Abstract
To test the hypothesis that sidedness of interfacial arginine (Arg) in apoA-I mimetic peptides, similar to that observed in apoA-I (Bashtovyy, D. et al. 2011. Sequence conservation of apolipoprotein A-I affords novel insights into HDL structure-function. J. Lipid Res. 52: 435–450.), may be important for biological activity, we compared properties of 4F and analogs, [K4,15>R]4F and [K9,13>R]4F, with Lys>Arg substitutions on the right and left side, respectively, of the 4F amphipathic helix. Intraperitoneal administration of these peptides into female apoE null mice (n = 13 in each group) reduced en face lesions significantly compared with controls; 4F and [K4,15>R]4F were equally effective whereas [K9,13>R]4F was less effective. Turnover experiments indicated that [K4,15>R]4F reached the highest, whereas [K9,13>R]4F had the lowest, plasma peak levels with a similar half life as the [K4,15>R]4F analog. The half life of 4F was two times longer than the other two peptides. The order in their abilities to associate with HDL in human plasma, generation of apoA-I particles with pre-β mobility from isolated HDL, lipid associating ability, and sensitivity of lipid complexes to trypsin digestion was: 4F>[K4,15,>R]4F>[K9,13>R]4F. These studies support our hypothesis that the sidedness of interfacial Arg residues in the polar face of apoA-I mimetics results in differential biological properties.
Keywords: atherosclerosis, peptide, paraoxonase 1, metabolism, animals, lipids, cholesterol
A careful computer analysis of the location of positively charged residues in the tandem amphipathic helical domains of human apoA-I shows the preponderance of arginine (Arg) residues on the right hand side of the polar face of the amphipathic helix (1). Because HDL particles containing apoA-I are major carriers of antioxidant enzymes (2), and the analysis of putative HDL-associating regions of paraoxonase 1 (PON1) showed the presence of clusters of aromatic amino acids (3), we hypothesized that the side-specific Arg residues in human apoA-I are involved in cation-π interactions with the aromatic residues present in the putative HDL binding region of PON1 (1).
We have shown that the interfacial lysine (Lys) residues in the class A amphipathic helical peptide 2F are in different microenvironments with sidedness in their pKa values; Lys residues located on the left hand side have lower pKa value than the Lys residues located on the right hand side of the amphipathic helix (4). In addition, we have shown that the apoA-I mimetic peptides are arranged in discoidal complexes in a head-to-tail manner in which the Lys residues with higher pKa values likely interact with phosphate in the lipid head group and Lys residues possessing lower pKa values are present in a more hydrophobic environment (5). Because Arg residues in apoA-I are predicted to be involved in cation-π interactions with PON1 (1), we tested the hypothesis that side-specific incorporation of Arg residues in the apoA-I mimetic peptide 4F results in peptides that exhibit differential biological properties. Thus, we synthesized (as shown in Fig. 1) two additional analogs of 4F, a peptide that has been extensively studied for its various anti-inflammatory properties by us as well as by others (6–10). Peptides [K4,15>R]4F and [K9,13>R]4F, with Arg residues substituted for Lys residues on the right and left side respectively (Fig. 1A), were compared for their ability to inhibit atherosclerosis in older female apoE null mice with established aortic atherosclerotic lesions. We further evaluated the relationship of their anti-atherogenic abilities to their lipid affinity, plasma turnover, and their ability to enhance PON1 activity in vivo and in vitro and to reduce reactive oxygen species (ROS) in plasma. Results of these studies show that the two Arg peptides possess different physicochemical and biological properties that are related to their ability to inhibit atherosclerosis.
Fig. 1.
WHEEL/HELNET analysis of 4F, [K4,15>R]4F, and [K9,13>R]4F. A: Helical wheel diagrams of 4F and its two Arg containing analogs. Lys and Arg residues are colored light and dark blue, respectively. Notice that in [K4,15>R]4F analog, Arg residues are located on the right hand side of the polar face (similar to that observed in human apoA-I helical wheels with the polar face pointing downwards), whereas in [K9,13>R]4F, Arg residues are located on the left hand side of the polar face. Color schemes for other residues are as follows: negatively charged amino acids, red; hydrophobic amino acids, black; Ala, no color; Lys, light blue; Arg dark blue. Nonpolar and polar faces of the amphipathic helices are labeled. B: Helical net diagrams of 4F, [K4,15>R]4F, and [K9,13>R]4F. Contour lines drawn at 0, 5, and 10 Å into the lipid milieu are indicated. C: WHEEL/HELNET derived helix parameters for 4F, [K4,15>R]4F, and [K9,13>R]4F.
EXPERIMENTAL PROCEDURES
Materials
Peptides 4F, [K4,15>R]4F, and [K9,13>R]4F (Fig. 1) were synthesized by the solid phase method as described earlier (11). Briefly, peptides were synthesized using an automated solid-phase Peptide Synthesizer (Model PS3, Protein Technologies, Inc., AZ) using 9-fluorenylmethoxycarbonyl (FMOC) amino acids and Rink Amide MBHA resin (100-200 mesh, cross linked with 1% divinyl benzene, 0.56 mmol/g, EMD Chemicals, Inc. NJ) as a solid support. The first amino acid from carboxyl terminus was derivatized to the solid support after deblocking the FMOC-protection used on the rink amide linker solid support with 20% (v/v) piperidine in dimethyl formamide (DMF), 10 ml/g of resin (5 min, 2 times each). The FMOC-group used as a temporary protection for α amino function was removed by 20% piperidine in DMF followed by the sequential addition of rest of the amino acids, as their FMOC-amino acid derivatives (3eq × 2 for 60 min each), in the presence of 2-(H-benzotriazole-1-Yl)-1,1,3,3,-tetramethlyuronium hexafluorophosphate (HBTU) and 0.4M, N-methlymorpholine (NMM) in DMF. The other protecting groups used for the side-chain functional amino acids were: tert-butyl for Tyr, Asp, and Glu; N-t-Boc for Lys; and 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl (Pbf) for Arg. After each coupling, the FMOC-protection was removed by treatment with 20% piperidine in DMF. After the final FMOC- protection removal at the N-terminal α amino function of the amino acid, the NH2 group was acetylated with acetic acid in 1:1 DMF-Dichloromethane (DCM) in the presence of HBTU/NMM and 7% acetic anhydride in DMF for 60 min. For 14C, peptides, N-terminal was acetylated with 14C acetic acid (American Radiolabeled Chemicals, Inc., St Louis, MO). The resin was preswelled in DCM before the start of the synthesis; coupling and deprotection steps during the synthesis were monitored by the Kaiser ninhydrin test. The peptide was released from the resin along with the removal of side-chain protections by stirring with trifluoroacetic acid (10ml/g of resin) containing thioanisole, water, phenol, and 1,2-ethanedithiol (82.5:5:5:5:2.5, reagent K[10]) for 2 h and filtered . The resulting acetyl peptide amides were precipitated by treating the filtrate with anhydrous cold ether.
The crude peptides were purified using a C-18 preparative reversed-phase HPLC (RP-HPLC) column (Varion 21.4 × 250 mm LxID, 5 micron) on a Beckman HPLC system (gradient of 25%–60% acetonitrile containing 0.1% TFA, (v/v) at a flow rate of 10 ml/min in 120 min). The pure fractions were pooled and converted to free base using AG 4-x4 Resin (100-200 mesh 2.8 mmol/dry g, free base form Bio-Rad Labs, Inc. CA), and lyophilized.
The purity and authenticity of synthetic peptides were confirmed by analytical HPLC on a Beckman system using C-18 column (Vydac 250 × 21.4 mm LxID, 5 micron) and mass spectral analysis using PE-Sciox APT-III triple-quadrupole ion-spray mass spectrometer.
Recombinant PON1 (rPON1, Clone G3C9) was expressed in Escherichia coli using expression plasmid kindly provided by Dr. Dan S. Tawfik, Weizmann Institute of Science, Israel. Expressed rPON1 was purified following the protocol developed in the laboratory of Dr. Tawfik (12).
Peptide concentrations were determined as described by us previously (11). 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) were purchased from Avanti Polar Lipids (Birmingham, AL).
CD spectroscopy
The circular dichroism (CD) spectra of peptides and peptide:POPC complexes, using 0.1 mg/ml concentration, were recorded at 37°C using JASCO J-815 CD spectrometer equipped with a Peltier type temperature control system (JASCO model PTC-423S/15) and interfaced to a personal computer. The instrument was calibrated with (1S)-(+)-10-camphorsulfonic acid. The CD spectra were measured from 260 nm to 190 nm every 0.5 nm with 4 s averaging per point and a 2 nm bandwidth. The secondary structure of the peptide was analyzed as described earlier (13, 14).
Size of the peptide:POPC complex
Apparent size of the peptide:POPC complex was determined using a fast protein liquid chromatography system (BioLogic, DuoFlow; Bio-Rad) and Superdex 200 10/300 GL column (GE Healthcare) run at a flow rate of 0.4 ml/min in PBS containing 0.02% sodium azide (pH 7.4). Complex elution was monitored using absorbance at 280 nm. The apparent Stokes diameter of the complex was estimated using high and low molecular weight calibration kits (GE Healthcare). The amounts of lipid and peptide in the eluted peak fraction were measured using an enzymatic colorimetric method (Phospholipids B; Wako Chemicals) and absorbance at 280 nm in the presence of 6M guanidine hydrochloride, respectively.
Fluorescence measurements
Fluorescence emission spectra in PBS (pH 7.4) were recorded using pc1 photon counting steady-state spectrofluorimeter (ISS, Inc., Champaign, IL) at 37°C. An excitation wavelength of 280 nm was used and emission spectra were recorded from 300 to 450 nm. The wavelength of maximum emission was determined from the recorded emission spectra. Unfolding of the peptide in the peptide:POPC complex was monitored by following red shift (shift to the longer wavelength) in the emission maximum as a function of guanidine hydrochloride concentration. Peptide:POPC complexes were incubated with various concentrations of guanidine hydrochloride, ranging from 0 to 4M, at room temperature in the dark for at least 15 h to allow samples to reach equilibrium. Free energy of unfolding was determined as described previously (15).
NMR spectroscopy
Large unilamellar vesicles (LUV) of POPC were prepared in 10 mM HEPES buffer (pH 7.4) by extruding multilamellar vesicles 20 times through a 100 nm pore size filter using LIPEX extruder (Northern Lipids, Inc., Vancouver, Canada). The peptide was added to POPC LUV at lipid to peptide ratio of 20:1 (wt:wt) and incubated overnight at room temperature in the dark. 1H NMR spectra of the resulting solutions were obtained using an Avance-500 NMR spectrometer at 37°C. Chemical shifts were referenced with respect to sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) (0.0 ppm) used as an internal standard. The NMR data were processed offline using the program FELIX (version 2007) (Felix NMR Inc., San Diego, CA).
Peptide administration and lesion quantitation
Peptide solutions were prepared in physiological saline and concentration was determined using A280 as described earlier (11). Female apoE null mice at 12 weeks of age were purchased from the Jackson Laboratory (Bar Harbor, ME) and were maintained on a chow diet (Ralston Purina). After acclimatization for two weeks, the animals were randomized into four groups. Peptides 4F, [K4,15>R]4F, and [K9,13>R]4F (100 μg/100 μl in saline/mouse) were administered intraperitoneally (ip) every day for 16 weeks; the control group received an equal volume of saline. At the end of the study protocol, animals were anesthetized using ketamine-xylazine and blood was collected by cardiac puncture. Quantification of lesion area was performed as described previously (16, 17). All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Plasma turnover of the peptides
Turnover studies were done as previously described (18). Briefly, 14C-labeled peptides (100 μg/mouse) were administered to apoE null mice ip, and the radioactivity in plasma was determined at 5, 10, 15, 30, and 45 min and at 1, 1.5, 2, 4, 6, 8, and 10 h. Three samples were taken at each time point. No more than three plasma samples were taken from each animal, and time points for animals were staggered so that no two animals had identical time point assignments. Turnover parameters were calculated on all data points (rather than averages) by GraphPad Prism (GraphPad Software Inc., La Jolla, CA).
Other methods
Total plasma cholesterol was determined using Infinity Total Cholesterol reagent (Thermo Scientific). ROS levels in the plasma of control and peptide-adminstered animals were determined using 2’,7’-dichlorodihydrofluorescein diacetate (DCFDA) (Invitrogen, Carlsbad, CA) (19). Paraoxonase and lactonase activities were determined using paraoxon and dihydrocoumarin, respectively, as substrates (20). The peptide:POPC complexes were prepared by mixing peptide and POPC at a 1:1 (wt:wt) ratio and incubating the mixture at 37°C for 16 h. The ability of peptide:POPC complexes to enhance the activity of rPON1 was determined as described by Gaidukov et al. (12) with some modification. rPON1 was incubated with peptide:POPC complexes at 1:100 (wt:wt) ratio for 3 h at 37°C before measuring the paraoxonase activity.
Trypsin (stock concentration 100 μg/ml) digestion of peptide:POPC complexes was carried out at 37°C using an enzyme to substrate ratio of 1:40 (wt:wt). At different time points, an aliquot was removed and analyzed for the undigested peptide by RP-HPLC as described earlier (21).
14C-labeled peptides (100 µg/ml) were incubated with human plasma (obtained from the American Red Cross) overnight at 37°C in the dark. Plasma was fractionated using a fast protein liquid chromatography system (BioLogic, DuoFlow; Bio-Rad) and Superdex 200 10/300 GL column (GE Healthcare) run at a flow rate of 0.4 ml/min in PBS containing 0.02% sodium azide (pH 7.4). Elution was monitored using absorbance at 280 nm. 1 ml fractions were collected and analyzed for radioactivity, cholesterol, and apoA-I.
Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (Ox-PAPC) was produced by air oxidation of unoxidized lipid for 72 h at room temperature in the dark (22, 23). Generation of oxidized lipids was monitored and confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Agarose gel electrophoresis was carried out using 0.75% agarose in TRIS-Barbital buffer (pH 8.5) at 100V for 1 h.
Western blot analysis was performed using a biotin-labeled polyclonal antibody to human apoA-I (Brookwood Biomedical, Birmingham, AL).
Statistical analysis
Groups were compared by one way ANOVA and posthoc two-tailed Student's t-tests were performed on groups found to be significantly different by ANOVA. Groups were considered to be significantly different when P < 0.05.
RESULTS
WHEEL/HELNET analysis
Helical wheel, helical net, and WHEEL/HELNET derived properties (24, 25) of the three peptides are shown in Fig. 1A, B, and C, respectively. Helical net (Fig. 1B) shows the depth of lipid penetration contour map (drawn at 0, 5, and 10 Å) as well. The overall lipid affinity of an amphipathic helix is partly dependent upon its depth of lipid penetration (11, 25). For many of the residues on the nonpolar face, depth of lipid penetration decreases in the following order: 4F>[K4,15>R]4F>[K9,13>R]4F (Fig. 1B). For example, depth of lipid penetration of Val10 is 10.6Å, 10.0Å, and 9.0Å for 4F, [K4,15>R]4F, and [K9,13>R]4F, respectively (Fig. 1B). Accordingly, the predicted lipid affinity (λ4) of the peptides decreases in the following order: 4F>[K4,15>R]4F>[K9,13>R]4F (Fig. 1C).
Secondary structure of peptides
Secondary structure analyses of CD spectra of the peptides in the absence of lipid indicated the following helical contents (estimated average values ± 10%): 4F, 66%; [K4,15>R]4F, 70%; [K9,13>R]4F, 58%. The helical contents of the three peptides in POPC complexes were estimated to be (estimated average values ± 10%): 4F, 77%; [K4,15>R]4F, 78%; [K9,13>R]4F, 75%. Thus, all the three peptides adopted a similar and predominantly helical secondary structure in their POPC complexes.
Sizes of the peptide:POPC complexes
The apparent Stokes diameters, estimated using high and low molecular weight calibration kits (GE Healthcare), are 75Å for 4F:POPC, 85Å for [K4,15>R]4F:POPC, and 80Å for [K9,13>R]4F:POPC. The lipid-to-peptide ratios (wt:wt) in the eluted peak fractions were as follows: 4F:POPC, 1.3; [K4,15>R]4F:POPC, 1.7; [K9,13>R]4F:POPC, 1.5. It is important to note that the starting lipid-to-peptide ratio (wt:wt) for each peptide was 1.0. Also, it is interesting to note that 4F forms larger particles with POPC (75 Å) compared with DMPC (67 Å) (26).
Fluorescence studies and guanidine hydrochloride-induced unfolding of peptides
Compared with identical wavelength of Trp fluorescence emission maximum (350 nm) in the absence of lipid, wavelength of Trp emission maximum of all the three peptides in peptide:POPC complex exhibited a blue shift (shift to a shorter wavelength). The extent of blue shift was as follows: 4F, 18 nm; [K4,15>R]4F, 18 nm; [K9,13>R]4F, 14 nm. Thus, in the POPC complex, Trp residues in 4F and [K4,15>R]4F are located in a more hydrophobic microenvironment than the Trp in [K9,13>R]4F. Guanidine hydrochloride denaturation experiments revealed the following estimates of the free energy of unfolding (kcal/mol) for the three peptides in peptide:POPC complexes: 4F:POPC, 2.00; [K4,15>R]4F:POPC, 1.95; [K9,13>R]4F:POPC, 1.66. Thus, whereas 4F forms most thermodynamically stable complex with POPC, [K9,13>R]4F forms least stable complex.
1H NMR studies of the effect of peptides on POPC LUV
A significant attenuation of methyl and methylene proton signals of POPC in LUV by 4F and [K4,15>R]4F, compared with [K9,13>R]4F, was observed (Fig. 2). This is presumably because of greater immobilization of methyl and methylene protons of POPC by 4F and [K4,15>R]4F compared with [K9,13>R]4F, in LUV.
Fig. 2.
Perturbation of methyl and methylene protons of POPC LUV by 4F, [K4,15>R]4F, and [K9,13>R]4F. Partial 1H NMR (500 MHz) spectra of POPC LUV in the absence and presence of 4F, [K4,15>R]4F, and [K9,13>R]4F. 1H NMR spectra were obtained at 37°C using a lipid to peptide ratio of 20:1 (wt:wt). Chemical structure of POPC and methyl (labeled as 1) and methylene (labeled as 2) protons are shown.
Effect of administration of peptides on lesion formation
Administration of peptides into female apoE null mice ip for 16 weeks significantly reduced aortic atherosclerotic lesions in all of the peptide groups compared with the control group as examined by aortic sinus (Fig. 3A) and en face (Fig. 3B) lesion analysis. Compared with control, both 4F and [K4,15>R]4F reduced aortic sinus lesion significantly; peptide [K9,13>R]4F showed a trend (P = 0.06) but did not reach statistical significance (Fig. 3A). Among the peptide treated groups, there was no statistically significant difference in the observed mean lesion area (Fig. 3A). However, en face analysis revealed that peptides 4F and [K4,15>R]4F reduced lesion equally and more effectively compared with [K9,13>R]4F (Fig. 3B).
Fig. 3.
Quantification of atherosclerotic lesions. A: Quantification of aortic sinus lesions. Oil Red O stained areas in the aortic sinus were measured in saline and 4F, [K4,15>R]4F, and [K9,13>R]4F treated groups at the end of treatment period. Data are expressed as mean ± SEM. Both 4F and [K4,15>R]4F, but not [K9,13>R]4F, reduced aortic sinus lesion significantly compared with control as determined by one-way ANOVA. B: En face preparations of the entire aorta from the aortic arch to the iliac bifurcation were done in mice treated with 4F, [K4,15>R]4F, and [K9,13>R]4F. The aorta was excised, cleaned, and opened longitudinally with extremely fine Vannas scissors then pinned flat on a black wax surface. The aorta was then stained with Oil Red O and lesions were quantified by video capture under a stereo dissecting microscope. Lesion and total areas were determined using SigmaScan (Systat) and lesion area was expressed as a percentage of total area.
Turnover of 14C-labeled peptides in female apoE null mice
Administration of 14C-labeled peptides ip into female apoE null mice (n = 5 in each group) showed that peptide 4F had the longest half life (t1/2 2.01 h) (Fig. 4A). The peptide [K9,13>R]4F has the shortest half life (t1/2 1.03 h) and the lowest peak plasma level (Fig. 4A). The peptide [K4,15>R]4F had the maximum peak plasma level (Fig. 4A) and thus, despite a shorter half life than 4F (t1/2 1.12 h), had a similar area under the curve (AUC; Fig. 4B). Among the three peptides, peptide [K9,13>R]4F had the smallest AUC (Fig. 4B). These differences in bioavailability of the Arg analogs may partially account for their ability to inhibit lesion formation.
Fig. 4.
Plasma turnover studies in female apoE null mice. (A) Bioavailability of 4F (■), [K4,15>R]4F (○), and [K9,13>R]4F (△). One hundred micrograms of 14C-labeled peptides in saline were administered ip to female apoE null mice. Blood was drawn at various time points as indicated. Radioactivity in plasma at different time points (n = 3 per time point) was expressed as percent of injected cpm. Data are expressed as mean ± SEM. B: Area under the curve was calculated for each peptide, and is expressed as % injected cpm × h.
Effect of administration of peptides on plasma cholesterol and ROS levels
Analogous to what was reported previously for 4F (27), administration of the new Arg analogs also did not alter plasma cholesterol levels. However, when the plasma of control and peptide-administered animals were analyzed by the DCFDA reagent to determine the possible effect of these peptides on ROS, all three peptides were equally effective in reducing ROS levels (Table 1).
TABLE 1.
Comparison of effects of 4F, [K4,15>R]4F, and [K9,13>R]4F on ROS levels in plasma obtained after peptide treatment (in vivo) and PON1 activity in vivo (in plasma obtained after peptide treatment) and in vitro
| Assay Name | Units | Saline | 4F (p vs. Saline) | [K4,15>R]4F (p vs. Saline) | [K9,13>R]4F (p vs. Saline) |
| 2’-7’-Dichlorodihydrofluorescein diacetate (Reactive oxygen species)* | Arbitrary Units ± SEM | 9726.250 ± 296.881 | 8115.091 ± 295.250 (0.005) | 7338.364 ± 556.491 (<0.001) | 8152.667 ± 350.334 (0.005) |
| Lactonase Activity (plasma samples)a | PON Units ± SEM | 119.424 ± 12.578 | 147.988 ± 6.176 (0.015) | 156.808 ± 1.945 (0.002) | 145.071 ± 2.541 (0.028) |
| Paraoxonase activity (in vitro)b | % of Control±SEM | — | 180 ± 2 | 231 ± 8 | 207 ± 2 |
There are no statistically significant differences among the peptides for these parameters.
Paraoxonase activity of rPON1 in the presence of peptide:POPC complex. rPON1 was added to the peptide:POPC complex at a ratio of 1:100 (wt:wt). Paraoxonase activity in the absence of peptide:POPC complex was assumed as 100%.
Stability of peptide:lipid complexes against proteolysis
This was studied by exposing the POPC complexes of the peptides to the proteolytic enzyme trypsin. The Arg peptides are more easily degradable than the Lys analogs (Fig. 5).
Fig. 5.
Trypsin digestion of POPC complexes of 4F (■), [K4,15>R]4F (○), and [K9,13>R]4F (△). One hundred micrograms/milliliter of the complex was digested with trypin (enzyme:complex, 1:40, wt:wt) at 37°C. At the indicated time points, an aliquot was taken out and frozen at −20°C for RP-HPLC analysis. Area under the peak was used to calculate the amount of undigested peptide in the complex. Error bars represent SD of two independent experiments.
Ability of peptide:POPC complex to enhance PON1 activity
Peptide:POPC (1:1, wt:wt) complexes were studied for their ability to activate rPON1 (12). The ratio of rPON1 to peptide:POPC complex was 1:100 (wt:wt). Results indicate that among the three peptide analogs, [K4,15>R]4F was most effective (Table 1).
Association of peptides with human HDL and formation of apoA-I containing particles with pre-beta mobility
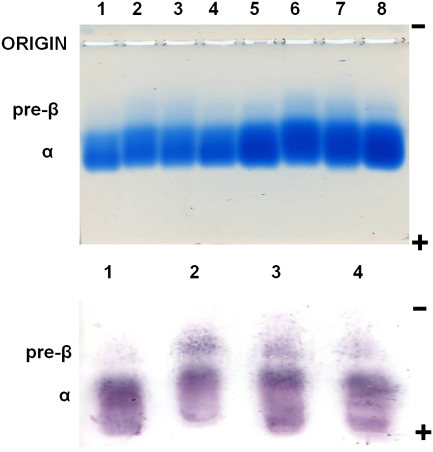
The following order was observed in the amount of 14C-labeled peptides associated with HDL in human plasma: 4F>[K4,15>R]4F>[K9,13>R]4F (Fig. 6). There was more radioactivity eluting in the post-HDL region and not associated with HDL in the case of [K9,13>R]4F compared with the other two peptides (Fig. 6). The ability of peptides to displace apoA-I from isolated human HDL also exhibited the above order (Fig. 7). Based on the Image J (version, 1.44; http://rsbweb.nih.gov/ij/) analysis of the Western blot (Fig. 7), the amount of apoA-I in the pre-β region (assuming 100% in the control HDL) was estimated to be: 4F, 640%; [K4,15>R]4F, 563%; [K9,13>R]4F, 208%.
Fig. 6.
Distribution of 14C-labeled 4F (■), [K4,15>R]4F (○), and [K9,13>R]4F (△) in human plasma. One hundred micrograms of 14C-labeled peptide were incubated with 1 ml human plasma at 37°C overnight in the dark. The plasma was fractionated using a fast protein liquid chromatography system (BioLogic, DuoFlow; Bio-Rad) and Superdex 200 10/300 GL column (GE Healthcare) run at a flow rate of 0.4 ml/min in PBS containing 0.02% sodium azide (pH 7.4). Complex elution was monitored using absorbance at 280 nm. One milliliter fractions were collected. Total cholesterol and radioactivity in each fraction were measured. Cholesterol values are indicated by red-filled circles. In addition to cholesterol, apoA-I was determined in FPLC fractions 12-20 using Western blot analysis. Compared with other fractions, fractions 13-15 were enriched in apoA-I.
Fig. 7.
Generation of apoA-I containing particles with preβ mobility by incubation of peptides with isolated human HDL. HDL (d = 1.063-1.210 g/ml) was isolated from human plasma using sequential density gradient ultracentrifugation. Peptides were incubated with HDL at HDL protein to peptide ratio of 5:1 (wt:wt) at 37°C overnight in the dark. Top panel shows Coomassie Blue stained gel and bottom panel shows a Western blot using a biotin-labeled polyclonal antibody to human apoA-I. In the top panel, lanes 1-4 and 5-8 represent 5 µL and 10 µL, respectively, of the sample volume loaded. Top panel: 1, control; 2, 4F; 3, [K4,15>R]4F; 4, [K9,13>R]4F; 5, control; 6, 4F; 7, [K4,15>R]4F; 8, [K9,13>R]4F. Bottom panel: 1, control; 2, 4F; 3, [K4,15>R]4F; 4, [K9,13>R]4F.
Ability of peptides to bind Ox-PAPC
Agarose gel electrophoresis was used to determine ability of the peptide to associate with Ox-PAPC (peptide to lipid ratio, 1:10, wt:wt). Because Ox-PAPC contains net negatively charged lipids, e.g., 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine (PGPC), lipid association should increase the electrophoretic mobility of the peptide toward positive pole (anode). Control experiments using POPC and PGPC complexes of 4F showed increased electrophoretic mobility of 4F:PGPC complex compared with 4F:POPC complex. Based on the agarose gel electrophoresis, all the three peptides were able to bind Ox-PAPC (Fig. 8). It is interesting to note that at pH 8.5, 4F exhibits higher electrophoretic mobility than the other two peptides. This is in accord with the lower pKa value of Lys than Arg. Thus, at pH 8.5, compared with 4F, the two Arg-containing peptides have higher net positive charge reducing their electrophoretic mobility (Fig. 8).
Fig. 8.
Agarose gel electrophoresis of peptides in the absence and presence of Ox-PAPC. The lipid to peptide ratio was 10:1 (wt:wt). 1, 4F; 2, [K4,15>R]4F; 3, [K9,13>R]4F; 4, 4F:Ox-PAPC; 5, [K4,15>R]4F:Ox-PAPC; 6, [K9,13>R]4F:Ox-PAPC.
DISCUSSION
We have shown that interfacial Lys residues of class A amphipathic helices contribute to their lipid-associating ability due to the ability of the alkyl side chain of Lys to snorkel (28, 29). However, not all Lys residues in an amphipathic helical peptide are similar in their properties when complexed with phospholipids. Among the four Lys residues in the primary sequence of 2F, residues 4 and 15 that are on the right side of the helix possess pKa values of 11 and 10.3, respectively (both are higher than the pKa value for the ϵ-NH2 (10.2) of the amino acid Lys in solution) whereas the Lys residues at positions 9 and 13 possess a pKa value of 9.4, a value less than the Lys pKa value (4). As shown previously, in the lipid-associated structure of 2F (and 4F), residues 4 and 15 are in proximity with the phosphate head group and residues 9 and 13 are in a more hydrophobic environment, thus explaining the reason for the sidedness of pKa values of the Lys residues (5, 26).
In the amphipathic helical structure of apoA-I as present in discoidal HDL, the side that is in proximity to phospholipid head groups (right-hand side) is rich in Arg residues (1). Because the guanidino group in Arg can be involved in multiple salt bridge formations, we propose that, in addition to interacting with the phosphate in the lipid head group, Arg residues may also be involved in protein-protein interaction with π-electron rich antioxidant enzymes such as PON1. As a first step to test this hypothesis, we synthesized side-specific Arg analogs of 4F; thus, the two new Arg analogs are [K4,15>R]4F and [K9,13>R]4F (Fig. 1). The amphipathicity and lipid affinity, derived from the WHEEL/HELNET (24) analysis, of the studied peptides are summarized in Fig. 1C. It is interesting to note that, as indicated by the hydrophobic moment/residue, both Arg analogs are more amphipathic than 4F (Fig. 1C). Among the two Arg analogs, [K4,15>R]4F is more amphipathic than [K9,13>R]4F (Fig. 1C). However, because the Lys side chain is more hydrophobic than Arg side chain (four methylene groups in the Lys side chain vs. three in the Arg side chain) (30), overall hydrophobicity of the Arg analogs is less than that of 4F (Fig. 1C). In contrast to 4F and [K9,13>R]4F, the angle of tilt (orientation of the amphipathic helix relative to the plane of the hydrated phospholipid that produces the maximum lipid affinity) (25) of [K4,15>R]4F is predicted to be zero (Fig. 1C).
The predicted lipid affinity (λ4), which partly depends upon the depth of penetration into the lipid milieu (11), of the three analogs decreases in the following order: 4F>[K4,15>R]4F> [K9,13>R]4F (Fig. 1C). In agreement with the predicted lowest lipid affinity of [K9,13>R]4F, 1H NMR data indicate that at a high lipid to peptide ratio (20:1, wt:wt), compared with 4F and [K4,15>R]4F,both of which are equally effective, [K9,13>R]4F is least able to perturb methyl and methylene proton signals of POPC LUV (Fig. 2). Although the predicted lipid affinity of 4F is higher than that of [K4,15>R]4F (Fig. 1C), amphipathicity of [K4,15>R]4F is highest among the three peptides. Helix amphipathicity has been shown to be far more important for interfacial binding than simple hydrophobicity (31). The ability of [K4,15>R]4F to perturb methyl and methylene proton signals of POPC LUV, similar to that of 4F, is perhaps related to its highest amphipathicity among the three peptides (Fig. 1C).
Compared with control, all the three analogs reduce plasma ROS levels and increased lactonase activity of PON1 to the same extent (Table 1). Based on CD studies, all the three analogs possess similar and predominantly helical secondary structure in the peptide:POPC complexes. However, among the three analogs, the POPC complex of [K4,15>R]4F was the best in enhancing paraoxonase activity of rPON1 (Table 1). Compared with 4F (75 Å), both [K4,15>R]4F (85 Å) and [K9,13>R]4F (80 Å) form larger POPC particles with higher lipid to peptide ratios (4F:POPC, 1.3; [K4,15>R]4F:POPC, 1.7; [K9,13>R]4F:POPC, 1.5). The ability to enhance rPON1 activity is directly related to the lipid to peptide ratio, i.e., [K4,15>R]4F:POPC>[K9,13>R]4F:POPC>4F:POPC (Table 1). It is likely that the higher lipid to peptide ratio of the complex is, at least in part, responsible for higher activation of rPON1. However, structural details of enhanced activation of rPON1 by POPC complexes of these peptides are presently unknown.
Perhaps due to the large well-established lesions in the aortic sinus of old apoE null mice, the lesion reduction between the peptide treated groups did not reach significance (Fig. 3A), although peptides 4F and [K4,15>R]4F both had significantly reduced sinus lesions compared with controls. However, as shown by en face analysis, the two Arg analogs differ in their ability to reduce atherosclerotic aortic lesions in female apoE null mice with [K4,15>R]4F analog being significantly more effective than [K9,13>R]4F analog (Fig. 3B). The [K4,15>R]4F analog is also better than the [K9,13>R]4F analog in its ability to enter the plasma compartment upon ip administration (Fig. 4A); thus, animals administered the [K4,15>R]4F peptide had significantly higher plasma peak levels than the other peptides (Fig. 4A). However, even though the amount of 4F that entered the plasma compartment is less compared with [K4,15>R]4F, this peptide possessed significantly slower half-time of clearance (2.01 h) (Fig. 4A). This resulted in AUC being maximum for [K4,15>R]4F, followed by 4F (Fig. 4B). Among the three analogs, the AUC was least for [K9,13>R]4F (Fig. 4B).
Lipid association has been shown to reduce the susceptibility of Lys and Arg residues in apoA-I toward trypsin digestion (32). Therefore, we hypothesized that among the two Arg peptide analogs, the peptide in which the Arg analogs interact with the phosphate group to stabilize the peptide:lipid complex would be less sensitive to proteolysis compared with the analog [K9,13>R]4F. In agreement with this, [K9,13>R]4F in the POPC lipid complex was more susceptible to trypsin digestion than [K4,15>R]4F; Peptide 4F in the POPC complex was the least sensitive (Fig. 5). This is in accord with the predicted lipid affinities of the three peptides, i.e., 4F>[K4,15>R]4F>[K9,13>R]4F.
It is interesting to note that the above order was also observed in the abilities of the three peptides to associate with HDL in human plasma (Fig. 6) as well as formation of apoA-I containing particles with preβ mobility when incubated with isolated HDL (Fig. 7).
In accord with their similar ability to reduce ROS levels in vivo (Table 1), all the three peptides were able to bind Ox-PAPC (Fig. 8).
Taken together, our results indicate that substitution of Lys residues with Arg on the right side of the helix that is closer to the phospholipid head group in the discoidal complex structure (5, 26) produces a peptide that has enhanced biological activity compared with the peptide with substitution on the opposite (left) side. Extrapolation of these results to the apoA-I structure may explain the significant role of the prevalence of Arg residues on the right hand side of the polar face of the amphipathic helix.
Acknowledgments
The authors thank Dr. Dan S. Tawfik, Weizmann Institute of Science, Israel, for providing clone G3C9 of PON1, Dr. Michael J Jablonsky for the use of spectropolarimeter, Dr. Kirill M Popov for the use of spectrofluorimeter, and Candyce E. Monroe and Tamara D. Keenum for their expert technical assistance.
Footnotes
Abbreviations:
- AUC
- area under the curve
- CD
- circular dichroism
- DCFDA
- 2’,7’-dichlorodihydrofluorescein diacetate
- ip
- intraperetoneal
- LUV
- large unilamellar vesicles
- PON1
- paraoxonase 1
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PAPC
- 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- Ox-PAPC
- oxidized PAPC
- ROS
- reactive oxygen species
REFERENCES
- 1.Bashtovyy D., Jones M. K., Anantharamaiah G. M., Segrest J. P. 2011. Sequence conservation of apolipoprotein A-I affords novel insights into HDL structure-function. J. Lipid Res. 52: 435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson W. S., Silva R. A. G., Chantepie S., Lagor W. R., Chapman M. J., Kontush A. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters. Arterioscler. Thromb. Vasc. Biol. 29: 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harel M., Aharoni A., Gaidukov L., Brumshtein B., Khersonsky O., Meged R., Dvir H., Ravelli R. B. G., McCarthy A., Toker L., et al. 2004. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat. Struct. Mol. Biol. 11: 412–419 [DOI] [PubMed] [Google Scholar]
- 4.Lund-Katz S., Phillips M. C., Mishra V. K., Segrest J. P., Anantharamaiah G. M. 1995. Microenvironments of basic amino acids in amphipathic alpha-helices bound to phospholipid: 13C NMR studies using selectively labeled peptides. Biochemistry. 34: 9219–9226 [DOI] [PubMed] [Google Scholar]
- 5.Mishra V. K., Anantharamaiah G. M., Segrest J. P., Palgunachari M. N., Chaddha M., Sham S. W. S., Krishna N. R. 2006. Association of a model class A (Apolipoprotein) amphipathic α-helical peptide with lipid. J. Biol. Chem. 281: 6511–6519 [DOI] [PubMed] [Google Scholar]
- 6.Vanella L., Li M., Kim D., Malfa G., Bellner L., Kawakami T., Abraham N. 2012. ApoA1: mimetic peptide reverses adipocyte dysfunction in vivo and in vitro via an increase in hemeoxygenase (HO-1) and Wnt10b. Cell Cycle. 11: 706–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou Z., Ou J., Ackerman A. W., Oldham K. T., Pritchard K. A. 2003. L-4F, an apolipoprotein A-1 mimetic, restores nitric oxide and superoxide anion balance in low-density lipoprotein-treated endothelial cells. Circulation. 107: 1520–1524 [DOI] [PubMed] [Google Scholar]
- 8.Wool G. D., Cabana V. G., Lukens J., Shaw P. X., Binder C. J., Witztum J. L., Reardon C. A., Getz G. S. 2011. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. FASEB J. 25: 290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wool G. D., Vaisar T., Reardon C. A., Getz G. S. 2009. An apoA-I mimetic peptide containing a proline residue has greater in vivo HDL binding and anti-inflammatory ability than the 4F peptide. J. Lipid Res. 50: 1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navab M., Shechter I., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Fogelman A. M. 2010. Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 30: 164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palgunachari M. N., Mishra V. K., Lund-Katz S., Phillips M. C., Adeyeye S. O., Alluri S., Anantharamaiah G. M., Segrest J. P. 1996. only the two end helixes of eight tandem amphipathic helical domains of human apo A-I have significant lipid affinity: implications for HDL assembly. Arterioscler. Thromb. Vasc. Biol. 16: 328–338 [DOI] [PubMed] [Google Scholar]
- 12.Gaidukov L., Viji R. I., Yacobson S., Rosenblat M., Aviram M., Tawfik D. S. 2009. ApoE induces serum paraoxonase PON1 activity and stability similar to apoA-I 3. Biochemistry. 49: 532–538 [DOI] [PubMed] [Google Scholar]
- 13.Mishra V. K., Palgunachari M. N., Hudson J. S., Shin R., Keenum T. D., Krishna N. R., Anantharamaiah G. M. 2011. Structure and lipid interactions of an anti-inflammatory and anti-atherogenic 10-residue class G(*) apolipoprotein J peptide using solution NMR. Biochim. Biophys. Acta. 1808: 498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenfield N. J. 2006. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1: 2876–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers D. P., Brouillette C. G., Engler J. A., Tendian S. W., Roberts L., Mishra V. K., Anantharamaiah G. M., Lund-Katz S., Phillips M. C., Ray M. J. 1997. Truncation of the amino terminus of human apolipoprotein A-I substantially alters only the lipid-free conformation. Biochemistry. 36: 288–300 [DOI] [PubMed] [Google Scholar]
- 16.Nayyar G., Handattu S. P., Monroe C. E., Chaddha M., Datta G., Mishra V. K., Keenum T. D., Palgunachari M. N., Garber D. W., Anantharamaiah G. M. 2010. Two adjacent domains (141–150 and 151–160) of apoE covalently linked to a class A amphipathic helical peptide exhibit opposite atherogenic effects. Atherosclerosis. 213: 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palinski W., Ord V. A., Plump A. S., Breslow J. L., Steinberg D., Witztum J. L. 1994. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler. Thromb. 14: 605–616 [DOI] [PubMed] [Google Scholar]
- 18.Handattu S. P., Garber D. W., Horn D. C., Hughes D. W., Berno B., Bain A. D., Mishra V. K., Palgunachari M. N., Datta G., Anantharamaiah G. M., et al. 2007. ApoA-I mimetic peptides with differing ability to inhibit atherosclerosis also exhibit differences in their interactions with membrane bilayers. J. Biol. Chem. 282: 1980–1988 [DOI] [PubMed] [Google Scholar]
- 19.Navab M., Hama S. Y., Hough G. P., Subbanagounder G., Reddy S. T., Fogelman A. M. 2001. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J. Lipid Res. 42: 1308–1317 [PubMed] [Google Scholar]
- 20.Draganov D. I., Stetson P. L., Watson C. E., Billecke S. S., La Du B. N. 2000. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation 123. J. Biol. Chem. 275: 33435–33442 [DOI] [PubMed] [Google Scholar]
- 21.Navab M., Ruchala P., Waring A. J., Lehrer R. I., Hama S., Hough G., Palgunachari M. N., Anantharamaiah G. M., Fogelman A. M. 2009. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J. Lipid Res. 50: 1538–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., et al. 2002. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277: 38517–38523 [DOI] [PubMed] [Google Scholar]
- 23.Watson A. D., Subbanagounder G., Welsbie D. S., Faull K. F., Navab M., Jung M. E., Fogelman A. M., Berliner J. A. 1999. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. J. Biol. Chem. 274: 24787–24798 [DOI] [PubMed] [Google Scholar]
- 24.Jones M. K., Anantharamaiah G. M., Segrest J. P. 1992. Computer programs to identify and classify amphipathic alpha helical domains. J. Lipid Res. 33: 287–296 [PubMed] [Google Scholar]
- 25.Segrest J. P., Jones M. K., Mishra V. K., Anantharamaiah G. M.2002. Experimental and computational studies of the interactions of amphipathic peptides with lipid surfaces. In Current Topics in Membranes Peptide-Lipid Interactions, A. S. Sidney, editor. Academic Press, San Diego, CA. 397–435.
- 26.Mishra V. K., Palgunachari M. N., Krishna N. R., Glushka J., Segrest J. P., Anantharamaiah G. M. 2008. Effect of leucine to phenylalanine substitution on the nonpolar face of a class A amphipathic helical peptide on its interaction with lipid. J. Biol. Chem. 283: 34393–34402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navab M., Anantharamaiah G. M., Hama S., Garber D. W., Chaddha M., Hough G., Lallone R., Fogelman A. M. 2002. oral administration of an apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 105: 290–292 [DOI] [PubMed] [Google Scholar]
- 28.Mishra V. K., Palgunachari M. N., Segrest J. P., Anantharamaiah G. M. 1994. Interactions of synthetic peptide analogs of the class A amphipathic helix with lipids. Evidence for the snorkel hypothesis. J. Biol. Chem. 269: 7185–7191 [PubMed] [Google Scholar]
- 29.Segrest J. P., De L. H., Dohlman J. G., Brouillette C. G., Anantharamaiah G. M. 1990. Amphipathic helix motif: classes and properties. Proteins. 8: 103–117 [DOI] [PubMed] [Google Scholar]
- 30.Livingstone J. R., Spolar R. S., Record M. T. 1991. Contribution to the thermodynamics of protein folding from the reduction in water-accessible nonpolar surface area. Biochemistry. 30: 4237–4244 [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Vidal M., Jayasinghe S., Ladokhin A. S., White S. H. 2007. Folding amphipathic helices into membranes: amphiphilicity trumps hydrophobicity. J. Mol. Biol. 370: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shore V. G., Sae A. S., Shore B. 1978. Surface exposure of apolipoproteins in high density lipoproteins: I. Reactivities with agarose-immobilized proteases. Biochim. Biophys. Acta. 529: 319–330 [DOI] [PubMed] [Google Scholar]