ± 0.003 | 9.80 | 0.025 | 77.6 |
| 1.00 | 108.9 | 1.40 | 1.41 ± 0.07 | 5.10 | 1.44 ± 0.10 | 7.23 | 0.25 | 81.3 | |
| 5.00 | 111.2 | 5.90 | 5.88 ± 0.24 | 4.14 | 6.08 ± 0.27 | 4.50 | 1.00 | 90.9 | |
The displayed accuracy is the mean of the assayed concentration (corrected by endogenous levels of the urine samples) in percent of the actual spiked concentration. For intraday precision, the mean concentrations and CVs of five individual samples are shown; for interday precision, mean concentrations and CVs of six individual samples are shown. For recovery, the mean percent from three individual extractions of recovered compound compared with spiked controls is shown.
TABLE 5.
Assessment of matrix effects
| Tetranor PGE-M | 2,3-Dinor-8-iso-PGF2α | 8-Iso-PGF2α | 2,3-Dinor-TXB2 | 11-Dehydro-TXB2 | LTE4 | 12-HETE | ||||||||
| Anal./IS | % iff. from cont. | Anal./IS | % Diff.rom cont. | Anal./IS | % Diff.from cont. | Anal./IS | % Diff. from cont. | Anal./IS | % Diff. from cont | Anal./IS | % Diff. from cont. | Anal./IS | % Diff. from cont. | |
| Low | 0.20 ng/ml | 1.00 ng/ml | 0.20 ng/ml | 1.00 ng/ml | 0.20 ng/ml | 0.10 ng/ml | 0.10 ng/ml | |||||||
| Control | 0.12 | — | 1.32 | — | 0.15 | — | 0.082 | — | 1.23 | — | 0.17 | — | 0.78 | — |
| Urine A | 0.13 | 7.2 | 1.45 | 9.7 | 0.14 | −4.8 | 0.071 | −13.6 | 1.12 | −8.8 | 0.18 | 1.7 | 0.85 | 8.2 |
| Urine B | 0.13 | 3.7 | 1.42 | 6.9 | 0.13 | −10.8 | 0.076 | −7.7 | 1.17 | −5.0 | 0.18 | 1.2 | 0.85 | 9.1 |
| Urine C | 0.12 | −2.5 | 1.37 | 3.1 | 0.13 | −11.9 | 0.073 | −11.1 | 1.11 | −9.3 | 0.19 | 6.3 | 0.82 | 5.4 |
| High | 5.00 ng/ml | 25.00 ng/ml | 5.00 ng/ml | 25.00 ng/ml | 5.00 ng/ml | 2.50 ng/ml | 2.50 ng/ml | |||||||
| Control | 2.86 | — | 32.38 | — | 3.66 | — | 2.00 | — | 3.47 | — | 4.24 | — | 19.27 | — |
| Urine A | 2.90 | 1.4 | 30.99 | −4.3 | 3.39 | −7.4 | 1.72 | −13.9 | 3.21 | −7.5 | 3.78 | −10.9 | 18.09 | −6.1 |
| Urine B | 3.02 | 5.4 | 32.93 | 1.7 | 3.71 | 1.2 | 1.81 | −9.4 | 3.49 | 0.5 | 4.17 | −11.6 | 18.85 | −2.2 |
| Urine C | 3.00 | 4.8 | 33.14 | 2.4 | 3.67 | 0.2 | 1.84 | −8.0 | 3.55 | 2.3 | 4.39 | 3.7 | 21.33 | 10.7 |
Displayed are the peak area ratios of spiked standards at two levels in urine samples and control and the percent difference compared with control. Anal., analyte; cont., control; diff; difference; IS, internal standard.
Sample stability was assessed (Table 6). For this purpose, urine aliquots were stored for 30 h at room temperature before samples were extracted. All analytes were stable under these conditions, except for tetranor-PGE-M (percent change of levels: −19.5; −24.8%), LTE4 (−28.5%; −13.4%), and 12-HETE (−29.3%; −29.4%). After going through six freeze/thaw cycles, only tetranor PGE-M showed some instability (~−15%). The postpreparation sample stability exhibited at least 14 days at 10°C in the autosampler.
TABLE 6.
Sample stabilities
| Concentration | Stability RT; conc. after 30 h | F/T stability; conc. after 6 cycles | Stability of prepared sample at 10°C; conc. after 14 d | |
| ng/ml urine | % | % | % | |
| Tetranor-PGE-M | 0.51 | 80.4 | 85.9 | 107.5 |
| 3.80 | 75.2 | 86.5 | 98.9 | |
| 2,3-Dinor-8-iso-PGF2α | 0.63 | 109.5 | 101.5 | 97.3 |
| 14.1 | 93.3 | 86.3 | 97.8 | |
| 8-iso-PGF2α | 0.19 | 109.8 | 108.8 | 105.5 |
| 1.89 | 89.3 | 87.1 | 105.1 | |
| 2,3-Dinor-TXB2 | 0.27 | 108.7 | 107.1 | 100.3 |
| 3.55 | 95.9 | 111.8 | 106.1 | |
| 11-Dehydro-TXB2 | 2.77 | 98.9 | 100.8 | 96.8 |
| 5.02 | 104.5 | 109.8 | 97.9 | |
| LTE4 | 0.13 | 71.5 | 103.2 | 111.1 |
| 1.18 | 87.6 | 102.3 | 102.6 | |
| 12-HETE | 0.19 | 70.7 | 102.5 | 108.7 |
| 1.31 | 70.6 | 112.4 | 103.5 |
Numbers represent percent concentrations after the indicated conditions and periods.
conc., concentration; F/T, freeze/thatw; RT, room temperature.
Method application
To test the suitability of our method for human biomonitoring purposes, eicosanoid levels in 24 h urine samples from smokers (n = 30) and nonsmoking controls (n = 30) were quantified. It is well known that nicotine enhances PGE2 generation through activation of COX-2 and that cigarette smoking increases oxidative stress (27, 41). To compensate for interindividual differences of urinary dilution, eicosanoid levels were normalized for creatinine.
We found that metabolite levels determined with our novel UPLC-SRM/MS method generally were in good agreement with concentration ranges determined by other groups (16, 24, 33, 34, 36, 42). Tetranor PGE-M, both PGF2α metabolites, and 2,3-dinor-TXB2 levels were significantly elevated in urine samples of smokers (Table 7). Mean tetranor PGE-M levels were 5.68 ng/mg crea in smokers, compared with 2.78 ng/mg crea in controls (P < 0.000). 2,3-Dinor-8-iso-PGF2α levels were 2 times higher in smokers than in nonsmokers (P < 0.001). Generally, 2,3-dinor-8-iso-PGF2α concentrations were about 10 times higher than 8-iso-PGF2α concentrations. The latter were also affected by cigarette smoking (about 1.4-fold higher in smokers; P < 0.05). Among all tested lipid mediators, we found the largest difference between smokers and nonsmokers for 2,3-dinor-TXB2 concentrations (2.21 vs. 11.28 ng/mg crea; P < 0.000). Surprisingly, 11-dehydro-TXB2 and 12-HETE levels were not different between the two groups. Although LTE4 concentrations were elevated in smokers (58.75 vs. 39.77 ng/mg crea), the differences did not reach statistical significance. Applying our novel UPLC-SRM/MS method, we could show that smoking affected urinary levels of tetranor PGE-M, 2,3-dinor-8-iso- and 8-iso-PGF2α, and 2,3-dinor-TXB2. Furthermore, these data demonstrate the importance of profiling urinary eicosanoid metabolites originating from different pathways rather than just measuring only one eicosanoid class.
TABLE 7.
Method application to smokers and nonsmokers
| Concentration | tetranor PGE-M | 2,3-dinor-8-iso-PGF2α | 8-iso-PGF2α | 2,3-dinor-TXB2 | 11-dehydro-TXB2 | LTE4 | 12-HETE |
| ng/mg Crea | ng/mg Crea | pg/mg Crea | ng/mg Crea | ng/mg Crea | pg/mg Crea | pg/mg Crea | |
| Nonsmokers (n = 30) | |||||||
| Mean | 2.78 | 1.08 | 178.21 | 2.21 | 3.24 | 39.77 | 220.25 |
| SD (range) | 3.21 (0.21–15.72) | 0.81 (0.00–4.05) | 100.32 (41.85–391.99) | 3.61 (0.32–-19.88) | 2.92 (1.29–16.62) | 26.46 (15.03–135.13) | 377.33 (0.83–1,729.80) |
| Smokers (n = 30) | |||||||
| Mean | 5.63 | 2.10 | 245.19 | 11.28 | 2.95 | 58.75 | 226.73 |
| SD (range) | 4.47 (1.20–22.47) | 1.47 (0.48–7.15) | 124.23 (0.65–562.35) | 16.44 (1.40–82.36) | 2.01 (0.80–9.16) | 51.50 (4.10–193.19) | 361.17 (0.44–1,088.36) |
| Mann-Whitney U test (Sig.) | <0.000 | 0.001 | 0.042 | <0.000 | 0.716 | 0.105 | 0.832 |
Displayed are the mean levels normalized to creatinine. The level of significance has been carried out with Mann-Whitney U Test. Significantly different values are underlined. Crea, creatinine. Sig., level of significance.
DISCUSSION
To evaluate and understand the physiological effects of lipid mediators, it is important to assess endogenous compounds originating from different pathways instead of single metabolites. Even though our newly developed method comprises not all eicosanoid species present in human urine (for TXB2 alone, more than 20 urinary species have been described [43]), it allows quantitative profiling metabolites of pathophysiological relevance. All chosen eicosanoid species were previously linked to diseases like CVD or various forms of cancer (1, 9).
A major challenge was to develop a sensitive and robust LC-SRM/MS multimethod approach suitable for all compounds of interest having individual chemical properties. All metabolites were ionized applying ESI in the negative mode. Collision-induced dissociation produced compound-specific product ions. Possible underlying chemical structures are shown in Fig. 1. However, whether these proposed structures are correct and the outlined detailed mechanisms of fragmentation have to be further elucidated. Murphy and colleagues proposed that the ion at m/z 305 (fragmentation of 11-dehydro-TXB2) might be a conjugated triene (Fig. 1), generated through loss of water and CO2 and the subsequent attack of the anionic site, formed at C-2 on the hydrogen at C-8 and rupture of the lactone ring (Fig. 1E) (43). For 12-HETE, the same group suggested that, for the fragment at m/z 179, the negative charge is located at C-12 (alkoxide anion; formed by charge migration) and not at the carboxyl moiety, as suggested Suzuki et al. (Fig. 1G) (24, 43).
Although we had to make several compromises for developing a multianalyte method, such as the choice of the sample preparation procedure or the ionization mode, we achieved excellent sensitivity (Table 3). In fact, we achieved the lowest LODs and LOQs for these analytes described until now (24, 31, 34, 36, 42, 44). In our view, this might be primarily due to three reasons: i) a very sensitive instrument was used for LC-SRM/MS analysis; ii) analyte separation was carried out on an UPLC column with a 1.7 µm particle size, leading to sharper peaks and thus to improved S/N ratios; and iii) LLE has been applied for sample preparation. Thus, the appropriate mass transitions showed less matrix interference. For tetranor PGE-M, 11-dehydro-TXB2, and 12-HETE, the S/N ratios were superior upon sample work-up with SPE (Table 2). For all other analytes, S/N was at least comparable to SPE-prepared samples. Further advantages of the chosen sample preparation are shorter processing and handling times. This is especially important for tetranor PGE-M, LTE4, and 12-HETE, which showed a certain level of instability at room temperature (Table 6).
The total running time for our LC-SRM/MS method is shorter than for many other eicosanoid multianalyte methods (14 min vs. > 20 min) (36, 38, 44). Although we could achieve a good separation of all analytes, the peak detected for 12-HETE (Fig. 1G) is racemic 12-HETE. A separation of 12(R)- and 12(S)-HETE could only be achieved with chiral chromatography (runtimes from 10 to 60 min) (24, 45). The major isomer present in human urine might be 12(S)-HETE (24, 33).
Calibration was performed by adding stable isotope-labeled eicosanoid species (Table 3). When IS were available, maximum compensation for matrix effects and ionization response were achievable. This is of particular importance because we used gradient elution. To evaluate LC-SRM/MS method performance, validation according to the US Food and Drug Administration guidelines was conducted (46). We achieved excellent accuracies and precisions for all analyzed metabolites.
As a first application, we analyzed urinary eicosanoid levels of samples from smokers and nonsmokers. It is well known that cigarette smoking and nicotine consumption lead to increased circulating proinflammatory endogenous agents, including PGE2, TXA2, and PGF2, promoting development and progression of cancer (e.g., lung, gastrointestinal, and bladder) and CVD (27, 47, 48). The analyte levels were normalized to creatinine to correct for urine dilution. An advantage of creatinine is that its biological variation within a homogenous population is relatively small. However, disadvantages are its dependence on factors like diet, physical activity, age, or gender (49). Alternatives for creatinine as normalization parameter would be the urine volume or osmolality (50). We found significantly elevated levels of urinary tetranor PGE-M (metabolite of PGE2), both PGF2 metabolites 2,3-dinor-8-iso- and 8-iso-PGF2α, and 2,3-dinor-TXB2 (metabolite of TXA2). These results are in good agreement with findings from other groups (33, 36, 38, 51). Levels of 11-dehydro-TXB2 (also a metabolite of TXA2) were similar in urine of smokers and nonsmokers, which is somewhat surprising because elevated levels for smokers have been reported previously (52). On the basis of our results, one could hypothesize that 2,3-dinor-TXB2 is a better marker for TXA2 generation than 11-dehydro-TXB2. However, this assumption has to be further investigated. LTE4 concentrations were found elevated as shown previously (53). The reason that they were not significantly different between smokers and nonsmokers might be the fact that levels are very variable within the investigated groups.
In summary, we have developed a simple and robust UPLC-SRM/MS method for quantification of seven urinary eicosanoid species frequently used as biomarkers for various diseases and pathophysiological conditions within clinical studies. Our method allows a fast, sensitive, and robust profiling of these urinary lipid mediators. Future application of this method might contribute to a better understanding of the role and relevance of eicosanoid species for inflammatory diseases and conditions.
Footnotes
Abbreviations:
- COX
- cyclooxygenase
- CVD
- cardiovascular disease
- HETE
- hydroxyeicosatetraenoic acid
- IS
- internal standard
- LOD
- limit of detection
- LOQ
- limit of quantification
- LT
- leukotriene
- LLE
- liquid-liquid extraction
- PG
- prostaglandin
- RP
- reversed-phase
- SPE
- solid phase extraction
- SRM
- selected reaction monitoring
- TX
- thromboxane
- UPLC
- ultraperformance liquid chromatography
REFERENCES
- 1.Calder P. C. 2009. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 91: 791–795 [DOI] [PubMed] [Google Scholar]
- 2.Schmitz G., Ecker J. 2008. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47: 147–155 [DOI] [PubMed] [Google Scholar]
- 3.Dennis E. A., Cao J., Hsu Y. H., Magrioti V., Kokotos G. 2011. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111: 6130–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ecker J., Liebisch G., Englmaier M., Grandl M., Robenek H., Schmitz G. 2010. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc. Natl. Acad. Sci. USA. 107: 7817–7822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ecker J., Liebisch G., Grandl M., Schmitz G. 2010. Lower SCD expression in dendritic cells compared to macrophages leads to membrane lipids with less mono-unsaturated fatty acids. Immunobiology. 215: 748–755 [DOI] [PubMed] [Google Scholar]
- 6.Ecker J., Liebisch G., Scherer M., Schmitz G. 2010. Differential effects of conjugated linoleic acid isomers on macrophage glycerophospholipid metabolism. J. Lipid Res. 51: 2686–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder P. C., Albers R., Antoine J. M., Blum S., Bourdet-Sicard R., Ferns G. A., Folkerts G., Friedmann P. S., Frost G. S., Guarner F., et al. 2009. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 101(Suppl 1): S1–S45 [DOI] [PubMed] [Google Scholar]
- 8.Stanke-Labesque F., Moliere P., Bessard J., Laville M., Vericel E., Lagarde M. 2008. Effect of dietary supplementation with increasing doses of docosahexaenoic acid on neutrophil lipid composition and leukotriene production in human healthy volunteers. Br. J. Nutr. 100: 829–833 [DOI] [PubMed] [Google Scholar]
- 9.Smyth E. M., Grosser T., Wang M., Yu Y., FitzGerald G. A. 2009. Prostanoids in health and disease. J. Lipid Res. 50(Suppl): S423–S428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buczynski M. W., Dumlao D. S., Dennis E. A. 2009. Thematic review series: proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 50: 1015–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphey L. J., Williams M. K., Sanchez S. C., Byrne L. M., Csiki I., Oates J. A., Johnson D. H., Morrow J. D. 2004. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal. Biochem. 334: 266–275 [DOI] [PubMed] [Google Scholar]
- 12.Kadiiska M. B., Gladen B. C., Baird D. D., Germolec D., Graham L. B., Parker C. E., Nyska A., Wachsman J. T., Ames B. N., Basu S., et al. 2005. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 38: 698–710 [DOI] [PubMed] [Google Scholar]
- 13.Morrow J. D., Harris T. M., Roberts L. J., 2nd 1990. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal. Biochem. 184: 1–10 [DOI] [PubMed] [Google Scholar]
- 14.Devaraj S., Hirany S. V., Burk R. F., Jialal I. 2001. Divergence between LDL oxidative susceptibility and urinary F(2)-isoprostanes as measures of oxidative stress in type 2 diabetes. Clin. Chem. 47: 1974–1979 [PubMed] [Google Scholar]
- 15.Pratico D., V M. Y. L., Trojanowski J. Q., Rokach J., Fitzgerald G. A. 1998. Increased F2-isoprostanes in Alzheimer's disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 12: 1777–1783 [DOI] [PubMed] [Google Scholar]
- 16.Davi G., Chiarelli F., Santilli F., Pomilio M., Vigneri S., Falco A., Basili S., Ciabattoni G., Patrono C. 2003. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation. 107: 3199–3203 [DOI] [PubMed] [Google Scholar]
- 17.Chiabrando C., Valagussa A., Rivalta C., Durand T., Guy A., Zuccato E., Villa P., Rossi J. C., Fanelli R. 1999. Identification and measurement of endogenous beta-oxidation metabolites of 8-epi-Prostaglandin F2alpha. J. Biol. Chem. 274: 1313–1319 [DOI] [PubMed] [Google Scholar]
- 18.FitzGerald G. A. 1991. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am. J. Cardiol. 68: 11B–15B [DOI] [PubMed] [Google Scholar]
- 19.Ricciotti E., FitzGerald G. A. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31: 986–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davi G., Catalano I., Averna M., Notarbartolo A., Strano A., Ciabattoni G., Patrono C. 1990. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N. Engl. J. Med. 322: 1769–1774 [DOI] [PubMed] [Google Scholar]
- 21.Murphy R. C., Gijon M. A. 2007. Biosynthesis and metabolism of leukotrienes. Biochem. J. 405: 379–395 [DOI] [PubMed] [Google Scholar]
- 22.Rabinovitch N. 2007. Urinary leukotriene E4. Immunol. Allergy Clin. North Am. 27: 651–664 (vii) [DOI] [PubMed] [Google Scholar]
- 23.Kishi N., Mano N., Asakawa N. 2001. Direct injection method for quantitation of endogenous leukotriene E4 in human urine by liquid chromatography/electrospray ionization tandem mass spectrometry with a column-switching technique. Anal. Sci. 17: 709–713 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki N., Hishinuma T., Saga T., Sato J., Toyota T., Goto J., Mizugaki M. 2003. Determination of urinary 12(S)-hydroxyeicosatetraenoic acid by liquid chromatography-tandem mass spectrometry with column-switching technique: sex difference in healthy volunteers and patients with diabetes mellitus. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 783: 383–389 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Nunez D., Claria J., Rivera F., Poch E. 2001. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 37: 334–338 [DOI] [PubMed] [Google Scholar]
- 26.Yeung J., Holinstat M. 2011. 12-lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovasc. Hematol. Agents Med. Chem. 9: 154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang R. Y., Chen G. G. 2011. Cigarette smoking, cyclooxygenase-2 pathway and cancer. Biochim. Biophys. Acta. 1815: 158–169 [DOI] [PubMed] [Google Scholar]
- 28.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 29.Neale J. R., Dean B. J. 2008. Liquid chromatography-tandem mass spectrometric quantification of the dehydration product of tetranor PGE-M, the major urinary metabolite of prostaglandin E(2) in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 871: 72–77 [DOI] [PubMed] [Google Scholar]
- 30.Dumlao D. S., Buczynski M. W., Norris P. C., Harkewicz R., Dennis E. A. 2011. High-throughput lipidomic analysis of fatty acid derived eicosanoids and N-acylethanolamines. Biochim. Biophys. Acta. 1811: 724–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahl J. H., van Breemen R. B. 2010. Rapid quantitative analysis of 8-iso-prostaglandin-F(2alpha) using liquid chromatography-tandem mass spectrometry and comparison with an enzyme immunoassay method. Anal. Biochem. 404: 211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masoodi M., Nicolaou A. 2006. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20: 3023–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesaros C., Lee S. H., Blair I. A. 2009. Targeted quantitative analysis of eicosanoid lipids in biological samples using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2736–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Zhang G., Clarke P. A., Huang J. T., Takahashi E., Muirhead D., Steenwyk R. C., Lin Z. 2011. Simultaneous and high-throughput quantitation of urinary tetranor PGDM and tetranor PGEM by online SPE-LC-MS/MS as inflammatory biomarkers. J. Mass Spectrom. 46: 705–711 [DOI] [PubMed] [Google Scholar]
- 35.Lynch C. M., O'Kelly R., Stuart B., Treumann A., Conroy R., Regan C. L. 2011. The role of thromboxane A(2) in the pathogenesis of intrauterine growth restriction associated with maternal smoking in pregnancy. Prostaglandins Other Lipid Mediat. 95: 63–67 [DOI] [PubMed] [Google Scholar]
- 36.Yan W., Byrd G. D., Ogden M. W. 2007. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J. Lipid Res. 48: 1607–1617 [DOI] [PubMed] [Google Scholar]
- 37.Li H., Lawson J. A., Reilly M., Adiyaman M., Hwang S. W., Rokach J., FitzGerald G. A. 1999. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F(2)-isoprostanes in human urine. Proc. Natl. Acad. Sci. USA. 96: 13381–13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor A. W., Bruno R. S., Traber M. G. 2008. Women and smokers have elevated urinary F(2)-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids. 43: 925–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue H., Jansen S. A., Strauss K. I., Borenstein M. R., Barbe M. F., Rossi L. J., Murphy E. 2007. A liquid chromatography/mass spectrometric method for simultaneous analysis of arachidonic acid and its endogenous eicosanoid metabolites prostaglandins, dihydroxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and epoxyeicosatrienoic acids in rat brain tissue. J. Pharm. Biomed. Anal. 43: 1122–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liebisch G., Lieser B., Rathenberg J., Drobnik W., Schmitz G. 2004. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim. Biophys. Acta. 1686: 108–117 [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y., Wang Z. X., Tang M. P., Yao C. J., Xu W. J., Wang L. Y., Qiao Z. D. 2010. Nicotine induces cyclooxygenase-2 and prostaglandin E(2) expression in human umbilical vein endothelial cells. Int. Immunopharmacol. 10: 461–466 [DOI] [PubMed] [Google Scholar]
- 42.Armstrong M., Liu A. H., Harbeck R., Reisdorph R., Rabinovitch N., Reisdorph N. 2009. Leukotriene-E4 in human urine: comparison of on-line purification and liquid chromatography-tandem mass spectrometry to affinity purification followed by enzyme immunoassay. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 3169–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy R. C., Barkley R. M., Zemski Berry K., Hankin J., Harrison K., Johnson C., Krank J., McAnoy A., Uhlson C., Zarini S. 2005. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal. Biochem. 346: 1–42 [DOI] [PubMed] [Google Scholar]
- 44.Martin-Venegas R., Casillas R., Jauregui O., Moreno J. J. 2011. Rapid simultaneous analysis of cyclooxygenase, lipoxygenase and cytochrome P-450 metabolites of arachidonic and linoleic acids using high performance liquid chromatography/mass spectrometry in tandem mode. J. Pharm. Biomed. Anal. 56: 976–982 [DOI] [PubMed] [Google Scholar]
- 45.Bayer M., Mosandl A., Thaci D. 2005. Improved enantioselective analysis of polyunsaturated hydroxy fatty acids in psoriatic skin scales using high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 819: 323–328 [DOI] [PubMed] [Google Scholar]
- 46.US Department of Health and Human Services (US DHHS) F. a. D. A. F., Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) 2001. Guidance for industry: bioanalytical method validation. Accessed February 5, 2011, at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. [DOI] [PubMed]
- 47.McAdam B. F., Byrne D., Morrow J. D., Oates J. A. 2005. Contribution of cyclooxygenase-2 to elevated biosynthesis of thromboxane A2 and prostacyclin in cigarette smokers. Circulation. 112: 1024–1029 [DOI] [PubMed] [Google Scholar]
- 48.Morrow J. D. 2005. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 25: 279–286 [DOI] [PubMed] [Google Scholar]
- 49.Miller R. C., Brindle E., Holman D. J., Shofer J., Klein N. A., Soules M. R., O'Connor K. A. 2004. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin. Chem. 50: 924–932 [DOI] [PubMed] [Google Scholar]
- 50.Warrack B. M., Hnatyshyn S., Ott K. H., Reily M. D., Sanders M., Zhang H., Drexler D. M. 2009. Normalization strategies for metabonomic analysis of urine samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 547–552 [DOI] [PubMed] [Google Scholar]
- 51.Gross N. D., Boyle J. O., Morrow J. D., Williams M. K., Moskowitz C. S., Subbaramaiah K., Dannenberg A. J., Duffield-Lillico A. J. 2005. Levels of prostaglandin E metabolite, the major urinary metabolite of prostaglandin E2, are increased in smokers. Clin. Cancer Res. 11: 6087–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikonomidis I., Lekakis J., Vamvakou G., Andreotti F., Nihoyannopoulos P. 2005. Cigarette smoking is associated with increased circulating proinflammatory and procoagulant markers in patients with chronic coronary artery disease: effects of aspirin treatment. Am. Heart J. 149: 832–839 [DOI] [PubMed] [Google Scholar]
- 53.Duffield-Lillico A. J., Boyle J. O., Zhou X. K., Ghosh A., Butala G. S., Subbaramaiah K., Newman R. A., Morrow J. D., Milne G. L., Dannenberg A. J. 2009. Levels of prostaglandin E metabolite and leukotriene E(4) are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev. Res. 2: 322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]