Abstract
Microsomal triglyceride transfer protein (Mtp) inhibitors represent a novel therapeutic approach to lower circulating LDL cholesterol, although therapeutic development has been hindered by the observed increase in hepatic triglycerides and liver steatosis following treatment. Here, we used small interfering RNAs (siRNA) targeting Mtp to achieve target-specific silencing to study this phenomenon and to determine to what extent liver steatosis is induced by changes in Mtp expression. We observed that Mtp silencing led to a decrease in many genes involved in hepatic triglyceride synthesis. Given the role of diacylglycerol O-acyltransferase 2 (Dgat2) in regulating hepatic triglyceride synthesis, we then evaluated whether target-specific silencing of both Dgat2 and Mtp were sufficient to attenuate Mtp silencing-induced liver steatosis. We showed that the simultaneous inhibition of Dgat2 and Mtp led to a decrease in plasma cholesterol and a reduction in the accumulation of hepatic triglycerides caused by the inhibition of Mtp. Collectively, these findings provide a proof-of-principle for a triglyceride synthesis/Mtp inhibitor combination and represent a potentially novel approach for therapeutic development in which targeting multiple pathways can achieve the desired response.
Keywords: RNAi, combination therapies, low density lipoprotein cholesterol, dyslipidemia, atherosclerosis
Elevated low density lipoprotein cholesterol (LDL-c) is a risk factor for cardiovascular disease, and it is well established that lowering LDL-c can reduce the risk of coronary events, where a 1% decrease in LDL-c leads to a 1% drop in risk (1–3). Over the last several decades, significant progress has been made in understanding the underlying lifestyle and hereditary factors that influence circulating LDL-c, leading to effective treatment strategies that have significantly reduced the incidence of death due to coronary events (4). Despite the significant progress that has been made in attenuating LDL-c levels through lifestyle changes and pharmacological means, many individuals are unable to reach their target LDL-c levels, creating a need for additional therapies.
LDL-c consists of a single apolipoprotein B-100 (ApoB 100) molecule, which serves as a scaffold for lipids (cholesterol, cholesterol esters, and triglycerides) to attach to while circulating within blood (5). LDL-c is produced from very low density lipoprotein (VLDL), which is assembled and secreted by the liver (5). A novel therapeutic approach to lower LDL-c that is currently in clinical development involves blocking VLDL assembly and secretion by inhibiting the microsomal triglyceride transfer protein (Mtp) (Fig. 1). Mtp inhibitors have been shown to reduce circulating levels of VLDL and LDL in several animal models and in man, demonstrating the potential for an Mtp-targeted therapeutic (6–9). Yet, the promise of an Mtp-targeted therapeutic has been dampened by the observation that hepatic Mtp inhibition results in increased hepatic triglycerides, leading to liver steatosis (10–12). Nonabsorbable enterocyte-specific Mtp inhibitors have been developed as a means to circumvent liver steatosis, only to shift the problem to the small intestines (13–15). An attractive alternative would be to block the accumulation of hepatic triglycerides by the simultaneous inhibition of another target.
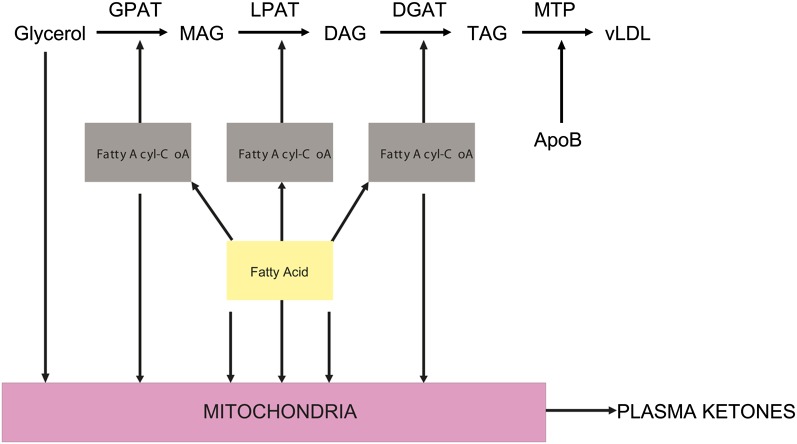
Fig. 1.
Triglyceride, VLDL, and plasma ketone production pathway. In the glycerol phosphate pathway, GPAT catalyzes the synthesis of mono-acyl glycerol (MAG), followed by conversion to diacyl glycerol (DAG) by LPAT, which in turn gets converted to triacylglycerols (TAG) by DGAT. MTP loads TAG onto ApoB to form the VLDL particles. The alternate pathway shown is fatty acid oxidation to plasma ketones. [Adapted from R. A. Coleman, Mol Cell Biol 22 (2002) page 8204]
We used siRNAs targeting Mtp to achieve target-specific silencing to determine to what extent hepatic steatosis is induced by changes in Mtp expression. We found that Mtp silencing led to a significant reduction in circulating cholesterol as well as an increase in hepatic triglycerides, leading to liver steatosis. Gene expression analysis of 96 genes involved in hepatic lipid metabolism revealed robust changes in many genes following Mtp silencing, including a decrease in many genes involved in hepatic triglyceride synthesis. Dgat2 catalyzes the last step in triglyceride synthesis, and Dgat2 silencing has been shown to alter multiple genes involved in hepatic triglyceride synthesis, suggesting that targeting Dgat2 may suppress multiple points within the triglyceride synthesis pathway. By utilizing two siRNAs specifically targeting Dgat2 and Mtp, we evaluated the ability of Dgat2 siRNA treatment to improve Mtp siRNA-induced liver steatosis, and we showed that the simultaneous inhibition of both Dgat2 and Mtp resulted in a decrease in plasma cholesterol and an attenuation of hepatic triglyceride accumulation, induced by Mtp silencing.
MATERIALS AND METHODS
siRNA design, synthesis, encapsulation, and selection
The siRNA sequences were designed and synthesized as described previously (16, 17). siRNA strands were annealed in an equimolar ratio. The duplexes were ultrafiltered and lyophilized. Duplex purity was evaluated using LC/MS and tested for the presence of endotoxin by standard methods. The purified duplexes were encapsulated using LNP compositions by jet mixing the siRNA and lipid solutions using a previously published protocol (18).
Screening and selection were performed as previously described (19). The sequence, chemical modifications, and in vitro screening results for the siRNAs used are listed in supplementary Table I. The selected siRNAs all demonstrated >80% knockdown (liver) three days after a single 3 mg/kg siRNA dose intravenously.
In vivo
Mice homozygous for the Ldlr gene (Ldlr+/−) were cross-bred with mice homozygous for the human CETP transgene (CETP+/−) driven by the endogenous ApoA1 promoter (19). This led to an elevation in LDL, providing a better window to assess reductions in LDL following siRNA treatment. Additionally, the cholesterol profile of this line more closely resembles the HDL-to-LDL ratio observed in humans (20). Male mice, 16–17 weeks of age, were group housed at 22°C on a 12:12 h light/dark cycle and fed ad libitum (Lab Diets 5020 9F, containing 9% crude fat and 0.021% cholesterol). Animals were intravenously injected with siRNAs and euthanized at the specified time points. Immediately after euthanasia, plasma was collected in heparinized plasma separator tubes. Livers were harvested and portioned into tubes containing either RNAlater or 10% neutral buffered formalin, or they were flash-frozen and stored for future analysis. All animal studies were conducted at Merck Research Laboratories and were approved by Institutional Animal Care and Use Committees (IACUC) and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
RNA isolation and qRT-PCR
Liver RNA isolation and quantitative real-time PCR were performed using Qiagen's RNeasy96 Universal Tissue Kit together with TaqMan Gene Expression reagents according to the supplied product protocol. TaqMan Gene Expression Assays (Applied Biosystems) were performed as described within the product protocol using the following primer probes: Mm00435015_m1 for Mtp, Mm00499536_m1 for Dgat2, Mm01545156_m1 for ApoB, and Mm99999915_g1 for Gapdh, which served as the reference. All reactions were performed in duplicate, and data were analyzed using the ddCt method relative to Gapdh and the negative control treatment (21). Data are represented as percentage expression relative to the control siRNA.
Plasma cholesterol, triglycerides, ketones, and free fatty acids analysis
Plasma cholesterol, triglycerides, ketones, and free fatty acids were determined within 3 to 4 h postcollection. Plasma total and HDL cholesterol levels were determined using Wako Diagnostic's total and HDL kits (431-52501, 439-17501) according to the supplied product protocol. Non-HDL was calculated by subtracting HDL from total cholesterol measurements. Plasma triglyceride levels were determined using Fisher Diagnostic Infinity Triglycerides Reagent (TR22321) and the supplied product protocol. Triglyceride levels were calculated from Sigma-Aldrich's Glycerol Standard Solution (G7793). Plasma ketone levels were determined using Wako Diagnostic's Total Ketone Bodies kit (415-73301, 411-73401) and the supplied product protocol. Ketone levels were calculated from Wako Diagnostics’ Ketone Body Calibrator 300 (412-73791). Plasma free fatty acids levels were determined using Wako Diagnostics’ NEFA-HR (2) kit (999-34691, 995-34791, 991-34891, 993-35191) and the supplied product protocol. Free fatty acid levels were calculated from Wako Diagnostic's NEFA Standard Solutions (276-76491, 997-76491).
Hepatic triglycerides
Mouse liver samples of ∼300 mg were frozen and stored at −80°C until analysis. Individual samples were extracted according to the Folch method, homogenized in 6 ml of chloroform:methanol (2:1), and then 4 ml water was added, thoroughly mixed, and centrifuged at 1,000 g for 15 min (22). The chloroform layer was removed and dried under nitrogen. The extracted lipids were redissolved in 1 ml chloroform, and 0.2 ml was transferred into HPLC sample vials. Samples were dried under nitrogen and redissolved in 2 ml hexane:isopropanol (98.8:1.2).
Liquid chromatography was performed as described previously using an isocratic mobile phase containing 98.8% hexane and 1.2% isopropanol at a flow rate of 0.5 ml/min through a Zorbax Sil (4.6 × 25 cm) silica column (Agilent Technologies #880952-701) (23). Lipids in a 5 μl injection were detected by absorbance at 206 nm and quantified by computer integration (Waters HPLC 2695 system with a 2996 PDA detector) of AUCs. Triglyceride concentrations were determined by comparison to standard curves using Nonpolar Lipid Mix-B (C/N 1130; Matreya Inc.).
Histology and hematology
Mouse livers were preserved in 10% neutral buffered formalin and paraffin embedded. Samples were sectioned 5 μm thick, and serial sections were stained with H and E (overall liver health), Masson's trichrome (fibrosis), and osmium (lipidosis). The H and E- and Masson's trichrome-stained samples were evaluated and scored by a board-certified veterinary pathologist. The osmium-stained sections were digitized and quantified using an Aperio Scanscope XT. An evaluation of liver function was performed by analyzing plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) from 75 μl of plasma using the ACE Alera Clinical Chemistry System (Alfa Wassermann Inc.).
Mathematical model
We constructed a reaction model similar to previously published models to gain insight into the effects of MTP inhibition on the production of VLDL (24). The mathematical model is a system of three ordinary differential equations that describes the rate kinetics of each process: hepatic triglyceride (TAG) synthesis from diacylglycerol pool (DAG) by Dgat2, transfer of TAG by Mtp onto ApoB, and formation of VLDL particles as well as ketone production and clearance.
We used three experimental datasets (Mtp, ApoB, and control siRNA datasets) to calibrate the mathematical model. We used Matlab-Simbiology software (MathWorks, Natick, MA) to solve the equations numerically. Model parameters were estimated by comparing the simulations with experimental data.
RESULTS
Sustained silencing of ApoB or Mtp led to an increase in hepatic triglycerides
To investigate the effects of prolonged siRNA-mediated silencing of ApoB or Mtp, we administered a 3 mg/kg siRNA dose intravenously every other week for up to 10 weeks to Ldlr+/−/hCETP+/− transgenic mice fed ad libitum. Liver samples were collected from separate animal cohorts that were euthanized on days 14, 28, 42, and 70. Greater than 75% knockdown of each target was observed at all time points, indicating that siRNA-mediated knockdown could be maintained for at least 10 weeks by twice-monthly administration (Fig. 2A, B).
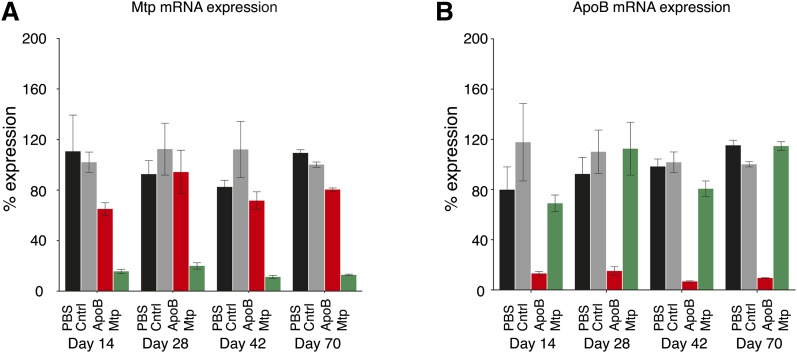
Fig. 2.
A 3 mg/kg siRNA dose intravenously every 14 days resulted in sustained mRNA silencing of Mtp or ApoB relative to control treatments [PBS or a control siRNA (Cntrl)]. A: Mtp and (B) ApoB mRNA expression is shown. Data represent individual groups of animals taken down on days 14, 28, 42, and 70. Data are shown as means ± SD bars (N = 8 per group).
Sustained knockdown of either ApoB or Mtp resulted in significant reductions in plasma non-HDL cholesterol levels at every time point (Fig. 3A). Plasma ketones were elevated on days 28, 42, and 70 for both Mtp and ApoB siRNA treatments, suggesting that fatty acid oxidation had increased (Fig. 3B). Plasma triglycerides were significantly reduced at the early time points but returned to control treatment levels by day 70 (Fig. 3C). Together, these data point to a similar change in plasma lipids following Mtp and ApoB siRNA treatments, and they suggest a possible compensatory response that results in plasma triglycerides increasing back to control treatment levels following prolonged silencing of either target.
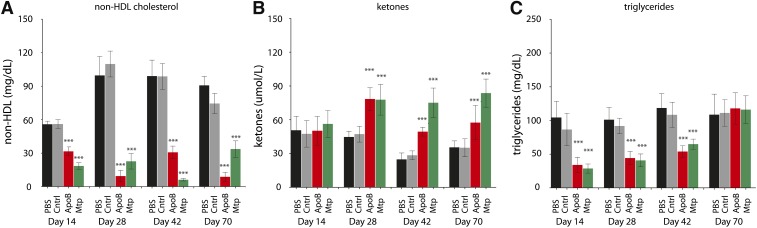
Fig. 3.
Analysis of plasma non-HDL (A), ketones (B), and triglycerides (C) following sustained silencing of either Mtp or ApoB mRNA expression. A: A 45%, 91%, 69%, and 88% reduction in non-HDL was observed for the ApoB siRNA-treated groups on days 14, 28, 42, and 70 relative to the control treatment. A 67%, 79%, 94%, and 55% reduction in non-HDL was observed for the Mtp siRNA–treated groups on days 14, 28, 42, and 70 relative to control. B: A 5.7%, 66%, 74%, and 64% increase in plasma ketones was observed for the ApoB siRNA-treated groups on days 14, 28, 42, and 70 relative to control. Mtp siRNA treatment resulted in a 19%, 65%, 164%, and 138% increase in plasma ketones on days 14, 28, 42, and 70 relative to control. C: ApoB siRNA treatment resulted in a 61%, 52%, 51%, and 6% reduction in plasma triglycerides on days 14, 28, 42, and 70. A 67%, 56%, 40%, and 4% increase in plasma triglycerides was observed for the Mtp siRNA treatment on days 14, 28, 42, and 70 relative to control. Data are shown as means ± SD bars (N = 8 per group). Significance was calculated using one-way ANOVA and Tukey's post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001.
Both ApoB-targeting siRNAs and MTP inhibitors have been shown to increase hepatic triglycerides (7, 9, 14, 18). MTP is required for loading triglycerides onto nascent lipoprotein particles (8). ApoB serves as the scaffold, ultimately transporting triglycerides out of the liver (5). Therefore, the observed increase in hepatic triglycerides following the knockdown or inhibition of either target likely results from a reduced rate of triglyceride breakdown or flux out of the liver relative to the rate of synthesis. We next evaluated hepatic triglyceride levels using both histopathological analysis of osmium-stained liver sections and biochemical analysis of hepatic triglyceride content to determine whether the transient decrease in plasma triglycerides corresponded to an increase in hepatic triglycerides. Image analysis of osmium-stained sections revealed similar levels of significant lipid accumulation for the ApoB and Mtp siRNA treatment groups relative to the PBS and control siRNA groups (Fig. 4A, B). This was supported by the observed elevation in hepatic triglycerides determined by HPLC analysis (Fig. 4C).
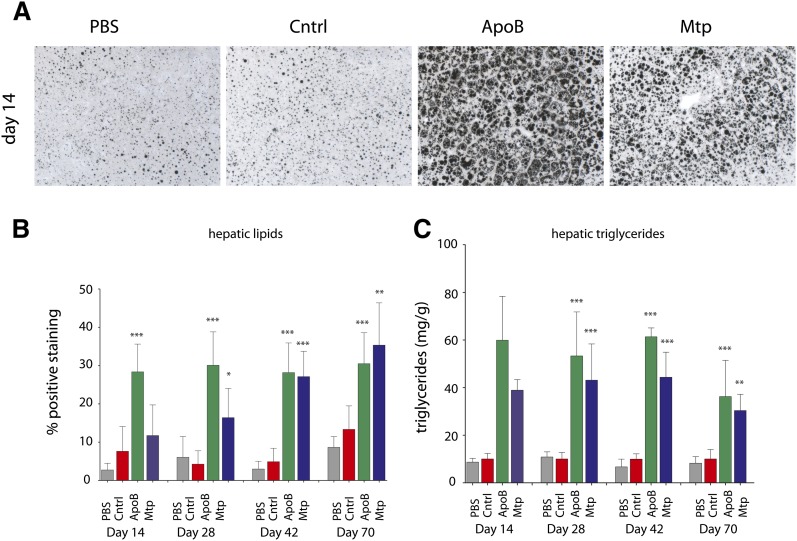
Fig. 4.
Analysis of hepatic lipid levels following sustained mRNA silencing of either Mtp or ApoB. A: Representative osmium-stained images for each group on day 14. B: Quantification of osmium-stained sections from two representative images for each animal within each group (N = 8 per group). Lipid accumulation on days 14, 28, 42, and 70 following a 3 mg/kg dose every 14 days increased 273%, 608%, 474%, and 129% relative to control for the ApoB-treated groups and increased 54%, 285%, 453%, and 165% relative to control for the Mtp-treated groups. C: Hepatic triglycerides analyzed by HPLC. Triglyceride accumulation on days 14, 28, 42, and 70 following a 3 mg/kg dose every 14 days increased 600%, 530%, 610%, and 360% relative to control for the ApoB-treated groups and increased 390%, 430%, 440%, and 300% relative to control for the Mtp-treated groups. B, C: Data are represented as means ± SD bars (N = 8 per group). Significance was calculated using one-way ANOVA and Tukey's post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001.
To investigate whether Mtp or ApoB siRNA treatment altered the expression level of genes within the triglyceride synthesis pathway, we measured the expression level of 96 genes involved in hepatic lipid metabolism using a qRT-PCR array. Interestingly, both Mtp and ApoB siRNA treatments resulted in a significant decrease in many genes that are key regulators of fatty acid and triglyceride synthesis as early as the day 14 time point (supplementary Table II). Yet, hepatic triglycerides remained elevated, suggesting either that there is a delayed response or that the resulting decrease in the expression of the regulators of hepatic triglyceride synthesis is not sufficient to alleviate triglyceride accumulation.
Dgat2 silencing attenuates liver steatosis induced by Mtp siRNA treatment
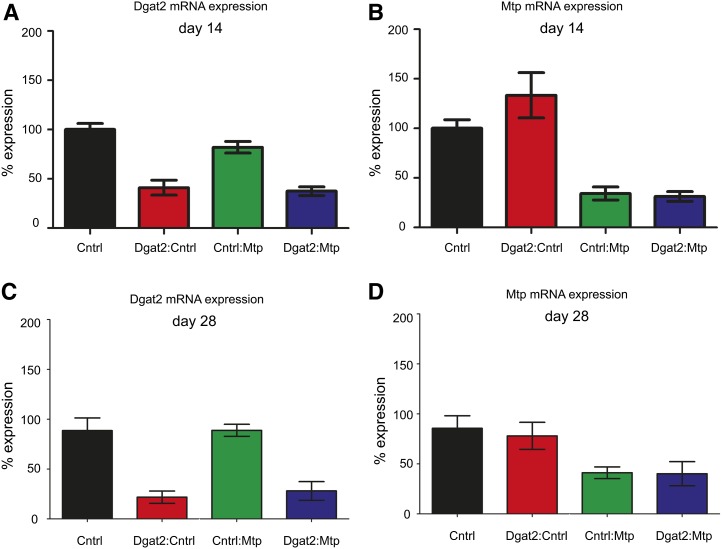
Dgat2 catalyzes the last step in triglyceride synthesis, and reducing Dgat2 expression reduces the expression of multiple genes within the hepatic triglyceride synthesis pathway (25). Given Dgat2’s role in regulating triglyceride synthesis, we then evaluated whether target-specific silencing of Dgat2 could attenuate the Mtp siRNA-induced accumulation of hepatic triglycerides by simultaneously administering siRNAs specifically targeting Dgat2 and Mtp. We coadministered intravenously siRNAs targeting Dgat2 and Mtp to Ldlr+/−/hCETP+/− transgenic male mice fed ad libitum. Mice received a 3 mg/kg total siRNA dose consisting of either a 3 mg/kg dose of the control siRNA or a 1.5:1.5 mg/kg siRNA mixture in a single injection (Dgat2:control siRNA, Mtp:control siRNA, or Dgat2:Mtp siRNA). Similar levels of knockdown were observed for Mtp+control and Mtp+Dgat2 treatments as well as for the Dgat2+control and Mtp+Dgat2 treatments at 14 days following a single treatment (Fig. 5A, B) and at 28 days following two treatments administered on days 0 and 14 (Fig. 5C, D), indicating that both targets could be silenced simultaneously.
Fig. 5.
Dgat2 and Mtp mRNA silencing following an individual or combination treatment. Dgat2 (A, C) and Mtp (B, D) mRNA expression compared with control-treated groups on day 14 (A, B) and day 28 (C, D). A single 1.5:1.5 mg/kg or 3 mg/kg dose was administered to the day 14 groups. A 1.5:1.5 or 3 mg/kg dose was administered on days 0 and 14 to the day 28 groups. Cntrl represents the control siRNA treatment. Groups labeled with two treatments were given a combination dose. Data are shown as mean % expression (bars) ± SD bars (N = 8 per group).
This treatment led to reductions in circulating cholesterol levels. Fourteen days following a single dose, similar reductions in non-HDL cholesterol were observed for the Mtp+control siRNA and Mtp+Dgat2 siRNA treatment groups, respectively (Fig. 6A). Similar results were observed on day 28 when siRNAs were administered on day 0 and day 14 (Fig. 6B).
Fig. 6.
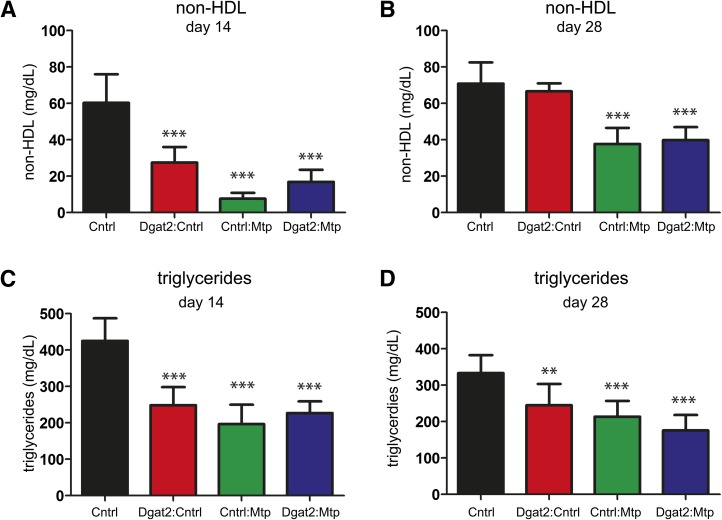
Analysis of plasma non-HDL (A, B) and triglycerides (C, D) following Dgat2 and Mtp siRNA treatment alone or in combination reveals similar reductions in both non-HDL and triglycerides on day 14 (A, C) and on day 28 (B, D) for the single and combination treatments. The day 14 groups received a single dose (day 0). The day 28 groups received two doses (day 0 and day 14). Data are shown as means ± SD bars (N = 8 per group). Significance was calculated using one-way ANOVA and Tukey's post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001.
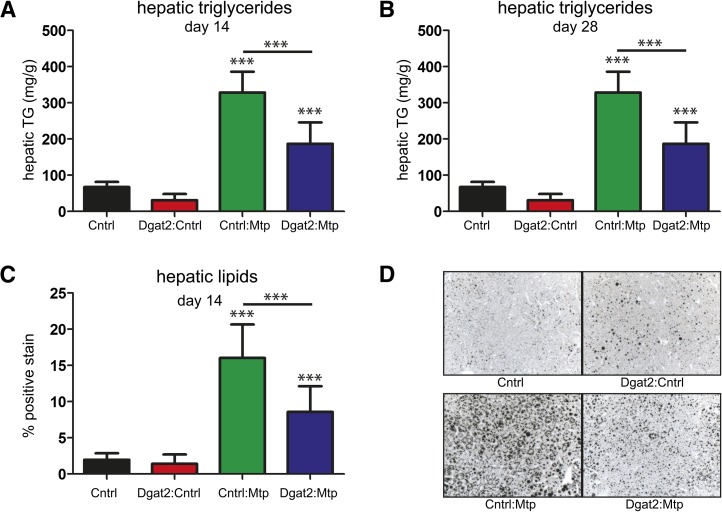
Plasma triglycerides were significantly reduced across all treated groups on day 14 following a single treatment (on day 0) and on day 28 following two treatments (on days 0 and 14) (Fig. 6C, D). When the Mtp siRNA was simultaneously administered with a siRNA targeting Dgat2, we failed to observe the same level of triglyceride accumulation within the liver, suggesting that Dgat2 silencing can attenuate Mtp siRNA-induced steatosis (Fig. 7A, B). Image analysis of osmium-stained liver sections served as an independent method to evaluate lipid accumulation within the liver and revealed a similar trend, with 53.5% less osmium staining in the Dgat2+Mtp group compared with the Mtp group 14 days following a single dose (Fig. 7C, D). Taken together, these data demonstrate the ability of Dgat2 silencing to reduce hepatic steatosis while maintaining the beneficial effects of lowering serum cholesterol and triglyceride levels induced by Mtp siRNA treatment.
Fig. 7.
Analysis of hepatic lipid levels following Dgat2 and Mtp siRNA treatments alone or in combination. Hepatic triglycerides were analyzed using enzymatic colorimetric method for samples collected on day 14 following a single treatment (A) and on day 28 following two treatments (B). C: Image analysis of osmium-stained liver sections and percentage positive osmium-stained liver sections at day 14. Quantification of osmium-stained sections was from two representative images for each animal within each group. A–C: Data are shown as means ± SD bars (N = 8 per group). Significance was calculated using one-way ANOVA and Tukey's post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. D: Representative images of osmium-stained control siRNA, Dgat2:control siRNA, Mtp:control siRNA, and Dgat2:Mtp siRNA livers.
DISCUSSION
The central role of Mtp in VLDL assembly has generated interest in Mtp as a therapeutic target for the treatment of hyperlipidemia. However, enthusiasm for Mtp-targeted therapeutics has waned given that Mtp inhibitors can lead to increased hepatic triglycerides and liver steatosis (10–12). The fact that we observed similar findings using a different modality (siRNAs) suggests that this is mediated by targeting Mtp and not a nonspecific effect.
We observed similar results for both Mtp and ApoB siRNA–mediated silencing, which is not surprising given that both are required for VLDL assembly. The level of hepatic steatosis observed for ApoB siRNA treatment is consistent with our previous results (20). It is worth noting, however, that liver steatosis was not reported when antisense oligonucleotides (ASO) were used to knock down ApoB (26). Following ApoB ASO treatment, modest but not significant increases in hepatic triglycerides were observed at week 6 that resolved by week 20 following biweekly administration (27). However, greater ApoB knockdown and cholesterol lowering occur with siRNA treatment, which may account for the discrepancy.
Both Mtp and ApoB silencing resulted in an accumulation of hepatic triglycerides. Non-HDL cholesterol remains significantly lower following sustained silencing of either ApoB or Mtp, although plasma triglycerides return to control treatment levels. The triglyceride source remains unclear. However, given that hepatic VLDL production is inhibited, it remains likely that VLDL production by the small intestine played a compensatory role. The diet used in these studies contained 9% crude fat and 0.021% cholesterol. Therefore, it is possible that the very low level of dietary cholesterol relative to fat and the increased contribution of the small intestine to VLDL production, which resulted in a shift in the cholesterol-to-triglyceride ratio within non-HDL, could explain why plasma non-HDL cholesterol remains significantly lower following sustained silencing of either ApoB or Mtp, whereas plasma triglycerides return to control treatment levels.
Both ApoB and Mtp knockdown resulted in a decrease in HDL. This is consistent with our previous results for siRNA-mediated knockdown of ApoB (19, 20). Although the cause of the decrease in HDL is unknown, it is likely that the HDL components provided by ApoB containing particles are reduced following knockdown, leading to a reduction in HDL levels (19, 28).
Nonabsorbable enterocyte-specific Mtp inhibitors are currently in development as a means to avoid inhibiting hepatic Mtp, thereby transferring the problem of triglyceride accumulation to the small intestine (13–15). An attractive alternative would be to block the accumulation of hepatic triglycerides by the simultaneous inhibition of another target. siRNAs are well positioned for evaluating the effect of simultaneously inhibiting multiple targets, and siRNA combinations may represent an attractive alternative to traditional small-molecule combination therapies, which rely on adding a novel compound on top of an approved drug. Although more work is needed, therapeutic development of siRNA combinations is well underway as highlighted by the fact that siRNA combinations for cancer are currently in clinical development.
Irrespective of the modality, these data provide a proof of principle for a triglyceride synthesis/Mtp inhibitor combination. Dgat2 catalyzes the last step in triglyceride synthesis, and Dgat2 knockdown has been shown to alter multiple genes involved in hepatic triglyceride synthesis, suggesting that targeting Dgat2 may suppress multiple points within the triglyceride synthesis pathway. In addition, reducing Dgat2 expression by ASO treatment has been shown to increase fatty acid oxidation (29). Consistent with these previous results, we found that Dgat2 silencing increased plasma ketone levels and that plasma ketones were significantly higher for the Mtp+Dgat2 combination relative to either the Mtp or Dgat2 siRNA treatments alone (data not shown). Therefore, Dgat2 silencing likely attenuates Mtp knockdown-induced liver steatosis by both decreasing triglyceride synthesis and increasing fatty acid oxidation.
It is worth noting that attenuation of hepatic steatosis induced by an MTP inhibitor was observed in mice overexpressing shRNA targeting either Dgat2 or Gpat (H. Zhou, personal communication). The Dgat2 shRNA × MTP inhibitor data represent an independent confirmation of our results with the Dgat2 and Mtp siRNAs. The findings are in line with the data reported here and suggest that inhibiting either the first step (Gpat) or the last step (Dgat2) of triglyceride synthesis will reduce hepatic triglyceride accumulation. Finally, it is worth noting that a GPAT inhibitor has been shown to increase fat metabolism and reduce hepatic lipid accumulation in db/db mice (30). The fact that both reduce MTP inhibition-induced hepatotoxicity suggests that hepatic inhibition of MTP may be a viable therapeutic when combined with either a GPAT or DGAT2 inhibitor. Because Dgat2 knockdown alters multiple genes involved in hepatic triglyceride synthesis, including Gpat, Dgat2 may be better suited for reducing many of the genes involved in triglyceride synthesis. We evaluated a Dgat2+ApoB combination and found that coadministration reduced ApoB silencing-induced hepatic steatosis, although the level of attenuation was not as great as observed with Mtp (data not shown).
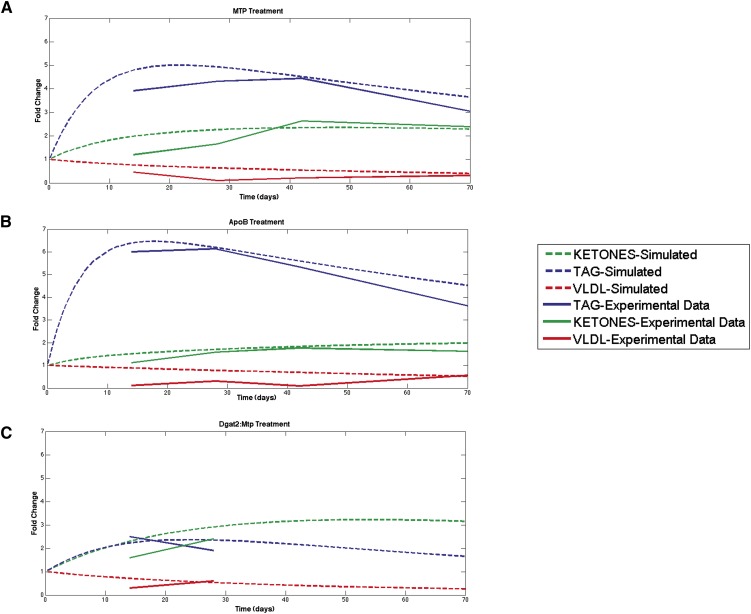
There are likely multiple therapeutically attractive targets for the alleviation of ApoB or Mtp inhibitor-induced steatosis. Toward a more systematic assessment of potential candidates, a mathematical model was built to better understand the relationship between the buildup of hepatic triglycerides and changes in plasma cholesterol, triglycerides, and ketone levels following Mtp and ApoB siRNA treatments. The fold change for each measurement was analyzed, and a simulation was generated to fit the data for both Mtp and ApoB treatments. Interestingly, the mathematical model predicts a transient buildup of hepatic triglycerides upon Mtp or ApoB silencing that occurs beyond the last time point measured in this study (Fig. 8A, B). It is important to note that, although we did not observe a significant decrease in the accumulation of hepatic triglycerides following the sustained knockdown of either ApoB or Mtp, it remains possible that the level of hepatic triglycerides eventually subsides.
Fig. 8.
Mathematical modeling of prolonged silencing effect on hepatic triglycerides, plasma ketone levels, and VLDL levels for Mtp, ApoB, and Dgat2:Mtp treatment groups. Model predictions compared with experimental data (Figs. 3, 4) of ApoB (A) and Mtp (B). C: Model predictions of Dgat2:Mtp combination upon prolonged silencing and the corresponding experimental data on days 14 and 28.
Using the parameters from the Mtp treatment, we simulated the effect of Dgat2 inhibition on triglyceride kinetics. We performed a parameter scan on k1 and fit the simulations to the experiment days 14 and 28 data. Consistent with our observations, the model predicts a decrease in the kinetics of triglyceride synthesis following Dgat2 silencing, which, when coupled with the kinetics of reduced VLDL production by Mtp silencing, resulted in an increase in plasma ketone levels and a blunted increase in hepatic triglycerides (Fig. 8C). Going forward, it is our hope to leverage and refine this model to test different therapeutic strategies to reduce the elevation of hepatic triglycerides caused by an Mtp-targeted therapeutic. The current model suggests that plasma ketones, an indirect measure of the level of fatty acid oxidation, may play an influential role in modulating the accumulation of hepatic triglycerides caused by reduced Mtp expression.
Supplementary Material
Acknowledgments
The authors thank Duncan Brown for siRNA design, Dipali Ruhela and her group for providing the LNP formulations, and Premier Laboratory for histological services and histopathological review. The authors also thank Harry Davis, Jr. and Stephen Previs for their knowledgeable input.
Footnotes
Abbreviations:
- ASO
- antisense oligonucleotide
- Dgat2
- diacylglycerol O-acyltransferase 2
- LDL-c
- low density lipoprotein cholesterol
- Mtp
- microsomal triglyceride transfer protein
- siRNA
- small interfering RNA
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Davis R. A., Hui T. Y. 2001. George Lyman Duff Memorial Lecture: atherosclerosis is a liver disease of the heart. Arterioscler. Thromb. Vasc. Biol. 21: 887–898 [DOI] [PubMed] [Google Scholar]
- 2.Skalen K., Gustafsson M., Rydberg E. K., Hulten L. M., Wiklund O., Innerarity T. L., Boren J. 2002. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 417: 750–754 [DOI] [PubMed] [Google Scholar]
- 3.Hulthe J., Fagerberg B. 2002. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler. Thromb. Vasc. Biol. 22: 1162–1167 [DOI] [PubMed] [Google Scholar]
- 4.Fox C. S., Evans J. C., Larson M. G., Kannel W. B., Levy D. 2004. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 110: 522–527 [DOI] [PubMed] [Google Scholar]
- 5.Davidson N. O., Shelness G. S. 2000. APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 20: 169–193 [DOI] [PubMed] [Google Scholar]
- 6.Wetterau J. R., Harrity T. W., Arbeeny C., Cap M., Connolly F., Chu C. H., George R. J., Gordon D. A., Jamil H., Jolibois K. G., et al. 1998. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science. 282: 751–754 [DOI] [PubMed] [Google Scholar]
- 7.Shiomi M., Ito T. 2001. MTP inhibitor decreases plasma cholesterol levels in LDL receptor-deficient WHHL rabbits by lowering the VLDL secretion. Eur. J. Pharmacol. 431: 127–131 [DOI] [PubMed] [Google Scholar]
- 8.Hussain M. M., Shi J., Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32 [DOI] [PubMed] [Google Scholar]
- 9.Chandler C. E., Pettini W. D., Savoy J. L., Petras Y. E., Chang S. F., Vincent G., Harwood J., Jr H. J. 2003. CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J. Lipid Res. 44: 1887–1901 [DOI] [PubMed] [Google Scholar]
- 10.Davidson M. H. 2009. Novel nonstatin strategies to lower low-density lipoprotein cholesterol. Curr. Atheroscler. Rep. 11: 67–70 [DOI] [PubMed] [Google Scholar]
- 11.Pereira I. V., Stefano J. T., Oliveira C. P. 2011. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 5: 245–251 [DOI] [PubMed] [Google Scholar]
- 12.Rizzo M., Wierzbicki A. S. 2011. New lipid modulating drugs: the role of microsomal transport protein inhibitors. Curr. Pharm. Des. 17: 943–949 [DOI] [PubMed] [Google Scholar]
- 13.Vu C. B., Milne J. C., Carney D. P., Song J., Choy W., Lambert P. D., Gagne D. J., Hirsch M., Cote A., Davis M., et al. 2009. Discovery of benzothiazole derivatives as efficacious and enterocyte-specific MTP inhibitors. Bioorg. Med. Chem. Lett. 19: 1416–1420 [DOI] [PubMed] [Google Scholar]
- 14.Kim E., Campbell S., Schueller O., Wong E., Cole B., Kuo J., Ellis J., Ferkany J., Sweetnam P. 2011. A small-molecule inhibitor of enterocytic microsomal triglyceride transfer protein, SLx-4090: biochemical, pharmacodynamic, pharmacokinetic, and safety profile. J. Pharmacol. Exp. Ther. 337: 775–785 [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal D., West K. L., Zern T. L., Shrestha S., Vergara-Jimenez M., Fernandez M. L. 2005. JTT-130, a microsomal triglyceride transfer protein (MTP) inhibitor lowers plasma triglycerides and LDL cholesterol concentrations without increasing hepatic triglycerides in guinea pigs. BMC Cardiovasc. Disord. 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majercak J., Ray W. J., Espeseth A., Simon A., Shi X-P., Wolffe C., Getty K., Marine S., Stec E., Ferrer M., et al. 2006. LRRTM3 promotes processing of amyloid-precursor protein by BACE1 and is a positional candidate gene for late-onset Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 103: 17967–17972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wincott F., DiRenzo A., Shaffer C., Grimm S., Tracz D., Workman C., Sweedler D., Gonzalez C., Scaringe S., Usman N. 1995. Synthesis, deprotection, analysis and purification of RNA and ribozymes. Nucleic Acids Res. 23: 2677–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihaila R., Chang S., Wei A. T., Hu Z. Y., Ruhela D., Shadel T. R., Duenwald S., Payson E., Cunningham J. J., Kuklin N., et al. 2011. Lipid nanoparticle purification by Spin Centrifugation-Dialysis (SCD): A facile and high-throughput approach for small scale preparation of siRNA-lipid complexes. Int. J. Pharm. 420: 118–121 [DOI] [PubMed] [Google Scholar]
- 19.Tadin-Strapps M., Peterson L. B., Cumiskey A. M., Rosa R. L., Mendoza V. H., Castro-Perez J., Puig O., Zhang L., Strapps W. R., Yendluri S., et al. 2011. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J. Lipid Res. 52: 1084–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ason B., Castro-Perez J., Tep S., Stefanni A., Tadin-Strapps M., Roddy T., Hankemeier T., Hubbard B., Sachs A. B., Michael Flanagan W., et al. 2011. ApoB siRNA-induced liver steatosis is resistant to clearance by the loss of fatty acid transport protein 5 (Fatp5). Lipids. 46: 991–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 22.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 23.Burrier R. E., Smith A. A., McGregor D. G., Hoos L. M., Zilli D. L., Davis, Jr H. R. 1995. The effect of acyl CoA: cholesterol acyltransferase inhibition on the uptake, esterification and secretion of cholesterol by the hamster small intestine. J. Pharmacol. Exp. Ther. 272: 156–163 [PubMed] [Google Scholar]
- 24.Adiels M., Packard C., Caslake M. J., Stewart P., Soro A., Westerbacka J., Wennberg B., Olofsson S-O., Taskinen M-R., Borén J. 2005. A new combined multicompartmental model for apolipoprotein B-100 and triglyceride metabolism in VLDL subfractions. J. Lipid Res. 46: 58–67 [DOI] [PubMed] [Google Scholar]
- 25.Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276: 38870–38876 [DOI] [PubMed] [Google Scholar]
- 26.Mullick A. E., Fu W., Graham M. J., Lee R. G., Witchell D., Bell T. A., Whipple C. P., Crooke R. M. 2011. Antisense oligonucleotide reduction of apoB-ameliorated atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 52: 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crooke R. M., Graham M. J., Lemonidis K. M., Whipple C. P., Koo S., Perera R. J. 2005. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J. Lipid Res. 46: 872–884 [DOI] [PubMed] [Google Scholar]
- 28.Lieu H. D., Withycombe S. K., Walker Q., Rong J. X., Walzem R. L., Wong J. S., Hamilton R. L., Fisher E. A., Young S. G. 2003. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 107: 1315–1321 [DOI] [PubMed] [Google Scholar]
- 29.Yu X. X., Murray S. F., Pandey S. K., Booten S. L., Bao D., Song X. Z., Kelly S., Chen S., McKay R., Monia B. P., Bhanot S. 2005. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 42: 362–371 [DOI] [PubMed] [Google Scholar]
- 30.Kuhajda F. P., Aja S., Tu Y., Han W. F., Medghalchi S. M., El Meskini R., Landree L. E., Peterson J. M., Daniels K., Wong K., et al. 2011. Pharmacological glycerol-3-phosphate acyltransferase inhibition decreases food intake and adiposity and increases insulin sensitivity in diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301: R116–R130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.