Abstract
Transcripts and protein for follicle-stimulating hormone receptor (FSHR) were demonstrated in abdominal adipose tissue of female chickens. There was no expression of the Fsh gene, but FSH and FSHR colocalized, suggesting that FSH was receptor bound. Partial correlations indicted that changes in abdominal fat (AF) content were most directly correlated with Fshr mRNA expression, and the latter was directly correlated with tissue FSH content. These relationships were consistent with FSH inducing Fshr mRNA expression and with the finding that FSH influenced the accumulation of AF in chickens, a novel role for the hormone. Chicken preadipocytes responded linearly to doubling concentrations of FSH in Fshr mRNA expression and quantities of FSHR and lipid, without discernable effect on proliferation. Cells exposed to FSH more rapidly acquired adipocyte morphology. Treatment of young chickens with chicken FSH (4 mIU/day, subcutaneous, days 7–13) did not significantly decrease live weight but increased AF weight by 54.61%, AF as a percentage of live weight by 55.45%, and FSHR transcripts in AF by 222.15% (2 h after injection). In cells stimulated by FSH, genes related to lipid metabolism, including Rdh10, Dci, RarB, Lpl, Acsl3, and Dgat2, were expressed differentially, compared with no FSH. Several pathways of retinal and fatty acid metabolism, and peroxisome proliferator-activated receptor (PPAR) signaling changed. In conclusion, FSH stimulates lipid biosynthesis by upregulating Fshr mRNA expression in abdominal adipose tissue of chickens. Several genes involved in fatty acid and retinal metabolism and the PPAR signaling pathway mediate this novel function of FSH.
Keywords: follicle-stimulating hormone action, Fshr expression, abdominal adipose tissue, development, chicken
Follicle-stimulating hormone (FSH) is a major glycoprotein hormone of the hypothalamus-pituitary-gonadal axis acting via its specific membrane receptor, FSHR, a member of the glycoprotein family of G protein coupled receptors (1–3). After FSH specifically binds to multileucine repeat sequences in the extracellular domain of FSHR (4), its signal is transduced by G proteins activating adenylyl cyclase with intracellular production of cAMP and activation of protein kinase A. The resultant protein phosphorylation achieves a series of physiological effects, including activating aromatase and inducing luteinizing hormone receptor, jointly acting to generate estrogen, which cooperates with FSH in follicular development (1).
FSH is the main controlling factor for Fshr gene expression (5). In rats from birth to 3 days of age, the ovaries do not respond to FSH, but there is rapid cAMP generation in response to FSH upon the transcription of full-length Fshr (1). In different follicular stages, the expression level of Fshr mRNA is negatively correlated with the size of follicles (6, 7), probably because the dependence of follicles on FSH differs throughout development: small follicles need more FSH stimulation, so the content of Fshr mRNA is highest in the granulosa cells. In addition, some paracrines, such as epidermal growth factor, fibroblast growth factor, insulin-like growth factor 1, and transforming growth factor-β, can participate in the regulation of Fshr gene expression (8–12).
FSH is mainly secreted by the pituitary gland to regulate gonadal development and function after delivery through the bloodstream (13–17). Additionally, FSH can be secreted from the stomach (18), but it is unclear whether this regulates reproductive or other functions. Consistent with the classic roles of FSH in steroidogenesis (19), Fshr is mainly expressed in the reproductive system (13–17). The receptor is also expressed in some nonreproductive organs and tissues (18, 20) where it plays roles in regulating the development of the skeletal system and others (21–24). There are no reports that Fsh mRNA and Fshr mRNA are expressed and regulate lipid deposition in adipose tissue, whereas estrogen receptor has been shown to be important for the regulation of lipid metabolism (22). Because FSHR and estrogen receptor have complementary function in reproduction (1), it was speculated that FSH and FSHR might play a role in the regulation of lipid metabolism of abdominal adipose tissue.
In the present study, the expression of the Fshr gene in adipose tissue was verified using Beijing-you (BJY) chickens, an indigenous Chinese breed with superior meat quality. The expression of Fshr mRNA was shown to be upregulated by FSH and mediated FSH-stimulated lipid deposition via multiple signaling pathways.
MATERIALS AND METHODS
Animals and tissue collection
All experimental procedures, using female BJY chickens from the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (Beijing, China), were performed in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). One-day-old hatchlings with the same genetic background were reared in stair-step caging under continuous lighting using standard conditions of temperature, humidity, and ventilation. Feed was formulated to meet requirements (Nutrient Requirements of Chinese Color-feathered Chickens, 2004) and was available ad libitum, as was water.
On each of days 1, 21, 42, 90, and 120, six birds of similar weight were selected, stunned, and euthanized using approved procedures. Abdominal fat (AF) was rapidly dissected, weighed, snap-frozen in liquid nitrogen, and stored at −80°C. AF content was expressed as a percentage of live body weight. From the collections on day 42, subsamples of the AF from three of the six birds were fixed in 4% paraformaldehyde. Additional abdominal adipose tissue was collected aseptically on day 42 to prepare preadipocytes.
RNA extraction
Total RNA was isolated from the frozen tissues using Trizol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). After removal of any genomic DNA, RNA was dissolved at 1 μg/μl and stored at −80°C for use in RT-PCR and quantitative PCR (q-PCR).
RT-PCR
Total RNA (2.5 μg) was used to generate cDNA in a final volume of 25 μl according to the manufacturer's instructions (Promega, Madison, WI). PCR was performed in the presence of 1.5 mM MgCl2, 200 μM dNTP mixtures, 1.5 IU Taq polymerase, and 50 pmol each of the forward and reverse primers in a final volume of 50 μl. Primers for the Fshr gene and Fsh gene (Table 1) were designed (Primer Premier 5.0) from the GenBank sequences to further minimize possible confounding from cDNA. The primers were synthesized by Shanghai ShengGong Biological Co. (Shanghai, China). Optimal PCR conditions are shown in Table 1. Parallel amplifications included cDNA from chicken ovary or pituitary to serve as positive controls for Fshr mRNA and Fsh mRNA, respectively. Aliquots of the PCR products were assessed by electrophoresis in 1.5% agarose gels, and amplicons were sequenced (Takara Bio, Shiga, Japan) to verify authenticity.
TABLE 1.
The specific primers for RT-PCR and q-PCR in this study
| Gene | Sequence | Product size (bp) | Cycle profile | Accession number | |
| RT-PCR | Fshr | F:5′-TAATGGAACCTGCCTGGATG-3′ | 261 | 95°C/30 s, 63°C /30 s, 72°C /30s (40 cycles) | NM_205079 |
| R:5′-GCACAGCAATGGCTAGGATAG-3′ | |||||
| Fsh | F:5′-CAGGAAGGCACAGCACTCTA-3′ | 497 | 95°C/30 s, 62°C /30 s, 72°C /30s (40 cycles) | NM_204257 | |
| R:5′-CAGCCTGTTCAATACCCTTATC-3′ | |||||
| q-PCR | Fshr | F:5′-TAATGGAACCTGCCTGGATG-3′ | 261 | 95°C for 15 s and 63°C for 35 s (40 cycles) | NM_205079 |
| R:5′-GCACAGCAATGGCTAGGATAG-3′ | |||||
| Acsl3 | F:5′-ACCAGGGCTGAGTGGATGAT-3′ | 192 | 95°C for 15 s and 63°C for 35 s (40 cycles) | NM_028817 | |
| R:5′-CAGACGTGGGACCAAAGAGAC-3′ | |||||
| Rdh10 | F:5′-GCTGTTCGTGGTCACATTCCG-3′ | 209 | 95°C for 15 s and 63°C for 35 s (40 cycles) | NM_001199459 | |
| R:5′-TCTCCTCGTTGCTCTGCGTGT-3′ | |||||
| Lpl | F:5′-AGGAGAAGAGGCAGCAATA-3′ | 222 | 95°C for 15 s and 63°C for 35 s (40 cycles) | AB016987 | |
| R:5′-AAAGCCAGCAGCAGATAAG-3′ | |||||
| RarB | F:5′-ACCTCGTGTTCACCTTTGCC-3′ | 197 | 95°C for 15 s and 63°C for 35 s (40 cycles) | NM_205326 | |
| R:5′-GTGAGGCTTGTTGGGTCGTC-3′ | |||||
| Dgat2 | F:5′-AAACCCACAACCTGCTGACCAC-3′ | 287 | 95°C for 15 s and 63°C for 35 s (40 cycles) | NW_001474485 | |
| R:5′-TGCTCCTCCCACCACGATGA-3′ | |||||
| AdipoQ | F:5′-GCCAGGTCTACAAGGTGTCA-3′ | 86 | 95°C for 15 s and 63°C for 35 s (40 cycles) | NM_206991 | |
| R:5′-CCATGTGTCCTGGAAATCCT-3′ |
q-PCR
The abundance of Fshr mRNA in abdominal adipose tissue at each age and in adipocytes incubated with FSH (see below) was quantified by q-PCR using the same primers in RT-PCR. Each 25 μl PCR mixture contained 12.5 μl of 2× iQ™ SYBR Green Supermix, 0.5 μl (10 mM) of each primer, and 1 μl of cDNA. Mixtures were incubated in an iCycler iQ Real-time Detection system (Bio-Rad, Hercules, CA). Quantification of the transcripts was performed using a standard curve with 10-fold serial dilutions of cDNA. A melting curve was constructed to verify that only a single PCR product was amplified. Samples were assayed in triplicate with standard deviations of threshold cycle values not exceeding 0.5 on a within-run basis, and each analysis was repeated at least twice. Negative (without template) and positive controls (ovary) were included within each assay.
In situ hybridization
Paraffin-embedded sections (10 μm) from three birds were prepared. Sequential sections were divided into three sets and were used for Fshr mRNA in situ hybridization (ISH), following established methodology (25). The antisense probe hybridized to bp 625–885 of the Fshr mRNA coding region (GenBank acession no. NM-205079).
Immunohistochemistry
Paraffin-embedded sections (10 μm) from three birds were dewaxed in xylene (2 × 5 min) and 100% ethanol (2 × 2 minutes) and rehydrated through a graded ethanol series (95, 70, 50, and 30%), each for 2 min, and finally with distilled water. For antigen retrieval (26), slides were incubated in citrate buffer (10 mM citric acid, 0.05% Tween 20 [pH 6.0]) at 95–100°C for 10 min. After washing twice with PBS, slides were blocked with 3% BSA in PBS for 30 min. Slides were incubated in a humidified chamber overnight with 1:100 dilutions of mouse anti-chicken FSH or anti-chicken FSHR (Huijia Biological Technology Co., Ltd, Xiamen China) at 4°C. After thorough washing, they were treated with 1:100 dilutions of HRP-conjugated goat anti-mouse immunoglobulin for 1 h at room temperature, washed three times in PBS, and developed with diaminobenzidine for 10–15 min in the dark. Sections were then counterstained with hematoxylin.
Measurement of FSH and FSHR levels
Concentrations of FSH and FSHR in abdominal fat (AF) and adipocytes that were induced by FSH were measured using a chicken-specific FSH ELISA kit (Zhonghao Biological Technology Co., LTD, Beijing, China) and a FSHR ELISA kit (Huijia Biological Technology Co., LTD, Xiamen, China). Samples were homogenized at room temperature and centrifuged (1000 g, 20 min) at 4°C to separate debris and the fat cake, and the infranatant was frozen immediately at −80°C until assay. The assay was performed according to the manufacturer's instructions after dilution to optimize accuracy.
Preadipocyte cell culture
Preadipocytes of abdominal adipose tissue were collected from day 42 female BJY chickens following published methods (27, 28). Preadipocytes were plated in 96-well and 6-well cultures dishes. The culture medium was DMEM/F12 (1:1) containing 10% FBS and with 0.25% oleic acid to induce differentiation and was changed every 2 days for a total of 8 days of culture. Starting on day 0, chicken FSH (Zhonghao Biological Technology Co., LTD, Beijing, China) was included in the media at concentrations of 0, 5, 10, or 20 mIU/ml. Every 2 days, cell morphology, proliferation (29), and lipid content (30) were determined in triplicate wells from each treatment. Total RNA was also prepared from three wells and stored for q-PCR and microarray analysis.
Microarray analyses and q-PCR
Microarray hybridization analysis was performed using RNA from cells treated with 0 and 20 mIU/ml FSH for 6 day according to the Agilent Expression Analysis Technical Manual (GeneTech Biotechnology Limited Co., Shanghai, China). Differentially expressed (DE) genes were identified by ≥ 1.5-fold-changes and P value < 0.05 in ANOVA. Gene ontology (GO) enrichment analysis of the DE genes was performed using the GOEAST software toolkit. The significance level of the GO term enrichment was set as a false discovery rate-adjusted P value < 0.1 by the Yekutieli method, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway information was used in this analysis. Pathways that were significantly enriched in DE genes were identified by the hypergeometric test using R packages (P < 0.1; false discovery rate adjusted). Minor pathways with less than two known chicken genes were discarded. For several genes, the expression of which was markedly altered by treatment with FSH, the microarray data were verified by q-PCR using specific primers (Table 1). q-PCR was performed as indicated above.
FSH treatment of female chickens
To examine changes in plasma concentrations resulting from exogenous FSH, three 7 day old BJY chickens were injected subcutaneously (dorsal neck) with 0.5 ml physiological saline or saline containing 4 mIU chicken FSH (Zhonghao Biological Technology Co., Ltd, Beijing, China). Blood samples (≈150 μl) were collected 2 h before and 2, 8, and 24 h after injection, plasmas were separated, and FSH concentrations were measured by ELISA, as noted earlier.
Eighteen 7 day old female BJY chickens were randomly distributed into FSH-treatment and control groups. Birds were raised with feed and water provided ad libitum during the experiment. Birds in the treated group were injected with 4 mIU FSH in saline, and the control group received saline daily for 7 days. Three FSH-treated and three control birds were euthanized 2 h after injection on day 13, and AF samples were collected to measure the tissue contents of FSH hormone and Fshr mRNA. The methods used were as described above. The remainders of the birds were weighed then euthanized 24 h after injection on day 13, giving a total of six birds per group for measurements of AF weight and AF as a percentage of live weight.
Statistical analyses
Comparisons across sampled ages were made by one-way ANOVA using Statistical Analysis Systems software (Version 8.2, SAS Institute, Cary, NC) to determine significance (accepted at P < 0.05 or P < 0.01). Multiple regressions were used to examine relationships between AF (dependent variable) and tissue FSH, FSHR protein, and Fshr mRNA, and partial correlation coefficients were then derived. Effects of FSH concentrations on cultured cells were evaluated, on a within-day basis, by ANOVA. The data are means ± SD.
RESULTS
Fshr mRNA and its protein are expressed in adipose tissue of chicken
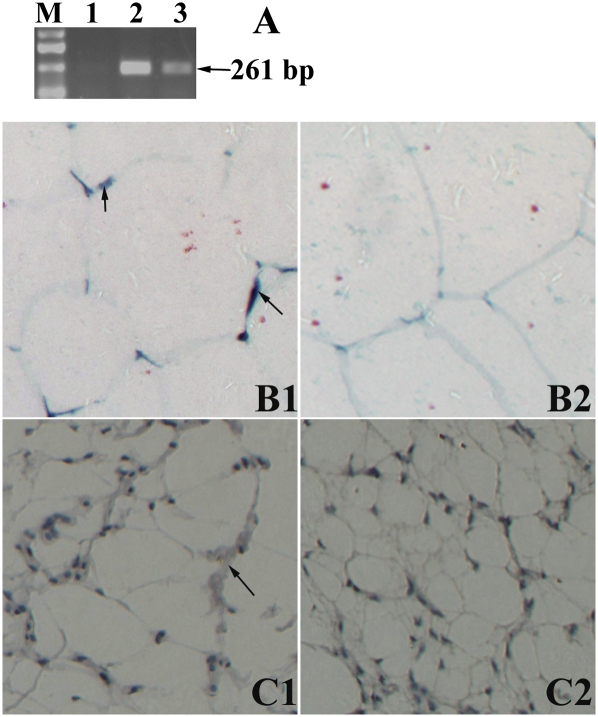
To determine if Fshr gene is expressed in chicken adipose tissue, total RNA were isolated from AF, and Fshr mRNA expression was detected at the transcript and protein level. After RT-PCR, the expected 261 bp product was verified by electrophoresis, and the product was confirmed by sequencing. The results show that Fshr mRNA is present in AF (Fig. 1A). ISH was detected Fshr transcripts in the thin shell of cytoplasm peripheral to the lipid locule (Fig. 1B), whereas the FSHR protein was more narrowly detected, by immunocytochemistry, to be likely in the plasmalemma (Fig. 1C). These results demonstrate unequivocally that Fshr gene is expressed in chicken AF.
Fig. 1.
Expression of Fshr mRNA in chicken abdominal adipose tissue. A: Fshr mRNA expression was detected by RT-PCR. Lanes 1–3: negative control, positive control (ovary), abdominal adipose tissue, respectively. M, DL2000 Marker. The expected 261 bp product was verified by electrophoresis in abdominal adipose tissue. B1: Fshr mRNA ISH: positive hybridized signals (arrows) were showed in abdominal adipose tissue, which indicate expression of Fshr mRNA (inverted microscope, 200×). C1: immunocytochemical detection of FSHR protein in abdominal adipose tissue: positive signal appears as brown staining surrounding the fat locule (inverted microscope, 200×). B2 and C2, the negative controls.
The Fsh gene was not expressed, but FSH was detected in adipose tissue
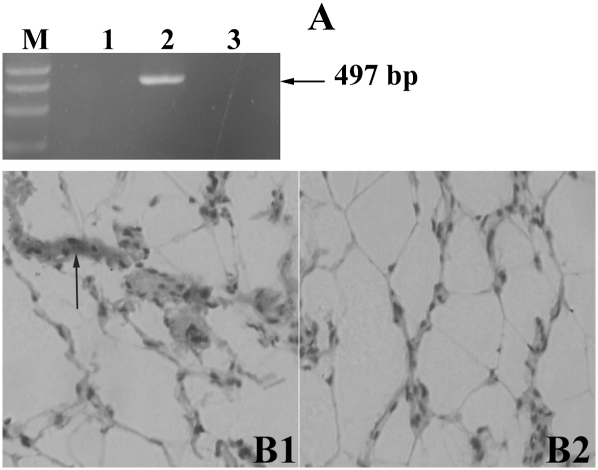
The possible expression of Fsh gene and presence of FSH protein in adipose tissue were assessed. RT-PCR, using cDNA from the pituitary as a positive control, failed to detect Fsh transcripts in AF (Fig. 2A). In contrast, there was consistent immunocytochemical evidence for the presence of the FSH protein, again peripheral to the fat locules, probably in the plasmalemma (Fig. 2B).
Fig. 2.
Fsh gene is not expressed but the hormone is detectable in chicken abdominal adipose tissue. A: Fsh mRNA expression was detected by RT-PCR. Lanes 1–3: negative control, positive control (pituitary), and abdominal adipose tissue, respectively. M, DL2000 marker. No expected product was detected in abdominal adipose tissue. B1: Immunocytochemical detection of FSH protein in abdominal adipose tissue: positive signal appears as brown staining surrounding the fat locule (inverted microscope, 200×). B2: The negative control.
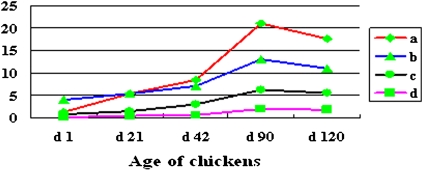
Consistent changes in content of Fshr transcripts, FSHR protein, FSH protein, and AF content occur across development
AF content increased with development from 0 at day 1 to peak at about 2% of live body weight at day 90 and then declined slightly at day 120. The contents of Fshr mRNA, FSHR, and FSH followed the same trend (Fig. 3). Excluding data from day 1, when there was no AF, changes in all of these variables were highly interrelated. Partial correlations were derived to gain insight into the strongest direct relationships (Table 2). The most important findings were that AF was very highly correlated with Fshr mRNA levels, independently of receptor protein and tissue FSH (r12.34 = 0.957) and that Fshr mRNA remained highly correlated with tissue FSH (r24.13 = 0.963) after accounting for AF and receptor protein. The direct relationship between Fshr mRNA and its protein was only moderate (r23.14 = 0.61). Thus, 93% of the variation in Fsh mRNA was associated with variation in tissue FSH, and 91.6% of the variation in AF was associated with variation in Fshr mRNA. If these strong associations reflect causation, FSH in the tissue appeared to upregulate Fshr mRNA expression, which, in turn, was a major positive influence on adipose tissue.
Fig. 3.
Similar trends occur across development in changes in AF content, FSH content, FSHR content, and the expression of Fshr mRNA. Fshr mRNA is shown as the number of copies (×105) per μg total RNA (a), FSH as mIU/kg tissue (b), FSHR as ng/kg tissue (c), and AF as a percent of live weight (%) (d). Data are means ± SD (n = 6). d = day.
TABLE 2.
Simple and partial correlations between variables measured in abdominal fat. Data are from 24 measurements (four stages of development; n = 6 birds at each age)
| Simple correlation matrix | ||||
| 1 | 2 | 3 | 4 | |
| 1 | 1 | 0.9928 | 0.9815 | 0.9827 |
| 2 | 1 | 0.9932 | 0.9974 | |
| 3 | 1 | 0.9914 | ||
| 4 | 1 | |||
| Partial correlations | ||||
| Estimate | P Value | |||
| r12.34a | 0.957 | <0.0001 | ||
| r13.24 | −0.513 | 0.0146 | ||
| r14.23 | −0.891 | <0.0001 | ||
| r23.14 | 0.61 | 0.0026 | ||
| r24.13 | 0.963 | <0.0001 | ||
| r34.12 | −0.427 | 0.0475 | ||
1 = fat content, 2 = receptor mRNA, 3 = receptor protein, and 4 = tissue FSH.
The partial correlation between fat content (1) and receptor mRNA (2), accounting for covariation with receptor protein (3) and tissue FSH content (4)
FSH stimulates lipid biosynthesis in vitro and in vivo by upregulating the expression of its receptor
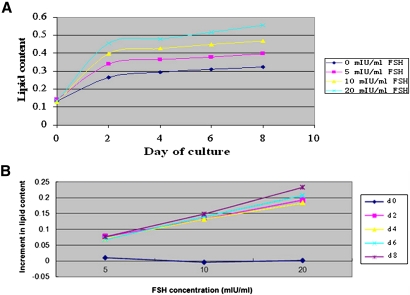
When preadipocytes were cultured with different concentrations of FSH, cell numbers increased from day 0 to day 6 and then were slightly reduced at day 8; there was no significant effect of FSH (P > 0.05, data not shown). For all treatments, including the 0 FSH controls, the lipid content per cell increased abruptly by day 2 and then more steadily between day 2 and day 8 (Fig. 4A). Differences between FSH concentrations were all significant (P < 0.05 or P < 0.01). The stimulatory effect of FSH was most apparent when the increment in lipid content per cell, over that measured in the absence of FSH, was plotted against the log-concentration of FSH (Fig. 4B). Because FSH did not influence cell numbers but did increase lipid content in a log-concentration dependent manner, FSH clearly affected lipid metabolism in favor of increased lipid accumulation.
Fig. 4.
FSH increases the lipid content of adipocytes in a log-dose related manner. A: Changes in lipid content, normalized for cell numbers, with four concentrations of FSH over days of culture. Lipid content was expressed as OD value at 490 nm. B: The lipid contents are plotted as increments over that measured in cells not exposed to FSH against doubling concentrations of FSH. Data are means ± SD (n = 3 wells). d = day.
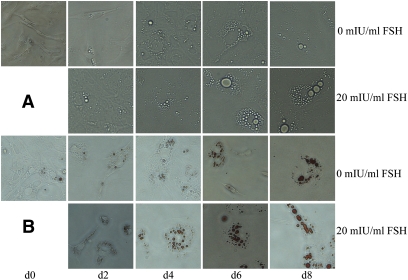
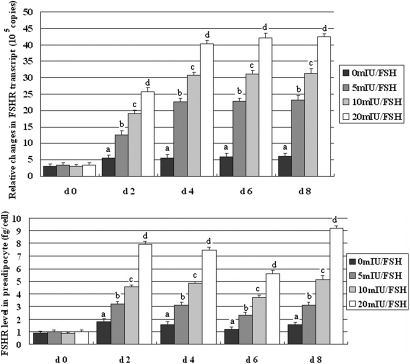
During culture, the cells changed from being spindle-like toward a round or oval morphology (Fig. 5A), and the number of lipid locules progressively decreased while they became larger, especially evident in cells exposed to 20 mIU/ml FSH when compared with those not treated with FSH (Fig. 5B). Lipid accumulation in response to FSH treatment resulted in cells at day 8 with typical characteristics of fully differentiated adipocytes with interiors dominated by a single large locule. As shown in Fig. 6, treatment with FSH significantly up-regulated expression of Fshr mRNA relative to that measured in cells not exposed to FSH; comparable increases in FSHR protein were detected. On a within-day basis, differences in mRNA and protein across the FSH concentrations were almost all significant. Consistent with the changes observed across stages of development, these in vitro results demonstrate that FSH brings about increased expression of the Fshr gene in differentiating preadipocytes and stimulates lipid accumulation.
Fig. 5.
Morphological changes and lipid deposition induced by 20 mIU/ml FSH in preadipocytes during differentiation in vitro (inverted microscope, 200×). A: The change from cells being spindle-shaped to larger oval or rounded forms was accelerated in those exposed to 20 mIU/ml FSH when compared with those not treated with FSH. B: Lipid, stained with Oil red O, accumulated as fewer but much larger locules in cells exposed to 20 mIU/ml FSH when compared with those not treated with FSH.
Fig. 6.
FSH treatment increases the abundance of Fshr transcripts and amount of the receptor in preadipocytes undergoing differentiation. A: The expression of Fshr mRNA in adipocytes was assessed by q-PCR. Consistent increases occurred in the abundance of Fshr transcripts. B: Concentration of FSHR in adipocytes was measured using ELISA. Consistent increases occurred in the quantity of the receptor in cells exposed to FSH in culture (i.e., after day 0). On a within-day basis, all differences between FSH treatments were significant. Data are means ± SD (n = 3 wells). d = day. q-PCR indicates quantitative real-time PCR.
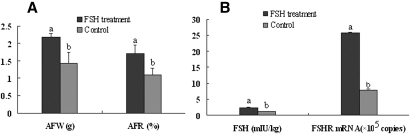
The in vivo experiment showed that the average plasma concentration of FSH in 7 day old female BJY chickens was 3.93 mIU/ml. Concentrations increased to 7.73, 5.35, and 4.18 mIU/ml at 2, 8, and 24 h after a subcutaneous injection of 4 mIU chicken FSH (Table 3), with significantly higher (P < 0.01) concentrations at 2 h and 8 h than before injection. Treatment with 4 mIU FSH daily for 7 days did not significantly decrease live weight but significantly increased (P < 0.01) AF weight by 54.61%, AF as a percentage of live weight by 55.45% (P < 0.01) (Fig. 7A), and FSHR transcripts in AF by 222.15% (P < 0.01) (Fig. 7B). These results confirm the in vitro finding that FSH stimulated lipid accumulation through upregulating the expression of Fshr mRNA in chicken AF.
TABLE 3.
Plasma concentrations of FSH in 7-day BJY chickens before and after a subcutaneous injection of 4 mIU FSH
| Time | Pre-2 h | Post-2 h | Post-8 h | Post-24 h |
| Treatment (mIU/ml) | 3.93 ± 0.18 | 7.73 ± 0.14 | 5.35 ± 0.15 | 4.18 ± 0.13 |
| Control (mIU/ml) | 3.89 ± 0.11 | 3.95 ± 0.20 | 3.84 ± 0.17 | 3.94 ± 0.18 |
Injections were 0.5 ml of saline (controls) or the same volume containing 4 mIU chicken FSH. Blood was sampled 2 h before injection then at 2, 8, and 24 h after injection; plasma was separated, and FSH was measured by ELISA. Different uppercase superscripts indicate significant differences (P < 0.01) in each column, and different lowercase superscripts indicate significant differences (P < 0.01) within the row. Data are means ± SD (n = 3).
Fig. 7.
FSH treatment of young chickens increased the AF accumulation and FSH contents and Fshr mRNA in AF. Chickens received 4 mIU FSH daily from day 7 to day 13. A: Birds were weighed, and AF was collected 24 h after injection on day 13. AFW and AFR in FSH-treated birds were significantly higher than those in controls (P < 0.01). Data are means ± SD (n = 6). B: FSH content and Fshr mRNA expression were detected by q-PCR and ELISA in abdominal adipose tissue collected 2 h after injection on day 13. FSH content and Fshr mRNA levels in AF from FSH-treated birds were significantly higher than those in controls. Data are means ± SD (n = 3).
FSH regulates the lipid biosynthesis through multiple pathways
The Agilent gene expression microarray was used to explore the pathways by which FSH stimulates lipid deposition in cultured preadipocytes. Differentially expressed (DE) gene profiles were determined between cells treated with 20 mIU/ml FSH and control cells not exposed to FSH. Using standard thresholds, a total of 829 DE genes were found (369 upregulated and 460 downregulated; Supplementary Fig. I). The functional classification of 583 identified DE genes was determined using GO classification, showing that the majority of enriched GO terms among the DE genes were in lipid metabolism (viz. fatty acid oxidation, brown fat cell differentiation, lipid transport, and positive regulation of fatty acid and lipid metabolism) and others, including gene expression, protein metabolism, immune function, and energy metabolism (Supplementary Fig. II).
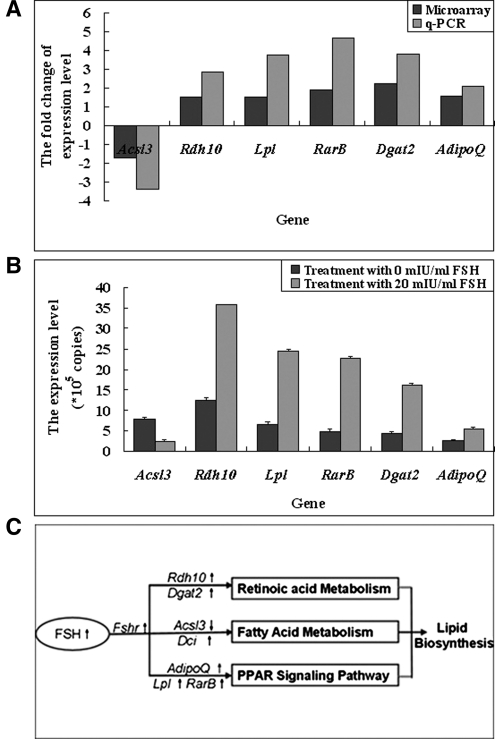
Genes related to lipid metabolism were differentially expressed (n = 39), 19 of which were significantly upregulated and 20 of which were downregulated (P < 0.05) in response to FSH (Supplementary Fig. III). Using pathway analysis, significant differences included fatty acid metabolism, retinol metabolism, and the peroxisome proliferator-activated receptor (PPAR) signaling pathway (P < 0.05), involved in some representative genes such as Retinol dehydrogenase 10 (Rdh10), Acyl-CoA synthetase long-chain family member 3 (Acsl3), Dodecenoyl-Coenzyme A delta isomerase (Dci), Retinoic acid receptor, beta (RarB), Adiponectin Q(AdipoQ), Lipoprotein lipase(Lpl), and Diacylglycerol O-acyltransferase homolog 2 (Dgat2). (Table 4 and Supplementary Figs. IV–VII), so qPCR was performed to verify the expression of these seven representative DE genes. Results from q-PCR, also using RNA from cells treated with 0 and 20 mIU/ml FSH, authenticated those from the microarray analysis (Fig. 8A, B).
TABLE 4.
Some representative differentially expressed genes (P < 0.05) related to lipid metabolism and to fatty acid and retinal metabolism pathways
| Name | Fold change | P | |
| Fatty acid metabolism related genes | |||
| Acsl3 | Acyl-CoA synthetase long-chain family member 3 | −1.74 | 0.01373 |
| Dci | Dodecenoyl-CoenzymeA delta isomerase | 1.88 | 0.00405 |
| Retinal metabolism related genes | |||
| Rdh10 | Retinol dehydrogenase 10 (all-trans) | 1.53 | 0.00629 |
| Lipid metabolism related genes | |||
| Lpl | Lipoprotein lipase | 1.51 | 0.01855 |
| Dgat2 | Diacylglycerol O-acyltransferase homolog 2 | 2.24 | 0.01351 |
| AdipoQ | Adiponectin Q | 1.54 | 0.02836 |
| RarB | Retinoic acid receptor, β | 1.90 | 0.04296 |
Fig. 8.
Exploration of pathways by which FSH stimulated lipid deposition in cultured preadipocytes using microarray. Microarray hybridization analysis was performed using RNA from cells treated with 0 (control) and 20 mIU/ml FSH for 6 days. A: Validation of differentiated expressed genes determined by microarray (Acsl3, Rdh10, Lpl, RarB, Dgat2, and Adipo Q) using q-PCR. Values are expressed as fold-change when comparing expression level detected in treatment cells (20 mIU/ml FSH) with control cells without FSH. B: Expression levels of the indicated mRNA in cells treated with 20 mIU/ml FSH were all significantly higher (P < 0.05) than cells in control. Data are means ± SD (n = 3). C: The proposed pathways and key genes influenced by FSH induction, according to GO term and KEGG pathway analysis. FSH probably regulates lipid deposition in adipocytes through increasing/decreasing the expression of genes (e.g., Rdh10, Acsl3, Dci, RarB, AdipoQ, Lpl, and Dgat2) in the fatty acid metabolism pathway, retinol metabolism pathway, and PPAR signaling pathway. Up arrow indicates increased; down arrow indicates decreased. Italic = genes.
It appears that FSH probably regulates lipid deposition in adipocytes through increasing the expression of genes (e.g., Rdh10, Acsl3, Dci, RarB, AdipoQ, Lpl, and Dgat2) in the fatty acid metabolism pathway, retinol metabolism pathway, and PPAR signaling pathway (Fig. 8 C and Supplementary Figs. IV–VII).
DISCUSSION
In addition to its well known role in the classic hypothalamo-pituitary-gonadal axis, FSH is synthesized in extrapituitary locations (18) and is now believed to have direct actions in a variety of nongonadal tissues (21–24), consistent with the demonstration of Fshr mRNA expression in nonreproductive tissues (20). Given the cooperativity between actions of FSH and estrogen, for example in follicular development (1), and the broad functions of estrogen in lipid metabolism, this study set out to investigate the possibility that FSH might also play a regulatory role in adipose tissue, a previously unexplored function.
This investigation first demonstrated the expression of the avian Fshr gene in AF tissue from female BJY chickens. In addition to the presence of Fshr transcripts detected by RT-PCR and ISH indicating the presence of transcripts in the perilocular domain, immunocytochemical detection of the FSHR protein in a discrete peripheral location of the adipocytes was consistent with the receptor being an integral component of the plasmalemma. The gene for the cognate ligand, FSH, was not expressed in abdominal adipose tissue, but the FSH protein was found immunocytochemically, with similar cellular location to the receptor. This suggests that FSH, probably originating from the pituitary, is extracted from blood and is bound to receptors in the abdominal adipose tissue. As AF accumulates, from negligible quantities in newly hatched birds to quite substantial quantities (∼2% of body weight) by day 90, changes in Fshr mRNA expression, the FSHR protein, and FSH and AF content were quantified at four stages of development. All variables were highly interrelated, and second-order partial correlation analyses indicated that AF content was most directly correlated with Fshr mRNA content and that the latter was most directly correlated with tissue content of FSH. In other tissues, FSH is known to be a key inducer of FSHR (5). These findings are consistent with chicken abdominal adipose tissue being a target for the action of FSH and are highly suggestive of the involvement of FSH/FSHR in determining, in part, the quantity of this tissue, a previously unknown biological role for FSH.
A model in vitro system, using differentiating avian preadipocytes, directly tested this suggested action of FSH. Adding FSH to the culture media had no effect on differentiation, triggered by oleic acid, or on short-term survival of the cells, but it distinctly promoted their maturation and the extent of lipid accumulation. In a clearly log-dose responsive manner, FSH accelerated the transformation of cells to enlarged rounded morphologies with a single fat locule dominating the interior, both typical characteristics of mature adipocytes. Exposure to increasing doses of FSH resulted in significant upregulation of the expression of Fshr mRNA, evident at each day of culture. These findings clearly demonstrate that FSH is able to act on adipocytes by inducing its own receptor and then likely favoring net lipogenesis, resulting in increased fat accumulation.
The in vivo experiment demonstrated that supplemental FSH (4 mIU/day, days 7–13) significantly increased AF accumulation over that in control chickens. The abundance of Fshr mRNA and FSH content in the AF were also significantly higher in FSH-treated than those in control chickens 2 h after injection on day 13. These findings support the interpretation that, in young female BJY chickens, FSH plays a positive role in abdominal lipid deposition through upregulating the expression of Fshr mRNA in abdominal adipose tissue. This novel role for FSH is consistent with lipid deposition being enhanced in ovariectomized rats (31), where increased secretion of FSH would be expected.
To further explore the molecular mechanism underlying the regulatory function of FSH on lipid accumulation, gene expression profiles were examined in cultured preadipocytes with and without FSH treatment. Using KEGG pathway analysis, large numbers of differentially expressed genes were found to be enriched in fatty acid metabolism, retinol metabolism, and the PPAR signaling pathway; some representative genes were Rdh10, Acsl3, Dci, RarB, AdipoQ, Lpl, and Dgat2. Rdh10 is the dehydrogenase converting retinol to retinoic acid (32). After synthesis, retinoic acid enters the lipid metabolic pathway, influenced by retinoic acid receptor /retinoid X receptor and PPAR (33). Acsl3 is known to be involved in de novo synthesis of fatty acids (34, 35) and Dci is an isomerase of fatty acid metabolism (36). DGAT2 is the enzyme enabling the final esterification in lipid synthesis (37). AdipoQ is the abundant adipocytokine, adiponectin, regulated in part by the PPAR signaling pathway.
In conclusion, this study, using female chickens, has demonstrated that abdominal adipose tissue is a target for the action of FSH, a previously undocumented function. This tissue was shown to express the receptor gene, Fshr gene, and to contain the receptor, likely in the plasmalemma of adipocytes. Similarly, FSH was present in the same location, but the Fsh gene was not expressed in adipose tissue. Developmental changes from hatchlings to 120 days indicated that the content of abdominal adipose tissue in the birds was closely correlated with the level of Fshr mRNA expression and FSH measurable in the tissue. Treatment of young chickens with FSH (4 mIU/day, subcutaneous, days 7–13) significantly increased AF weight, AF as a percentage of live weight, and Fshr transcripts in AF (2 h after injection). Using preadipocytes differentiated by oleic acid, acquisition of the typical morphology of mature adipocytes and lipid accumulation were promoted by FSH in a log-dose related fashion. At the same time, FSH significantly upregulated the expression of Fshr mRNA and differential expression, compared with no exposure to FSH, was demonstrated for 829 transcripts. A number of these gene products play roles in retinol and PPAR signaling and lipid metabolism and provide preliminary insight into how FSH might act in this novel function.
Supplementary Material
Acknowledgments
The authors thank W. Bruce Currie (Emeritus Professor, Cornell University) for contributions to the data analysis and preparation of the manuscript.
Footnotes
Abbreviations:
- AF
- abdominal fat
- BJY
- Beijing-you
- DE
- differentially expressed
- FSH
- follicle-stimulating hormone
- FSHR
- follicle-stimulating hormone receptor
- GO
- gene ontology
- ISH
- in situ hybridization
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- PPAR
- peroxisome proliferator-activated receptor
- q-PCR
- real time quantitative polymerase chain reaction
The research was supported by the project of the State Key Laboratory Of Animal Nutrition (2004DA125184G1101), the National Key Technology R&D Program (2011BAD28B03), and the earmarked fund for modern agro-industry technology research system (nycytx-42).
The online version of this article (available at http://www.jlr.org) contains supplementary data.
REFERENCES
- 1.Rannikki A. S., Zhang F. P., Huhtaniemi I. T. 1995. Ontogeny of follicle stimulating hormone receptor gene expression in the rat testis and ovary. Mol. Cell. Endocrinol. 107: 199–208 [DOI] [PubMed] [Google Scholar]
- 2.Heckert L. L., Griswold M. D. 2002. The expression of the follicle-stimulating hormone receptor in spermatogenesis. Recent Prog. Horm. Res. 57: 129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simoni M., Gromoli J., Niesclag E. 1997. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 18: 739–773 [DOI] [PubMed] [Google Scholar]
- 4.Braun T., Schofield P. R., Sprengel R. 1991. Amino-terminal leucine-rich repeats in gonadotropin receptors determine hormone selectivity. EMBO J. 10: 1885–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilly J. L., LaPolt P. S., Hsuech A. J. W. 1992. Hormonal regulation of follicle stimulating hormone receptor messenger ribonucleic acid level in cultured rat granulose cells. Endocrinology. 130: 1296–1302 [DOI] [PubMed] [Google Scholar]
- 6.Cardenas H., Pope W. F. 2002. Androgen receptor and follicle-stimulating hormone receptor in the pig ovary during the follicular phase of the estrous cycle. Mol. Reprod. Dev. 62: 92–98 [DOI] [PubMed] [Google Scholar]
- 7.Liu J., Aronow B. J., Witte D. P. 1998. Cyclic and maturation-dependent regulation of follicle-stimulating hormone receptor and luteinizing hormone receptor messenger ribonucleic acidexpression in the porcine ovary. Biol. Reprod. 58: 648–658 [DOI] [PubMed] [Google Scholar]
- 8.Burger L. L., Haisenleder D. J., Aylor K. W., Dalkin A. C., Prendergast K. A., Marshall J. C. 2004. Regulation of luteinizing hormone-beta and follicle-stimulating hormone (FSH)-beta gene transcription by androgens: testosterone directly stimulates FSH-beta transcription independent from its role on follistatin gene expression. Endocrinology. 145: 71–78 [DOI] [PubMed] [Google Scholar]
- 9.Shen S. T., Yu J. Y. 2002. Cloning and gene expression of a cDNA for the chicken folliele-stimulating hormone (FSH)-beta-subunit. Gen. Comp. Endocrinol. 125: 375–386 [DOI] [PubMed] [Google Scholar]
- 10.Shupnik M. A. 1996. Gonadotropin gene modulation by steroids and gonadotrpoin- releasing hormone. Biol. Reprod. 54: 279–286 [DOI] [PubMed] [Google Scholar]
- 11.Weiss J., Guendner M. J., Halvorson L. M., Jameson J. L. 1995. Transcriptional activation of the follicle-stimulating hormone beta-subunit gene by activin. Endocrinology. 136: 1885–1891 [DOI] [PubMed] [Google Scholar]
- 12.Ciccone N. A., Dunn I. C., Boswel I. T., Tsutsui K., Ubuka T., Ukena K., Sharp P. J. 2004. Gonadotrophin inhibitory hormone depresses gonadotrophin alpha and follicle- stimulating hormone beta subunit expression in the pituitary of the domestic chicken. J. Neuroendocrinol. 16: 999–1006 [DOI] [PubMed] [Google Scholar]
- 13.Méduri G., Charnaux N., Driancourt M. A., Combettes L., Granet P., Vannier B., Loosfelt H., Milgrom E. 2002. Follicle stimulating hormone receptors in oocytes? J. Clin. Endocrinol. Metab. 87: 2266–2276 [DOI] [PubMed] [Google Scholar]
- 14.Patsoula E., Loutradis D., Drakakis P., Michalas L., Bletsa R., Michalas S. 2003. Messenger RNA expression for the follicle-stimulating hormone receptor and luteinizing hormone receptor in human oocytes and preimplantation-stage embryos. Fertil. Steril. 79: 1187–1193 [DOI] [PubMed] [Google Scholar]
- 15.Zheng W., Magid M. S., Kramer E. E., Chen Y. T. 1996. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am. J. Pathol. 148: 47–53 [PMC free article] [PubMed] [Google Scholar]
- 16.Mizrachi D., Shemesh M. 1999. Follicle-stimulating hormone receptor and its messenger ribonucleic acid are present in the bovine cervix and can regulate cervical prostanoid synthesis. Biol. Reprod. 61: 776–784 [DOI] [PubMed] [Google Scholar]
- 17.Cui H. X., Zhao S. M., Cheng M. L., Guo L., Ye R. Q., Liu W. Q., Gao S. Z. 2009. Cloning and expression levels of genes relating to the ovulation rate of the Yunling Black goat. Biol. Reprod. 80: 219–226 [DOI] [PubMed] [Google Scholar]
- 18.Mandrekar P. S., Sheth A. R., Doctor V. M., Zaveri J. P., Sheth N. A. 1990. Immuno-cytochemical localization of follicle stimulating hormone in normal human stomach. Anat. Rec. 227: 334–339 [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., Rudd M. D., Hernandez G. I., Gonzalez R. I., Fan H. Y., Zeleznik A. J., Richards J. S. 2009. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol. Endocrinol. 23: 649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu J. F., Huang W. Q., Wang S. E., Chu C. Y. 2004. Studies on distribution of FSH, LH and colocalization with GnRHR in rat submaxillary glands. Acta Anatomica Sinica. 35: 652–655 [Google Scholar]
- 21.Farhat M. Y., Lavigne M. C., Ramwel P. W. 1996. The vascular protective effects of estrogen. FASEB J. 10: 615–624 [PubMed] [Google Scholar]
- 22.Gruber C. J., Tschugguel W., Schneeberger C., Huber J. C. 2002. Production and actions of estrogens. N. Engl. J. Med. 346: 340–352 [DOI] [PubMed] [Google Scholar]
- 23.Sowers M. R., McConnell D., Jannausch M., Buyuktur A. G., Hochberg M., Jamadar D. A. 2006. Estradiol and its metabolites and their association with knee osteoarthritis. Arthritis Rheum. 54: 2481–2487 [DOI] [PubMed] [Google Scholar]
- 24.Oshima Y., Matsuda K., Yoshida A., Watanabe N., Kawata M., Kubo T. 2007. Localization of estrogen receptors alpha and beta in the articular surface of the rat femur. Acta Histochem. Cytochem. 40: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gjerdrum L. M., Lielpetere I., Rasmussen L. M., Bendix K., Hamilton D. S. 2001. Laser-assisted microdissection of membrane-mounted paraffin sections for polymerase chain reaction analysis. J. Mol. Diagn. 3: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otali D., Stockard C. R., Oelschlager D. K., Wan W., Manne U., Watts S. A., Grizzle W. E. 2009. The combined effects of formalin fixation and individual steps in tissue processing on immuno-recognition. Biotech. Histochem. 84: 223–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S., Wang L., Wang N., Wang Y. X., Shi H., Li H. 2009. Oleate induces trans-differentiation of chicken fibroblasts into adipocyte-like cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 154: 135–141 [DOI] [PubMed] [Google Scholar]
- 28.Ramsay T. G., Rosebrough R. W. 2003. Hormonal regulation of postnatal chicken preadipocyte differentiation in vitro. Comp. Biochem. Physiol. B Mol. Integr. Physiol. 136: 245–253 [DOI] [PubMed] [Google Scholar]
- 29.Kawada K., Yonei T., Ueoka H., Kiura K., Tabata M., Takigawa N., Tanimoto M. 2002. Comparison of chemosensitivity tests: clonogenic assay versus MTT assay. Acta Med. Okayama. 56: 129–134 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Mu Y., Li H., Ding N., Wang Q., Wang Y., Wang S., Wang N. 2008. Peroxisome proliferator-activated receptor-γ gene: a key regulator of adipocyte differentiation in chickens. Poult. Sci. 87: 226–232 [DOI] [PubMed] [Google Scholar]
- 31.Meli R., Pacilio M., Raso G. M., Esposito E., Coppola A., Nasti A., Carlo C. D., Nappi C., Carlo R. D. 2004. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 145: 3115–3121 [DOI] [PubMed] [Google Scholar]
- 32.Picozzi P., Marozzi A., Fornasari D., Benfante R., Barisani D., Meneveri R., Ginelli E. 2003. Genomic organization and transcription of the human retinol dehydrogenase 10 (RDH10) gene. FEBS Lett. 554: 59–66 [DOI] [PubMed] [Google Scholar]
- 33.Szatmari I., Pap A., Rühl R., Ma J. X., Illarionov P. A., Besra G. S., Rajnavolgyi E., Dezso B., Nagy L. 2006. PPAR gamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 203: 2351–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao H. B., Ye J. 2008. Long chain Acyl-CoA synthetase 3-mediated phosphatidyl-choline synthesis is required for assembly of very low density lipoproteins in human hepatoma Huh7 cells. J. Biol. Chem. 283: 849–854 [DOI] [PubMed] [Google Scholar]
- 35.Chang Y. S., Tsai C. T., Huangfu C. A., Huang W. Y., Lei H. Y., Lin C. F., Su I. J., Chang W. T., Wu P. H., Chen Y. T., et al. 2011. ACSL3 and GSK-3β are essential for lipid upregulation induced by endoplasmic reticulum stress in liver cells. J. Cell. Biochem. 112: 881–893 [DOI] [PubMed] [Google Scholar]
- 36.Janssen U., Fink T., Lichter P., Stoffel W. 1994. Human mitochondrial 3,2-trans-enoyl-CoA isomerase (DCI): gene structure and localization to chromosome 16p13.3. Genomics. 23: 223–228 [DOI] [PubMed] [Google Scholar]
- 37.Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., et al. 1998. Identification of a gene encoding an acyl CoA: diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA. 95: 13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.