Abstract
Agonist-induced lipolysis of adipose fat is robustly inhibited by insulin or by feedback inhibition by the long-chain fatty acids (LCFA) produced during lipolysis. However, the mode of action of LCFA in suppressing adipose lipolysis is not clear. β,β’-Tetramethyl hexadecanedioic acid (Mββ/ EDICA16) is a synthetic LCFA that is neither esterified into lipids nor β-oxidized, and therefore, it was exploited for suppressing agonist-induced lipolysis in analogy to natural LCFA. Mββ is shown here to suppress isoproterenol-induced lipolysis in the rat in vivo as well as in 3T3-L1 adipocytes. Inhibition of isoproterenol-induced lipolysis is due to decrease in isoproterenol-induced cAMP with concomitant inhibition of the phosphorylation of hormone-sensitive lipase and perilipin by protein kinase A. Suppression of cellular cAMP levels is accounted for by inhibition of the adenylate cyclase due to suppression of Raf1 expression by Mββ-activated AMPK. Suppression of Raf1 is further complemented by induction of components of the unfolded-protein-response by Mββ. Our findings imply genuine inhibition of agonist-induced adipose lipolysis by LCFA, independent of their β-oxidation or reesterification. Mββ suppression of agonist-induced lipolysis and cellular cAMP levels independent of the insulin transduction pathway may indicate that synthetic LCFA could serve as insulin mimetics in the lipolysis context under conditions of insulin resistance.
Keywords: cAMP, AMPK, Raf1, diabetes
Lipolysis of adipose fat stores results in the production of nonesterified long-chain fatty acids (LCFA) in response to changes in energy requirements and availability (reviewed in Ref. 1). Fatty acids derived by adipose fat lipolysis may either be reesterified into adipose fat, or serve as major source of oxidizable substrate for muscle activity and as precursor for the hepatic production of triacylglycerol-rich lipoproteins. Agonist-induced lipolysis is mediated by consecutive activation of the adenylate cyclase (AC), resulting in cAMP production and protein kinase A (PKA) activation by cAMP, followed by PKA-induced phosphorylation of hormone-sensitive lipase (HSL) at three serine residues (563, 659, and 660), resulting in its activation and translocation from the cytosol to the lipid-droplet surface. Concomitant with HSL phosphorylation, the phosphorylation of perilipin by PKA at multiple sites (S81, S222, S276, and S517) results in a dynamic restructuring of the lipid-droplet surface, in facilitating the translocation of phosphorylated HSL to the lipid droplet, and in activating its hydrolyzing activity. Perilipin phosphorylation by PKA further results in releasing CGI-58 from the lipid droplet, in its association with adipose triacylglycerol lipase (ATGL), and in activating its lipolytic activity. Activated ATGL, HSL, and monoglyceride lipase may act now consecutively in hydrolyzing adipose triacyglycerols to diacylglycerol, monoacylglycerol, and finally to free glycerol and LCFA. Agonist-induced cAMP and lipolysis is restrained by insulin due to cAMP hydrolysis by insulin-activated phosphodiesterase3B (PDE3B) (2), combined with insulin-induced reesterification of LCFA into adipose fat (reviewed in Ref. 3). Indeed, increased adipose efflux of free LCFA, due to increased adipose lipolysis and/or suppression of adipose LCFA reesterification, is considered a cornerstone of diabetes. Agonist-induced lipolysis is further robustly inhibited by the LCFA generated during lipolysis (4, 5). In fact, agonist-induced lipolysis is made possible only by removing the free nonesterified LCFA product through their binding to medium albumin or by frequent medium replacement (4, 5). The mode of action of LCFA in suppressing agonist-induced lipolysis still remains to be investigated, being ascribed to product inhibition of HSL by its association with fatty acid–bound aP2 (6), or alternatively, to futile cycling of LCFA between lipolysis and reesterification (7).
MEDICA analogs (8–12) consist of long-chain methyl-substituted α,ω-dicarboxylic acids [e.g., HOOC-CH2-C(β)(CH3)2-(CH2)10-C(β)(CH3)2-CH2-COOH defined as MEDICA16/Mββ]. MEDICA analogs are not esterified into lipids, and the methyl substitutions at the β,β’ positions block their β-oxidation. Similar to LCFA, MEDICA analogs may be thioesterified endogenously into their respective monoacyl-CoA thioesters, but CoA-thioesterification of MEDICA analogs does not result in sequestration of CoA and does not limit the CoA-thioesterification of endogenous LCFA or their β-oxidation (13). Hence, MEDICA analogs may simulate LCFA effects in the lipolysis context while avoiding its own esterification into adipose fat. Indeed, treatment of animal models of diabesity (e.g., Zucker, cp/cp, db/db, ob/ob) with MEDICA analogs has been reported to result in pronounced decrease in plasma FFA, hepatic glucose and lipoprotein production, with concomitant increase in total body glucose uptake and plasma lipoprotein clearance (9–12). In fact, the overall phenotype of MEDICA-treated animal models of diabesity appears to be essentially similar to that of HSL-knockouts (14), indicating that inhibition of agonist-induced lipolysis could play a role in total body sensitization to insulin by MEDICA analogs. The present report verifies the activity of Mββ in the context of agonist-induced lipolysis and dissects its mode of action in terms of modulating the AC/PKA transduction pathway of adipose lipolysis. Our findings may further indicate that synthetic LCFA may serve as insulin mimetics in the lipolysis context, while bypassing the insulin transduction pathway.
EXPERIMENTAL PROCEDURES
Animals
Male Wistar rats weighing 250–275 g and fed standard rodent diet (Teklad 2018) were daily dosed by gavage for five consecutive days with 200 mg Mββ/kg body weight in 1% carboxy methyl cellulose (CMC) or with vehicle only, in line with previously reported dose-efficacy studies (8–12). Fasting animals were then implanted with PE50 jugular vein cannula under ketamine/xylazine anesthesia and left to recover for 24 h. On the following day, the animals were dosed with Mββ or vehicle, followed 120 min later by single intraperitoneal injection of 10 mg isoproterenol/kg body weight. Blood samples (200 μl) were collected in EDTA at time points as indicated, and plasma glycerol levels were determined by using free glycerol reagent kit (#F6428, Sigma-Aldrich). Basal plasma glycerol levels were 0.26 ± 0.8 and 0.17 ± 0.1 μmol/ml for CMC- and Mββ-treated animals, respectively (not significant). Animal care and experimental procedures were in accordance with the accredited animal ethics committee of the Hebrew University.
Cell culture
3T3-L1 preadipocytes were first cultured in 10 cm Falcon plates in medium A, consisting of Dulbecco modified Eagle medium (DMEM) supplemented with 25 mM glucose, 10% bovine serum (Biological Industries), 2.0 mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 µg/ml amphotericin B (Biological Industries). When reaching 60–70% confluence, the cells were further cultured to confluence in 12-well plates (Cell Star) in medium A. For conversion to adipocytes, two-day postconfluent cultures were further cultured for three days in medium B, where the 10% bovine serum of medium A was replaced by 10% fetal calf serum (FCS) (Biological Industries), supplemented with 2.5 U/ml human insulin (Homulin, Eli Lilly), 4 μg/ml dexamethasone (Merck), and 0.5 mM 1-methyl-3-isobutyl-1-methylxantine (IBMX). Cells were further cultured for two days in the presence of 2.5 U/ml human insulin, followed by 7–10 days with insulin-free medium, being replaced with fresh medium every two days. For measuring lipolysis, 3T3-L1–differentiated adipocytes were cultured in DMEM medium supplemented with 10% FCS, in the presence of additions as indicated. One hour prior to glycerol measurement, the culture medium was replaced with Phenol Red-free DMEM medium, supplemented with 2% fatty-acid-free BSA (Roche) and additions as indicated. Glycerol release (arbitrary units) into the medium was determined by using free glycerol reagent kit (#F6428, Sigma-Aldrich). Basal glycerol release of 3T3-L1 cells cultured in 12-well plates were in the range of 0.85–2.1 µg/well.
COS-1 and HeLa cells were cultured in 12- or 24-well plates in DMEM, 1.0 mM glutamine, 100 U penicillin/ml, and 0.1 mg streptomycin/ml medium, supplemented with 10% FCS. All cultures were maintained at 37°C in a humidified atmosphere of 7% CO2 in air.
Cell extracts
3T3L-1 adipocytes were washed with cold phosphate-buffered saline (PBS) and extracted with cold 150 μl lysis buffer consisting of 10 mM Tris buffer (pH 8.0), 150 mM NaCl, 1% Triton X-100 (w/w), 0.24 mM sodium orthovanadate, 60 mM sodium fluoride, 60 mM octyl β-D-glucopyranoside (#O-8001, Sigma-Aldrich), 1.0 mM phenylmethylsulphonylfluoride (PMSF), 0.1 μg/ml okadaic acid, 40 nM bis-peroxo(1,10 phenanthroline) oxovanadate(V) (bpV(phen), and 1 μl/ml protease inhibitor mix (Sigma-Aldrich). The lysate was immediately sonicated on ice for 5 s using a tip sonicator (Ultrasonics), then further incubated in lysis buffer for 30 min at 4°C, and then centrifuged at 23,000 g for 15 min at 4°C. The aqueous phase, in between the cell precipitate and the floating upper lipid phase, was collected and stored at −70°C.
Extracts of COS-1 or HeLa cells were prepared by sonicating the cells in 3 vol of lysis buffer, followed by further incubation in lysis buffer for 30 min at 4°C. Lysates were cleared by centrifugation at 15,000 g for 10 min at 4°C, and the supernatant was kept at −70°C.
Protein content of cellular extracts was determined by the BCA protein assay (#23225, Pierce Biotechnology).
cAMP
3T3-L1–differentiated adipocytes (cultured in 12-well plates) or COS-1 cells (cultured in 24-well plates) were incubated with additions as indicated. cAMP was determined using an enzyme immunoassay kit (#RPN225, Amersham) according to manufacturer instructions.
Western blot analysis
Samples of 15–45 μg protein were resolved by 7–12.5% SDS-PAGE under reducing conditions and were then transferred onto polyvinylidene difluoride (Millipore) or cellulose nitrate membranes (Schleicher and Schuell). PKA-phosphorylated HSL (P-HSL) and perilipin (P-perilipin) were determined by anti-phosphoPKA-concensus site antibodies. Phosphorylated and total protein blots were carried out using the same lysates. Blots were probed with the indicated first antibody, followed by horseradish peroxidase–labeled second antibody. Bands were analyzed by ImageQuant software (Molecular Devices). Three or more experiments were used in presenting respective histograms.
Transfection
COS-1 cells, cultured in DMEM containing 10% FCS, were transfected with pEGFP-Raf-1 or pEGFP expression plasmids (T. Balla) (15) using TransIT-LT1 transfection reagent (Mirus Bio). Following 6 h of transfection, the cells were incubated in fresh medium for 18 h to allow for the expression of transfected plasmids, followed by 24 h in the presence of additions as indicated.
Real time PCR
Total RNA was prepared using the TRI reagent (Sigma-Aldrich). First-strand cDNA used as template was synthesized by reverse transcription using oligo(dT) as primer and the Reverse-iTMAX First Strand Kit (ABgene). Raf1, CHOP, and BiP transcripts normalized by tubulin were quantified by real-time PCR (Rotor Gene RG-3000A) using SYBER green MasterMix (Absolute Syber Green ROX Mix, ABgene) and the following primers:
Mouse Raf1 [F: 5′-AGTCAGCCTGAAGCATTGATGTC-3′, R: 5′-ATCCTGTCTTCCATCGAGCTGCTT-3′]; mouse CHOP [F: 5′-GTCCTGTCCTCAGATGAAATTGG-3′, R: 5′-GCAGGGTCAAGAGTAGTGAAGGTT-3′]; mouse BiP [F: 5′-A [CCTATTCCTGCGTCG-3′, R: 5-GCATCGAAGACCGTGT-3]; Tubulin [F: 5′-TAGCAGAGATCACCAATGCC-3′, R: 5′-GGCAGCAAGCCATGTATTTA-3′].
AMPKα1 silencing by siRNA
3T3-L1 adipocytes, cultured for three to five days after insulin removal, were transiently transfected with a pool of three siRNA oligonucleotides against mouse AMPKα1 (SC-29674), while scrambled siRNA (SC-37007, Santa Cruz Biotechnology) served as negative control. Briefly, 7.5 µl of siRNA and 4 µl of transfection reagent (Lipofectamine 2000, Invitrogen) were each diluted with 25 µl of serum-free media (Opti-MEM, Invitrogen), mixed, and further incubated for 20 min at room temperature. The transfection mixture was added drop by drop to each culture well containing 450 µl of serum-free media, reaching final siRNA concentrations of 150 nM. Transfected cells were incubated for 4 h, followed by adding to each well 500 µl of DMEM medium supplemented with 20% FBS. Following incubation for additional 24 h, the medium was replaced by DMEM medium supplemented with 10% FCS. The transfected cells were incubated for 24 h to allow for AMPKα1 silencing, followed by 24 h in the presence of additions as indicated. Cells were lysed as described above.
Materials
Rabbit polyclonal anti-mouse acetyl-CoA carboxylase (ACC) (#3662), rabbit polyclonal anti-mouse P-ACC(S79) (#3661S), rabbit polyclonal anti-mouse phosphoPKA-concensus site (#9261S), rabbit anti-mouse AMPK (#2532), rabbit anti-mouse P-AMPK(T172) (#2531S), rabbit polyclonal anti-human/mouse eIF2α (#9722), and P-eIF2α(S51) (#9721S) antibodies were from Cell Signaling Technology. Rabbit polyclonal anti-mouse Raf1 antibody (#SC-227) was from Santa Cruz Biotechnology. Rabbit polyconal anti-mouse HSL (#ab-45422) was from Abcam. Rabbit polyclonal anti-mouse perilipin A (#PA1-1051) antibody was from Affinity BioReagents. Mouse monoclonal anti-α-tubulin antibody (#T5168) was from Sigma-Aldrich. Horseradish peroxidase–labeled anti-mouse secondary antibody was from Jackson ImmunoResearch Laboratories. Forskolin, IBMX, N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (But2cAMP), 8-bromo-cAMP, 4-phenylbutyric acid (PBA), NG-methyl-L-arginine (L-NMMA), 5′-amino-5′-deoxy adenosine (AMDA), tunicamycin, thapsigargin, and cholera toxin (CTX) were from Sigma-Aldrich. Pertussis toxin (PTX), MDL 12,330A, and AICAR were from Calbiochem. Mββ (99.5% purity) was synthesized as previously described (8).
Data analysis
Statistical analysis was performed by one-way repeated measure ANOVA with Student-Newman-Keuls test. When only two groups were compared, significance was analyzed by paired t-test.
RESULTS
Inhibition of isoproterenol-induced lipolysis by Mββ
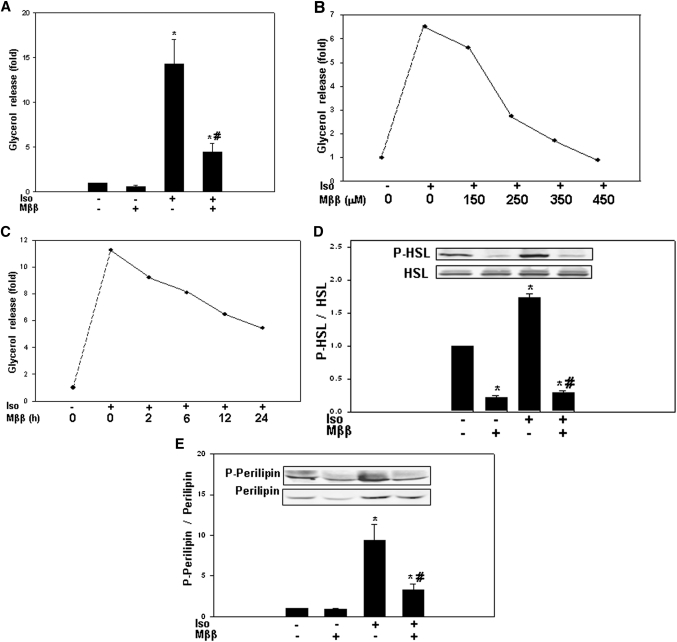
Suppression of adipose lipolysis by MEDICA in vivo was studied in Wistar rats treated with Mββ and then challenged with a single injection of isoproterenol (Fig. 1). Isoproterenol-induced glycerol release in vehicle-treated animals resulted in increase in plasma glycerol reaching its Cmax in about 60 min, followed by reverting gradually to basal glycerol levels. Treatment with Mββ resulted in shorter time to maximal level (Tmax), lower maximal level (Cmax), and 50% decrease in the total amount of glycerol release, implying inhibition of isoproterenol-induced adipose lipolysis in vivo.
Fig. 1.
Mββ inhibits isoproterenol-induced lipolysis in vivo. Wistar rats were treated with Mββ or CMC for six consecutive days followed by intraperitoneal isoproterenol challenge and plasma glycerol sampling as described in Experimental Procedures. A: Isoproterenol-induced increase in plasma glycerol over respective basal levels. Representative experiment consisting of CMC-treated animal (filled circles) and Mββ-treated animal (open circles). B: AUC(0–4 h) of plasma glycerol for the data sets of five independent experiments. Mean ± SE . *Significant compared with CMC-treated animals (P < 0.05).
In line with inhibition of adipose lipolysis by Mββ in vivo (Fig. 1) and in analogy to natural LCFA in vitro (4, 5), isoproterenol-induced lipolysis of 3T3-L1 adipocytes was robustly inhibited by Mββ (Fig. 2A), as well as by other MEDICA analogs (not shown). Inhibition by Mββ was concentration dependent, with an apparent IC50 of 200 μM (Fig. 2B). The µM concentrations of Mββ reflect the high binding affinity of MEDICA analogs to medium albumin [estimated to be higher than 99%, independent of Mββ concentrations in the range of 0–0.9 mM (unpublished results)], resulting in nanomolar concentrations of the free MEDICA acid in the culture medium. Inhibition by Mββ was progressive throughout the first 24 h of incubation (Fig. 2C), being already evident within the first 2 h. Inhibition of isoproterenol-induced lipolysis by Mββ was accompanied by inhibition of isoproterenol-induced phosphorylation of HSL (P-HSL) (Fig. 2D) and perilipin (P-perilipin) (Fig. 2E) by PKA.
Fig. 2.
Mββ inhibits isoproterenol-induced lipolysis in 3T3-L1 adipocytes. 3T3-L1 adipocytes were incubated for 24 h in the absence or presence of 350 μM Mββ, and in the absence or presence of 100 nM isoproterenol (Iso) added to the incubation medium 1 h prior to sampling. Lipolysis (A–C) was quantified by fold glycerol release as described in Experimental Procedures. P-HSL, HSL, P-perilipin, and perilipin (D, E) were determined by SDS-PAGE/Western blot analysis as described in Experimental Procedures. A: Inhibition of isoproterenol-induced lipolysis by Mββ. Glycerol release of nontreated cells is defined as 1.0. Mean ± SE of six independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with respective isoproterenol-treated cells (P < 0.05). B: Inhibition of isoproterenol-induced lipolysis with increasing Mββ concentrations. Glycerol release of nontreated cells is defined as 1.0. Representative experiment. C: Inhibition of isoproterenol-induced lipolysis by Mββ, time curve. Glycerol release of nontreated cells is defined as 1.0. Representative experiment. D, E: Inhibition of HSL and perilipin phosphorylation by Mββ. Densitometric intensity ratios of P-HSL/HSL (D) and P-perilipin/perilipin (E). Densitometric intensity of nontreated cells is defined as 1.0. Mean ± SE of seven independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with respective isoproterenol-treated cells (P < 0.05). Inset: Representative blot.
Inhibition of isoproterenol-induced lipolysis by Mββ-activated AMPK
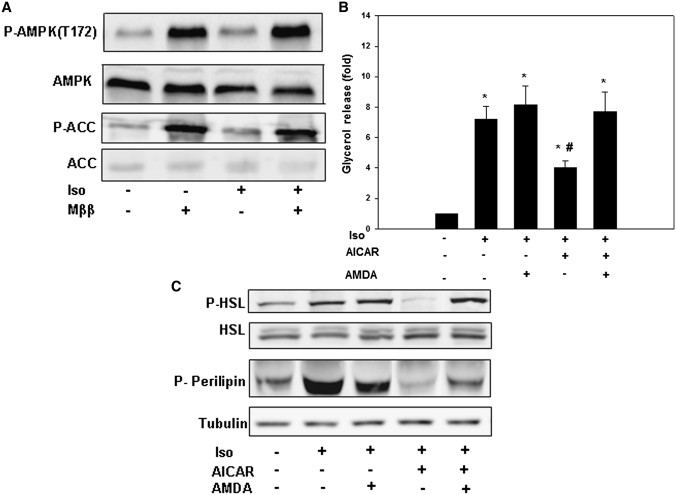
We have recently reported that LCFA and their MEDICA analogs activate AMP-protein kinase (AMPK) through increase in intracellular AMP/ATP ratio by the free LCFA/MEDICA acid, complemented by activation of LKB1 phosphorylation of AMPKα(Thr172) by the respective CoA monothioester (12). AMPK activation by LCFA/MEDICA prompted us to probe the putative role played by AMPK in mediating inhibition of lipolysis by Mββ. Inhibition of lipolysis by Mββ was compared with that induced by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR)/ZMP–activated AMPK (16–19). AMPK of 3T3-L1 adipocytes was activated by Mββ (Fig. 3A) or AICAR (19) (not shown), as verified by the increase in phosphorylation of AMPK(T172) and its downstream P-ACC(S79) product, being already evident within the first 2 h of incubation. Also, similar to Mββ (Fig. 2) and in line with previous reports of suppression of lipolysis by activated AMPK (16–18), isoproterenol-induced lipolysis (Fig. 3B), P-HSL, and P-perilipin (Fig. 3C) were inhibited by AICAR, being abrogated by inhibition of the adenosine kinase by 5′-amino-5′-deoxyadenosine (AMDA) (20, 21), implying ZMP-activated AMPK. Inhibition of lipolysis by both AICAR/ZMP-activated AMPK and Mββ-activated AMPK may indicate causal relationship between AMPK activation and inhibition of agonist-induced lipolysis by Mββ.
Fig. 3.
Inhibition of isoproteranol-induced lipolysis by AMPK activation in 3T3-L1 adipocytes. 3T3-L1 adipocytes were incubated for 24 h in the presence of 350 μM Mββ, 750 μM AICAR, or 25 μM AMDA as indicated, and in the absence or presence of 100 nM isoproterenol (Iso) added to the incubation medium 1 h prior to sampling. Lipolysis (B) was quantified by glycerol release as described in Experimental Procedures. P-HSL, HSL, P-perilipin, perilipin, P-AMPK(T172), AMPK, P-ACC(S79), ACC, and tubulin (A, C) were determined by SDS-PAGE/Western blot analysis as described in Experimental Procedures. A: AMPK activation by Mββ. Representative blot. B: Inhibition of isoproterenol-induced lipolysis by AICAR. Glycerol release of nontreated cells is defined as 1.0. Mean ± SE of six independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with isoproterenol/AMDA-treated cells (P < 0.05). C: Inhibition of PKA-phosphorylated HSL and perilipin by AICAR. Representative blot.
Suppression of isoproterenol-induced cAMP by Mββ or AICAR
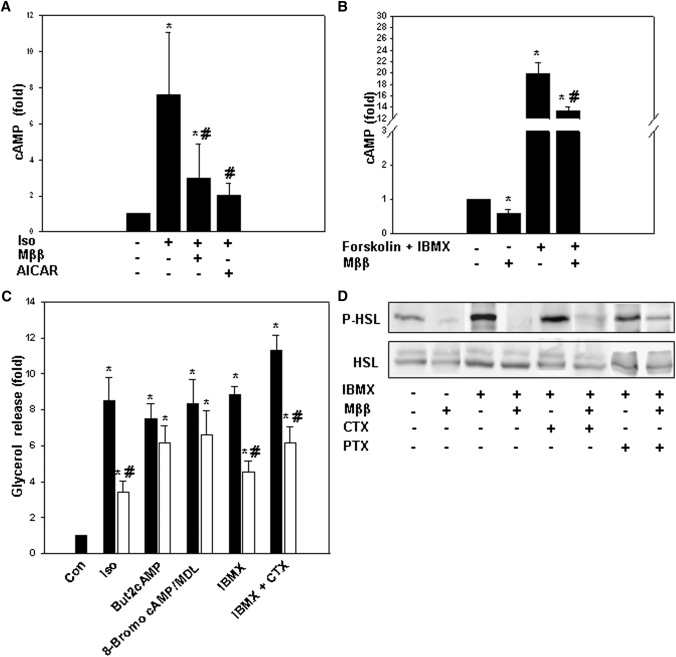
As HSL and perilipin are both phosphorylated by PKA in response to isoproterenol-induced cAMP (reviewed in Ref. 1), inhibition of agonist-induced lipolysis by Mββ or AICAR was further pursued by verifying respective cellular cAMP levels. Indeed, inhibition of agonist-induced lipolysis by Mββ or AICAR was accompanied by suppression of isoproterenol-induced cAMP levels (Fig. 4A) and forskolin/1-methyl-3-isobutyl-1-methylxantine (IBMX)–induced (Fig. 4B) cAMP levels in 3T3-L1 adipocytes as well as in other cell types (e.g., Cos-1, Jurkat, and HeLa). Suppression of isoproterenol-induced cAMP levels by Mββ was further pursued in terms of the putative activation of phosphodiesterase3B (PDE3B) and/or inhibition of the adenylate cyclase (AC) (1). cAMP levels induced by forskolin/IBMX-activated AC (Fig. 4B) as well as lipolysis induced by PDE3B inhibition by IBMX (Fig. 4C) were both robustly inhibited by Mββ, indicating that inhibition by Mββ prevailed under conditions of PDE3B inhibition. Also, under conditions of inhibiting the AC by MDL 12,330A (22), But2cAMP-induced lipolysis and 8-bromo-cAMP-induced lipolysis remained unaffected by Mββ (Fig. 4C), implying inhibition of cAMP production rather than its degradation by activated PDE3B. Hence, in contrast to insulin, where inhibition of agonist-induced cAMP and lipolysis is due to PDE3B activation, inhibition by Mββ may be ascribed to inhibition of the AC activity.
Fig. 4.
Suppression of agonist-induced cAMP by Mββ or AICAR. 3T3-L1 adipocytes or COS-1 cells were incubated in the presence of 350 μM or 250 μM Mββ, respectively, and with 750 μM AICAR, 50 ng/ml CTX or 100 ng/ml PTX for 24 h as indicated, 1 mM But2cAMP for4 h as indicated, 100 μM MDL for 2 h as indicated, 2 mM 8-Bromo cAMP for 1.5 h as indicated, 100 μM or 30 µM IBMX, respectively, for 1 h as indicated, and in the absence or presence of 100 nM isoproterenol (Iso) or 10 μM forskolin added to the incubation medium 1 h prior to sampling as indicated. Lipolysis (C) was quantified by glycerol release as described in Experimental Procedures. P-HSL and HSL (D) were determined by SDS-PAGE/Western blot analysis as described in Experimental Procedures. Cellular cAMP content (A, B) was determined as described in Experimental Procedures. A: Suppression of isoproterenol-induced cAMP by Mββ and AICAR in 3T3-L1 adipocytes. Mean cellular cAMP levels of nontreated adipocytes amounted to 1.64 fmol/μg protein and is defined as 1.0. Mean ± SE of three independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with respective isoproterenol-treated cells (P < 0.05). B: Suppression of forskolin/IBMX-induced cAMP by Mββ in COS-1 cells. cAMP levels of nontreated cells is defined as 1.0. Mean ± SE of four independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with forskolin/IBMX-treated cells (P < 0.05). C: Inhibition of isoproterenol (Iso)-, IBMX-, IBMX/CTX-, 8-bromo-, and But2cAMP-induced lipolysis (filled bars) by Mββ (open bars) in 3T3-L1 adipocytes. Glycerol release of nontreated cells is defined as1.0. Mean ± SE of four independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with non-Mββ respective cells (P < 0.05). D: Suppression of IBMX-, CTX-, and PTX-induced P-HSL by Mββ in 3T3-L1 adipocytes. Representative blot.
Mββ targets upstream of AC were pursued by evaluating first the effect of Mββ in the presence of added CTX or PTX, which activate the AC by persistent Gαs activation or Gαi inhibition, respectively (23). CTX-induced lipolysis (Fig. 4C) as well as CTX- or PTX-induced P-HSL (Fig. 4D) were robustly suppressed by Mββ, implying inhibition of the AC activity per se, rather than modulation of its upstream Gαs or Gαi targets. Also, suppression of cAMP levels by Mββ was not abrogated by inhibiting the NO-synthase activity by added NG-methyl-L-arginine (L-NMMA) (not shown), implying that AC inhibition by Mββ was not accounted for by nitrosylation of AC by AMPK-activated NO-synthase.
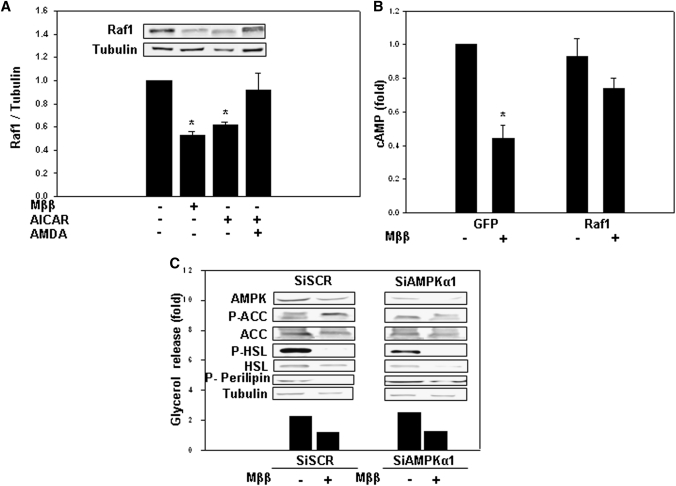
Raf1 suppression by Mββ and AICAR
Because Raf1 of the mitogen-activated protein kinase (MAPK) transduction pathway interacts with and activates the AC (24–26), suppression of the AC/cAMP/PKA transduction pathway by Mββ or AICAR was further pursued by evaluating Raf1 expression. Treating 3T3-L1 cells as well as other cell types (e.g., COS-1, Jurkat) with Mββ or AICAR resulted in suppressing Raf1 protein levels (Fig. 5A). Raf1 suppression by AICAR was abrogated by AMDA (Fig. 5A), implying causal relationship between ZMP-activated AMPK and Raf1 levels. Furthermore, overexpression of Raf1 resulted in abrogating suppression of cAMP levels by Mββ (Fig. 5B), implying causal relationship between Raf1 suppression and inhibition of isoproterenol-induced cAMP by Mββ.
Fig. 5.
Suppression of Raf1 expression by Mββ and AICAR. 3T3-L1 adipocytes or COS-1 cells were incubated for 24 h in the presence of 350 μM or 250 μM Mββ, respectively, 750 μM AICAR and 25 μM AMDA as indicated, and in the absence or presence of 100 nM isoproterenol (Iso), 30 μM IBMX or 10 μM forskolin added to the incubation medium 1 h prior to sampling as indicated. Lipolysis (C) was quantified by glycerol release as described in Experimental Procedures. P-HSL, HSL, P-perilipin, AMPK, P-ACC(S79), ACC, Raf1, and tubulin (A, C) were determined by SDS-PAGE/Western blot analysis as described in Experimental Procedures. Cellular cAMP content (B) was determined as described in Experimental Procedures. A: Suppression of Raf1 by Mββ and AICAR in 3T3-L1 adipocytes. Raf1 protein of nontreated cells is defined as 1.0. Mean ± SE of three independent experiments. *Significant compared with nontreated cells (P < 0.05). Inset: Representative blot. B: Rescue of Mββ-suppressed cAMP levels by overexpressed Raf1 in COS-1 cells. COS-1 cells were transfected with GFP-Raf1 expression vector or with the empty GFP plasmid as indicated, and were further cultured in the presence of forskolin/IBMX. cAMP values were corrected for transfection efficiency, estimated by the percentage of GFP expressing cells. Cellular cAMP content of nontreated controls transfected with the empty GFP plasmid is defined as 1.0. Mean ± SE of four independent experiments. *Significant compared with nontreated GFP-transfected cells (P < 0.05). C: Mββ suppression of isoproterenol-induced lipolysis, P-HSL, and P-perilipin prevails in AMPK-deficient 3T3-L1 adipocytes. 3T3-L1 adipocytes were transfected with siAMPK or scrambled Si (SCR). Representative Western blots and lipolysis (filled bars) of cells transfected with siAMPK or scrambled Si. Glycerol release of nontreated cells is defined as 1.0. Representative experiment.
The role played by putative AMPK-independent upstream targets of Mββ in suppressing Raf1 expression was verified in 3T3-L1 adipocytes transfected with Si-AMPK, thereby resulting in about 85% suppression of P-ACC(S79). Mββ still inhibited isoproterenol-induced lipolysis, P-HSL, and P-perilipin in 3T3-L1 cells transfected with Si-AMPK (Fig. 5C), implying an additional AMPK-independent activity of Mββ in suppressing Raf1 expression. An AMPK-independent activity of Mββ has been further verified in HeLa cells that lack LKB1 and where MEDICA analogs fail to activate AMPK (12). Treatment of HeLa cells with Mββ resulted in suppressing Raf1 expression (not shown), implying an upstream target for Mββ in suppressing Raf1 expression, in addition to AICAR- or Mββ-activated AMPK.
UPR activation by Mββ
LCFA have been reported to induce components of the unfolded protein response (UPR) in a variety of cell lines (27, 28), prompting us to search for a possible link between UPR components and suppression of Raf1 expression by Mββ. Treatment of 3T3-L1 adipocytes with Mββ indeed resulted in induction of the UPR markers CHOP and BiP transcripts, as well as P-eIF2α(S51) (Fig. 6A), to an extent similar to that induced by thapsigargin or tunicamycin. Also, suppression of Raf1 expression (Fig. 6B), isoproterenol-induced P-HSL (Fig. 6C), and agonist-induced glycerol release (Fig. 6D) by Mββ were partly rescued by abrogating UPR by the chemical chaperone phenylbutyric acid (PBA) (29), pointing to a causal linkage between Mββ-induced UPR and suppression of isoproterenol-induced lipolysis by Mββ. In contrast to Mββ, AICAR/ZMP failed to induce the UPR marker CHOP or BiP (Fig. 6A), and PBA failed to abrogate suppression of Raf1 by AICAR (Fig. 6E), implying that suppression of isoproterenol-induced lipolysis by AICAR/ZMP may solely be ascribed to suppression of Raf1 expression by ZMP-activated AMPK.
Fig. 6.
Mββ-induced UPR. 3T3-L1 adipocytes were incubated for 24 h in the presence of 350 μM Mββ, 750 μM AICAR, 5 μg/ml tunicamycin or 0.3 μM thapsigargin as indicated, and in the absence or presence of 100 nM isoproterenol (Iso) added to the incubation medium 1 h prior to sampling as indicated. The PBA chaperone (5 mM) was added 6 h prior to Mββ. Lipolysis (D) was quantified by glycerol release as described in Experimental Procedures. P-HSL, HSL, Raf1, tubulin, eEIF2α, and P-eEIF2α(S51) (A–C, E) were determined by SDS-PAGE/Western blot analysis as described in Experimental Procedures. CHOP and BiP transcripts (A) were determined by quantitative RT-PCR as described in Experimental Procedures. A: Mββ-induced UPR. CHOP (filled bars) and BiP (open bars) transcript levels of nontreated adipocytes are defined as 1.0. Mean ± SE of three independent experiments. *Significant compared with nontreated cells (P < 0.05). Inset: Mββ-induced phosphorylation of eEIF2α(S51). Representative blot. B: Rescue of Mββ-suppressed Raf1 by PBA. Raf1 protein level of nontreated adipocytes is defined as 1.0. Mean ± SE of three independent experiments. *Significant compared with nontreated cells (P < 0.05). Inset: Representative blot. C: Rescue of Mββ-suppressed P-HSL by PBA. Representative blot. D: Rescue of Mββ-inhibited lipolysis by PBA. Glycerol release of nontreated cells is defined as 1.0. Mean ± SE of five independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with respective isoproterenol-treated cells (P < 0.05). E: AICAR suppression of Raf1 prevails in PBA-treated adipocytes. Raf1/tubulin ratio of extracts of nontreated cells is defined as 1.0. Mean ± SE of three independent experiments. *Significant compared with nontreated cells (P < 0.05); #Significant compared with respective isoproterenol-treated cells (P < 0.05). Inset: Representative blot.
DISCUSSION
Since the 1970s, it has been known that agonist-induced adipose lipolysis is inhibited by the LCFA generated through lipolysis and that it requires constant removal of free nonesterified fatty acids by their binding to medium albumin or by frequent medium replacement (4, 5). However, the mode of inhibition of agonist-induced lipolysis by its LCFA end product remained to be resolved. Moreover, no attempts were made to exploit the inhibitory efficacy of LCFA for suppressing lipolysis induced by adipose insulin resistance. MEDICA analogs may simulate the LCFA inhibition of agonist-induced lipolysis while avoiding their recycling into adipose fat. Apart from dissecting the mode of action of Mββ in suppressing agonist-induced lipolysis, the reported findings may indicate that synthetic LCFA may serve as insulin mimetics in the lipolysis context, while bypassing the insulin transduction pathway.
The proposed mode of action of Mββ in suppressing agonist-induced lipolysis is outlined in Fig. 7. Suppression of Raf1 expression by Mββ is proposed to be accounted for by both, Mββ-activated AMPK, complemented by Mββ-induced UPR components. Mββ-activated AMPK is proposed to account for the immediate activity of Mββ in suppressing Raf1 cellular content, while suppression of Raf1 expression by Mββ-induced UPR may account for the progressive activity of Mββ in suppressing agonist-induced lipolysis (Fig. 2C). Suppression of Raf1 by Mββ is proposed to result in inhibition of agonist-induced lipolysis due to inhibition of the adenylate cyclase activity, followed by decrease in agonist-induced cAMP and PKA-induced P-HSL and P-perilipin. It is noteworthy that perilipin phosphorylation by PKA may result in both, facilitating HSL contact with the lipid droplet as well as dissociation of CGI-58 from P-perlipin followed by its activation of ATGL (30). In contrast to Mββ, inhibition of agonist-induced lipolysis by AICAR is proposed to be solely accounted for by suppression of Raf1 expression by ZMP-activated AMPK. As MEDICA analogs simulate the characteristics of LCFA in terms of AMPK activation (12) and in inducing UPR (27, 28), we may assume that the mode of action proposed for MEDICA may account, at least partially, for inhibition of agonist-induced lipolysis by LCFA (4, 5). In light of AMPK activation by metformin or octanoate (31, 32), the proposed transduction pathway may also offer a mode of action for the recently reported activity of metformin (31) or octanoate (32) in suppressing isoproterenol-induced lipolysis in primary rat adipocytes or 3T3-L1 adipocytes, respectively.
Fig. 7.
Suppression of Raf1 expression by LCFA/MEDICA is proposed to be accounted for by activated AMPK, complemented by induced UPR. Raf1 suppression by AICAR is proposed to be solely accounted for by ZMP-activated AMPK. Suppression of Raf1 expression results in inhibition of the adenylate cyclase (AC) activity, followed by decrease in agonist-induced cAMP and in cAMP-induced PKA. Suppression of PKA-induced P-HSL and P-perilipin, followed by suppression of P-perlipin-induced CGI-58/ATGL, results in overall inhibition of agonist-induced lipolysis.
The proposed mode of action outlined in Fig. 7 is supported by the following findings: i) Raf1 suppression by AICAR was abrogated by AMDA (Fig. 5A), implying ZMP-activated AMPK. Raf1 suppression and inhibition of agonist-induced lipolysis by AICAR was not accompanied by induction of UPR components (Fig. 6A), and it was not abrogated by the PBA chaperone (Fig. 6E), implying a UPR-independent AICAR activity. AMPK activation by Mββ may imply that inhibition of agonist-induced lipolysis by Mββ may partly be accounted for by the AMPK/Raf1 cross-talk exemplified by AICAR. The mode of suppression Raf1 expression by activated AMPK still remains to be investigated. ii) Failure of Si-AMPK to abrogate suppression of agonist-induced lipolysis by Mββ (Fig. 5C) points to an additional Mββ target involved in suppressing lipolysis, independent of Mββ-activated AMPK. Indeed, suppression of Raf1 expression, isoproterenol-induced P-HSL, and lipolysis by Mββ was partly abrogated by the PBA chaperone (Fig. 6), indicating causal linkage between Mββ-induced UPR components, suppression of Raf1, and inhibition of agonist-induced lipolysis. Preliminary profiling of UPR markers induced by MEDICA analogs in cell lines and in vivo has indicated a specific profile of UPR markers rather than a canonical response (Hertz et al., unpublished). Lack of UPR activation by AICAR may indicate that induction of UPR components by Mββ is not accounted for by AMPK activation. iii) Suppression of isoproterenol-induced P-HSL, P-perilipin, and lipolysis by Mββ or AICAR was accompanied by decrease in cellular cAMP and was abrogated by added But2cAMP (Fig. 4), implying causal linkage between suppression of cellular cAMP levels and suppression of agonist-induced lipolysis by Mββ or AICAR. iv) Suppression of cellular cAMP by Mββ was abrogated in cells overexpressing Raf1 (Fig. 5B), implying causal linkage between Raf1 activity and cellular cAMP content. v) In light of PDE3B inhibition by IBMX, suppression of IBMX-induced lipolysis by Mββ (Fig. 4) implies inhibition of cAMP production rather than its hydrolysis by PDE activation. Similarly, lipolysis induced by 8-bromo-cAMP, under conditions of inhibiting the adenylate cyclase by MDL (Fig. 4C), remained unaffected by Mββ, further implying inhibition of cAMP production rather than its degradation. vi) The putative suppression of CGI-58/ATGL activity by Mββ due to suppression of perilipin phosphorylation still remains to be verified.
The findings reported here confirm previous studies in which constitutive-active AMPK was reported to suppress isoproterenol-induced lipolysis (17). However, our findings do not conform to other studies using AMPK inhibitors [e.g., DN-AMPK (33) and compound C (34)] or to those in which AMPK was claimed to be required for promoting lipolysis. Also, our findings may account for the mode of activation of AMPK by agonist-induced lipolysis (7, 33–35) (Fig. 3A), previously ascribed to cAMP-activated PKA or Epac1 (35) or, alternatively, to PKA-independent increase in intracellular AMP/ATP ratio due to ATP-consuming futile cycling of LCFA between lipolysis and reesterification (7). Thus, isoproterenol-induced AMPK may be ascribed to direct AMPK activation by the free LCFA generated during lipolysis (12). The primary role played by the acyl-CoA in activating AMPK (12) may account for the reported abrogation of isoproterenol-induced AMPK upon inhibiting the LCFA-CoA synthase by triacsin C (7).
Our findings may indicate that synthetic LCFA analogs may inhibit lipolysis while bypassing the insulin transduction pathway. The efficacy of Mββ in suppressing agonist-induced lipolysis may add to previously reported insulin-sensitizing and hypolipidemic activities of MEDICA analogs in suppressing hepatic glucose and lipoprotein production, and in enhancing total body glucose uptake and plasma lipoprotein clearance in animal models of diabesity (8–12).
Footnotes
Abbreviations:
- AC
- adenylate cyclase
- ACC
- acetyl CoA carboxylase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- AMDA
- 5′-amino-5′-deoxyadenosine
- CGI-58
- comparative gene identification-58
- CMC
- carboxy methyl cellulose
- CTX
- cholera toxin
- FCS
- fetal calf serum
- HSL
- hormone-sensitive lipase
- IBMX
- 1-methyl-3-isobutyl-1-methylxantine
- LCFA
- long-chain fatty acid
- L-NMMA
- NG-methyl-L-arginine
- LKB1
- liver kinase B1
- Mββ/MEDICA16
- β,β’-tetramethyl hexadecanedioic acid
- PDE
- posphodiesterase
- PKA
- protein kinase A
- PMSF
- phenylmethylsulphonylfluoride
- PTX
- pertusis toxin
- UPR
- unfolded protein response
Funded by Eurostars project E!5138.
REFERENCES
- 1.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. 2007. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27: 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitamura T., Kitamura Y., Kuroda S., Hino Y., Ando M., Kotani K., Konishi H., Mtsuzaki H., Kikkawa U., Ogawa W., et al. 1999. Insulin- induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell. Biol. 19: 6286–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilherme A., Virbasius J. V., Puri V., Czech M. P. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns T. W., Langley P. E., Terry B. E., Robinson G. A. 1978. The role of free fatty acids in the regulation of lipolysis by human adipose tissue cells. Metabolism. 27: 1755–1762 [DOI] [PubMed] [Google Scholar]
- 5.Fain J. N., Shepherd R. E. 1975. Free fatty acids as feedback regulators of adenylate cyclase and cyclic 3′:5′-AMP accumulation in rat fat cells. J. Biol. Chem. 250: 6586–6592 [PubMed] [Google Scholar]
- 6.Smith A. J., Thompson B. R., Sanders M. A., Bernlohr D. A. 2007. Interaction of the adipocyte fatty acid-binding protein with the hormone-sensitive lipase: regulation by fatty acids and phosphorylation. J. Biol. Chem. 282: 32424–32432 [DOI] [PubMed] [Google Scholar]
- 7.Gauthier M. S., Miyoshi H., Souza S. C., Cacicedo J. M., Saha A. K., Greenberg A. S., Ruderman N. B. 2008. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 283: 16514–16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Tana J., Rose-Kahn G., Srebnik M. 1985. Inhibition of lipid synthesis by beta beta’-tetramethyl-substituted, C14–C22, alpha, omega-dicarboxylic acids in the rat in vivo. J. Biol. Chem. 260: 8404–8410 [PubMed] [Google Scholar]
- 9.Bar-Tana J., Rose-Kahn G., Frenkel B., Shafer Z., Fainaru M. 1988. Hypolipidemic effect of beta, beta’-methyl-substituted hexadecanedioic acid (MEDICA 16) in normal and nephrotic rats. J. Lipid Res. 29: 431–441 [PubMed] [Google Scholar]
- 10.Mayorek N., Kalderon B., Itach E., Bar-Tana J. 1997. Sensitization to insulin induced by beta,beta’-methyl-substituted hexadecanedioic acid (MEDICA 16) in obese Zucker rats in vivo. Diabetes. 46: 1958–1964 [DOI] [PubMed] [Google Scholar]
- 11.Russell J. C., Amy R. M., Graham S. E., Dolphin P. J., Wood G. O., Bar-Tana J. 1995. Inhibition of atherosclerosis and myocardial lesions in the JCR:LA-cp rat by beta, beta’-tetramethylhexadecanedioic acid (MEDICA 16). Arterioscler. Thromb. Vasc. Biol. 15: 918–923 [DOI] [PubMed] [Google Scholar]
- 12.Za'tara G., Bar-Tana J., Kalderon B., Suter M., Morad E., Samovski D., Neumann D., Hertz R. 2008. AMPK activation by long chain fatty acyl analogs. Biochem. Pharmacol. 76: 1263–1275 [DOI] [PubMed] [Google Scholar]
- 13.Kalderon B., Sheena V., Shachrur S., Hertz R., Bar-Tana J. 2002. Modulation by nutrients and drugs of liver acyl-CoAs analyzed by mass spectrometry. J. Lipid Res. 43: 1125–1132 [DOI] [PubMed] [Google Scholar]
- 14.Park S. Y., Kim H. J., Wang S., Higashimori T., Dong J., Kim Y. J., Cline G., Li H., Prentki M., Shulman G. I., et al. 2005. Hormone-sensitive lipase knockout mice have increased hepatic insulin sensitivity and are protected from short-term diet-induced insulin resistance in skeletal muscle and heart. Am. J. Physiol. Endocrinol. Metab. 289: E30–E39 [DOI] [PubMed] [Google Scholar]
- 15.Balla A., Tuymetova G., Barshishat M., Geiszt M., Balla T. 2002. Characterization of type II phosphatidylinositol 4-kinase isoform reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 277: 20041–20050 [DOI] [PubMed] [Google Scholar]
- 16.Anthony N. M., Gaidhu M. P., Ceddia R. B. 2009. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (Silver Spring). 17: 1312–1317 [DOI] [PubMed] [Google Scholar]
- 17.Daval M., Diot-Dupuy F., Bazin R., Hainault I., Viollet B., Vaulont S., Hajduch E., Ferr`e P., Foufelle F. 2005. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 280: 25250–25257 [DOI] [PubMed] [Google Scholar]
- 18.Sullivan J. E., Brocklehurst K. J., Marley A. E., Carey F., Carling D., Ber R. K. 1994. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353: 33–36 [DOI] [PubMed] [Google Scholar]
- 19.Hardie D. G. 2007. AMP-activated protein kinase as a drug target. Annu. Rev. Pharmacol. Toxicol. 47: 185–210 [DOI] [PubMed] [Google Scholar]
- 20.Nakamaru K., Matsumoto K., Taguchi T., Suefuji M., Murata Y., Igata M., Kawashima J., Kondo T., Motoshima H., Tsuruzoe K., et al. 2005. AICAR, an activator of AMP-activated protein kinase, down regulates the insulin receptor expression in HepG2 cells. Biochem. Biophys. Res. Commun. 328: 449–454 [DOI] [PubMed] [Google Scholar]
- 21.Saitoh M., Nagai K., Nakagawa K., Yamamura T., Yamamoto S., Nishizaki T. 2004. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem. Pharmacol. 67: 2005–2011 [DOI] [PubMed] [Google Scholar]
- 22.Silinsky E. M., Vogel S. M. 1986. The effects of an adenylate cyclase inhibitor on the electrophysiological correlates of neuromuscular transmission in the frog. Br. J. Pharmacol. 88: 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. 1985. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 109: 566–572 [DOI] [PubMed] [Google Scholar]
- 24.Beazely M. A., Alan J. K., Watts V. J. 2005. Protein kinase C and epidermal growth factor stimulation of Raf1 potentiates adenylyl cyclase type 6 activation in intact cells. Mol. Pharmacol. 67: 250–259 [DOI] [PubMed] [Google Scholar]
- 25.Ding Q., Gros R., Gray I. D., Taussig R., Ferguson S. S., Feldman R. D. 2004. Raf kinase activation of adenylyl cyclases: isoform-selective regulation. Mol. Pharmacol. 66: 921–928 [PubMed] [Google Scholar]
- 26.Feldman R. D., Gros R. 2007. New insights into the regulation of cAMP synthesis beyond GPCR/G protein activation: implications in cardiovascular regulation. Life Sci. 81: 267–271 [DOI] [PubMed] [Google Scholar]
- 27.Cnop M., Igoillo-Esteve M., Cunha D. A., Ladriere L., Eizirik D. L. 2008. An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochem. Soc. Trans. 36: 909–915 [DOI] [PubMed] [Google Scholar]
- 28.Wei Y., Wang D., Topczewski F., Pagliassotti M. J. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. 291: E275–E281 [DOI] [PubMed] [Google Scholar]
- 29.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Gorgun C. Z., Hotamisligil G. S. 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 313: 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi T., Omatsu N., Morimoto E., Nakashima H., Ueno K., Tanaka T., Satouchi K., Hirose F., Osumi T. 2007. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J. Lipid Res. 48: 1078–1089 [DOI] [PubMed] [Google Scholar]
- 31.Zhang T., He J., Xu C., Zu L., Jiang H., Pu S., Guo X., Xy G. 2009. Mechanisms of metformin inhibiting lipolytic response to isoproterenol in primary rat adipocytes. J. Mol. Endocrinol. 42: 57–66 [DOI] [PubMed] [Google Scholar]
- 32.Lei T., Xie W., Han J., Corkey B. E., Hamilton J. A., Guo W. 2004. Medium-chain fatty acids attenuate agonist-stimulated lipolysis, mimicking the effects of starvation. Obes. Res. 12: 599–611 [DOI] [PubMed] [Google Scholar]
- 33.Yin W., Mu J., Birnbaum M. J. 2003. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 278: 43074–43080 [DOI] [PubMed] [Google Scholar]
- 34.Koh H. J., Hirshman M. F., He H., Li Y., Manabe Y., Balschi J. A., Goodyear L. J. 2007. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem. J. 403: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omar B., Zmuda-Trzebiatowska E., Manganiello V., Goransson O., Degerman E. 2009. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell. Signal. 21: 760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]