Abstract
The lipidation of apoA-I in liver greatly influences HDL biogenesis and plasma HDL levels by stabilizing the secreted apoA-I. Niacin is the most effective lipid-regulating agent clinically available to raise HDL. This study was undertaken to identify regulatory mechanisms of niacin action in hepatic lipidation of apoA-I, a critical event involved in HDL biogenesis. In cultured human hepatocytes (HepG2), niacin increased: association of apoA-I with phospholipids and cholesterol by 46% and 23% respectively, formation of lipid-poor single apoA-I molecule-containing particles up to ∼ 2.4-fold, and pre β 1 and α migrating HDL particles. Niacin dose-dependently stimulated the cell efflux of phospholipid and cholesterol and increased transcription of ABCA1 gene and ABCA1 protein. Mutated DR4, a binding site for nuclear factor liver X receptor alpha (LXR α ) in the ABCA1 promoter, abolished niacin stimulatory effect. Further, knocking down LXR α or ABCA1 by RNA interference eliminated niacin-stimulated apoA-I lipidation. Niacin treatment did not change apoA-I gene expression. The present data indicate that niacin increases apoA-I lipidation by enhancing lipid efflux through a DR4-dependent transcription of ABCA1 gene in HepG2 cells. A stimulatory role of niacin in early hepatic formation of HDL particles suggests a new mechanism that contributes to niacin action to increase the stability of newly synthesized circulating HDL.
Keywords: high density lipoproteins, apolipoprotein A-I, ATP binding cassette transporterA1, direct repeat 4, HDL biogenesis, human hepatoblstoma cell line
A growing body of evidence indicates that HDLs protect against the development of atherosclerotic coronary heart disease (CHD). In fact, a low HDL-cholesterol level remains an important and independent risk factor for CHD even in patients with low basal or therapeutically lowered LDL-cholesterol levels (1–4). Antiatherogenic properties of HDL are believed to be mediated by the ability of apoA-I, the major protein of HDL, and possibly other apolipoproteins to not only to mediate reverse cholesterol transport from peripheral tissues but also through its anti-inflammatory and anti-oxidation properties (4–9).
Niacin has long been used in the treatment of dyslipidemia and cardiovascular disease (CVD) (9–13). In pharmacologic doses, niacin reduces cholesterol, triglycerides, and LDL, and increases HDL. A number of clinical trials have indicated that treatment with niacin, alone or in combination with other lipid-regulating agents, significantly reduced total mortality and coronary events and retarded the progression and induced the regression of coronary atherosclerosis (1–4, 12, 13). In a recent trial (AIM-HIGH), administration of niacin to patients with atherosclerotic cardiovascular disease receiving intensive statin therapy did not provide incremental clinical benefit to statin therapy during a 3 year follow-up period (14). The reasons for the unexpected outcome of this trial are unclear and remain to be confirmed by another ongoing much larger trial, HPS2-THRIVE, assessing the long-term clinical effects of extended release niacin/laropiprant in patients with established cardiovascular disease receiving simvastatin alone or with ezetimibe (ClinicalTrials.gov Identifier: NCT00461630). Also subgroup analyses of the AIM HIGH trial have not yet been presented.
Niacin is the most potent currently available pharmacologic agent to increase HDL (10–13). Interestingly, niacin selectively increases plasma HDL particles containing mainly apoA-I (LP-AI, HDL subclasses with cardio-protective properties) but not HDL particles containing both apoA-I and A-II (LP-AI+AII) (15). However, the molecular mechanisms of its action on HDL levels, composition, and functional properties are poorly understood. Previous kinetics studies in normolipemic humans using 125I-labeled apoA-I as a tracer indicated that niacin treatment decreased the fractional catabolic rate (FCR) of apoA-I but did not alter its synthetic rate (16, 17). Another study with infusion of deuterated leucine as a tracer in combined hyperlipidemic subjects showed that niacin treatment was associated with an increase in HDL apoA-I concentration and production (18).This result is in contrast to all previous kinetic studies and also with in vitro data on niacin's action on apoA-I and apoA-II in cultured hepatocytes. The reasons for this discrepancy are unclear and may relate to differences in patient population studied or methodology and require further research. Our in vitro studies demonstrate that niacin inhibits the uptake of 125I-labeled HDL protein but not 3H-labeled HDL cholesterol ester by cultured HepG2 cells without affecting apoA-I synthesis (19). Further, our recent studies indicate that the inhibitory effect of niacin is mediated by inhibition of the cell surface expression of ATP synthase β chain, resulting in reduced hepatic uptake of 125I-labeled HDL protein (20). This data, along with earlier turnover trials (16, 17), indicate that decreased HDL catabolism is probably a major mechanism by which niacin increases circulating apoA-I and HDL.
Most plasma apoA-I molecules exist in the lipidated form associated with HDL particles because the lipid-free apoA-I is not stable, and is quickly removed from the circulation through filtration in the kidneys (21, 22). The initial lipidation of newly secreted apoA-I by phospholipids and cholesterol, mainly promoted by the membrane protein ABCA1 activity at the cell surface, is critical during the early formation of HDL particles, and for stabilizing apoA-I as biologically active molecules (23–26). There is a significant link between the ABCA1-mediated cholesterol efflux in the initial formation of HDL and with its stability in plasma (24–27). Liver-specific inactivation of ABCA1 in mice resulted in a 2-fold increase in the catabolic rate of HDL protein (27). Mutations in the ABCA1 gene with defective ABCA1-mediated cellular cholesterol/phospholipid efflux have been shown to result in the total loss or extremely low levels of HDL in patients with Tangier disease (28–31). Thus the apoA-I catabolism can be significantly influenced by the lipidation of apoA-I in the early formation of HDL particles. We hypothesized that niacin may increase nascent HDL formation by enhancing the hepatic lipidation of apoA-I thus preventing premature in vivo clearance of poorly lipidated apoA-I. In this study, we tested this hypothesis by examining the effect of niacin on ABCA1 expression and its participation in HDL biogenesis in HepG2 cells.
MATERIALS AND METHODS
HepG2 cell culture and treatment
HepG2 cells were maintained in DMEM containing 10% FBS, supplemented with 100 units/ml penicillin G, and 100 μ g/ml streptomycin sulfate at 37°C in a humidified atmosphere of 95% air, 5% CO2. In this study, we have used niacin at doses of 0.1–1.0 mM for incubation with HepG2 cells. In humans, plasma level of niacin was found to be about 0.3 mM after oral ingestion of 2 g of niacin (32). In our extensive clinical experience with niacin, the plasma concentrations of niacin will be in the range of 0.1–0.5 mM after oral administration of 1–3 g doses of niacin (the dose commonly used in humans). Also, because of the first pass effect and transport through the portal venous system, niacin concentrations in liver tissue will be much higher than in plasma levels after oral administration of 1–3 g of niacin. These actual portal vein concentrations have not been reported. Thus, the doses of niacin used in our in vitro experiments will be clinically relevant and comparable to the niacin concentrations observed in human plasma and probably in the liver after oral administration of niacin in clinical doses.
ApoA-I lipidation assay
HepG2 cells were metabolically labeled in DMEM containing 5 mg/ml of fatty acid-free BSA with [Methyl-3H]Choline Chloride (1 uCi/ml) for phospholipids, or [1,2-3H(N)]Cholesterol, and [5-3H(N)]mevalono-lactone-Rs (2 uCi/ml) (PerkinElmer Life Sciences) was used for endogenously synthesized cholesterol for ∼ 24–48 hConstructions such as . ApoA-I-containing particles from the culture medium were immunoprecipitated by poyclonal anti-human apoA-I antibody (Calbiochem), and harvested by protein A-Sepharose beads. After washing with PBS, 3H-phospholipids or 3H-cholesterol associated with apoA-I was quantified by scintillation counting. The lipidation of apoA-I by phospholipids or cholesterol is expressed as cpm per μ g cell protein. A rabbit IgG (Invitrogen) was used as control for immuno-precipitation and subtracted as background counts.
Immuno-precipitation and Western blotting
Immuno-precipitation of apoA-I from HepG2 culture medium and Western blotting analysis of apoA-I and ABCA1 were essentially performed as described previously (33, 34).
Native gradient PAGE and 2D gel analysis
HepG2 cells were incubated with various amounts of niacin (0–1 mM Nicotinic acid, Sigma), which is in clinically relevant range of doses, for 24 h in DMEM containing 1 mg/ml of BSA free of fatty acids. Approximately 10–40 ul of sample ( ∼ 10 μ g protein) were applied to the ∼ 3–30% gradient polyacrylamide gel. Electrophoresis was carried out in TBE buffer at 50v for 22 h at 4°C. Proteins on PAGE gel were transferred to polyvinylidene fluoride (PVDF) membranes and immunoblotted with anti apoA-I antibody, and developed with the enhanced chemiluminescent reagents (Amersham). For two-dimensional (2D) gel analysis, the medium samples ( ∼ 10 μ g protein) were first loaded on the 0.7% agarose gel and run for 3 h at 100v at 4°C in Tris-Tricine buffer pH8.6. The gel strips from the first electrophoresis were cut off and transferred to the second gel of ∼ 3–30% of gradient native polyacrylamide and run for 22 h at 50v in TBE buffer at 4°C. Molecular size standards (Amersham, 17044501) were applied in the second gel electrophoresis. Proteins on the native gel were transferred to PVDF membranes and immuonblotted with anti apoA-I antibody.
Cholesterol and phospholipid efflux
HepG2 cells ( ∼ 70% confluence) were incubated with niacin for ∼ 24–48 h and then labeled with 2uCi/ml 3H-choline or 3H-cholesterol in DMEM containing 5 mg/ml BSA free of fatty acids for 24 h. After washing with PBS three times, the efflux of 3H-labeled phospholipids or cholesterol from cells was conducted in the same medium containing niacin plus 15 μ g/ml of recombinant human apoA-I for 4 h. The cellular lipid efflux activity from HepG2 cells is expressed as cpm per μ g cell protein. The apoA-I-specific cholesterol efflux is the value that has subtracted the value of efflux in the absence of the exogenously added apoA-I, and the efflux activity is expressed as the percent of cpm in medium over the total (medium plus cells).
Plasmids
The human ABCA1 gene promoter DNA sequence (GenBank accession #AJ252201, -927∼+115bp, using the transcription start site as +1) was isolated by PCR amplification from human genomic DNA (Clontech), and cloned into pGL3(R2.1) basic vector harboring the luciferase repoter cDNA. The primers used are: 5 ′ -TCT T AC GCG T AA GTT GGA GGT CTG GAG TGG C (sense) and 5 ′ -CGC AGA TCT TGA GAA CCG GCT CTG TTG GTG (anti-sense). Underlined are restriction sites for MluI and BglII, respectively. Mutations in the ABCA1 promoter were made by using the Quick Change Mutagenesis kit (Stratagene). The human apoAI promoter reporter was made as described previously (33).
Dual luciferase assay
Cell transfection and the luciferase assay for ABCA1 and apoA-I promoter were essentially performed as described previously (33).
RT and quantitative real-time PCR
Total RNA extracted from cells was reverse transcribed with 100 units of SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was performed by using the QuantiTect SYBG Green PCR reagents (Qiagen) in iCycler with gene specific primer sets: 5 ′ -TGA GCT GCA ACT CAA TGA TGC C and 5 ′ -CCA CAT ATG TGT GCT GCA GCC for liver X receptor alpha (LXR α ); 5 ′ -ACT GAT GAG GCC AGC TCA GC and 5 ′ -CCA CAG ACA CGG CAA AGC TC for LXR β ; 5 ′ -GCG CCA TCG TCC TCT TTA ACC C and 5 ′ -GGT ACT TGT GCT TGC AGT AGG C for RXR α ; 5 ′ -CCC AGA GCA AAA AGC GAC TC and 5 ′ -GGT CAT CAT CAC TTT GGT CCT TG for human ABCA1; and 5 ′ - GCA TCG TCA CCA ACT GGG ACG AC and 5 ′ - CAT GGC TGG GGT GTT GAA GGT CTC for β actin; or 5 ′ -CGG CGA CGA CCC ATT CGA AC and 5 ′ -GAA TCG AAC CCT GAT TCC CCG T for 18S rRNA as endogenous control gene. In regular RT-PCR, primers for apoA-I are 5 ′ -AGA GAC TGC GAG AAG GAG GTG and 5 ′ -CAG ATC CTT GCT CAT CTC CTG.
RNA interference assay
HepG2 cells are transfected by using Fugene 6 (Roche) for 24 h with 50 nM of small interfering RNA (siRNA) for LXR α (Ambion Silence validated siRNA ID 5458) or ABCA1 (Invitrogen, Cat# SI03025190), and scrambled siRNA control, separately. The target sequence for ABCA1 is TTGGGACTTGGTGGGACGAAA (5939-5959bp, GenBank accession NM_005502), and for LXR α (NR1H3), GGAGATAGTTGACTTTGCT (1037-1019bp, Genbank accession NM_005693). The transfectants were washed and then treated with niacin in DMEM medium containing 5 mg/ml of BSA free of fatty acids, and metabolically labeled for ∼ 24–48 h. The assays for the apoA-I lipidation were performed as described above.
Statistical analysis
Student's t-test was used in data analysis and a P-value <0.05 was considered significant.
RESULTS
Niacin increased the lipidation of apolipoprotein A-I by phospholipids and cholesterol
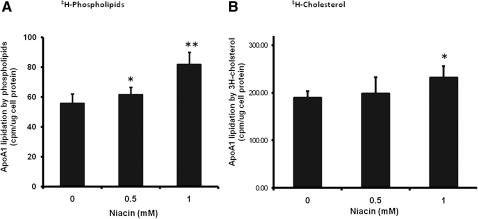
To investigate the effect of niacin on HDL biogenesis, we first examined apoA-I lipidation by phospholipids and cholesterol, an initial step in HDL biogenesis. As shown in , niacin at 0.5 and 1 mmol/L increased the apoA-I-associated 3H-phospholipids by 10% and 46%, respectively, and the apoA-I-associated 3H-cholesterol by 5% and 23%, respectively, in the culture medium that has not added exogenous apoA-I. We have used 3H-mevalonate in initial experiments to test the lipidation by endogenous synthesized cholesterol, which showed similar results. Niacin increased the lipidation of apoA-I by endogenous synthesized cholesterol by 27% (4.95 ± 0.56 vs. 6.30 ± 0.73; P = 0.027) in the presence of 1 mM niacin. These data suggest that niacin increased the lipidation of apoA-I, and the lipidation by phospholipids appears to be more sensitive to niacin treatment.
Fig. 1.
Niacin increased lipidation of apolipoprotein A-I by phospholipids (A) and cholesterol (B). HepG2 cells were metabolically labeled with 1 uCi/ml of 3H-choline or 2 uCi/ml of 3H-cholesterol in DMEM containing 5 mg/ml BSA free of fatty acids for 48 h in the presence of various amount of niacin as indicated. ApoA-I-containing particles from the culture medium were immunoprecipitated by anti-human apoA-I antibody, and harvested by protein A-Sepharose beads. Phospholipids or cholesterol associated with apoA-I was quantified by β -counting. The lipidation of apoA-I by phospholipids or cholesterol is expressed as cpm per ug cell protein. A rabbit IgG was used as control for immunoprecipitaion and subtracted as background counts ( ∼ 0.5–2 cpm/ μ g cell protein). Data are expressed as mean ± SD of three experiments; *P < 0.05; **P < 0.01.
Furthermore, analysis of 14C-labeled acetate or 3H-labeled palmitate incorporation followed by the TLC assay in our previous study (Ref. 35 and unpublished observations) indicate that niacin does not alter biosynthesis of cholesterol and phospholipids under similar cell culture conditions (incorporation of acetate into cholesterol, cpm/mg cell protein × 1000: control = 105.6 ± 7.8, 1 mM niacin = 102.8 ± 7.2; incorporation of palmitate into phosphatidylcholine, cpm/mg cell protein × 1000: control = 255.3 ± 9.0, 1 mM niacin = 261.6 ± 8.0). Together, these data indicate that niacin increases the lipidation by increasing the secretion of cholesterol and phospholipids that are associated with apoA-I in the medium but does not change their biosynthesis in HepG2 cells.
Niacin increased the formation of lipid-poor apoA-I particles and pre beta 1 migrating HDL particles
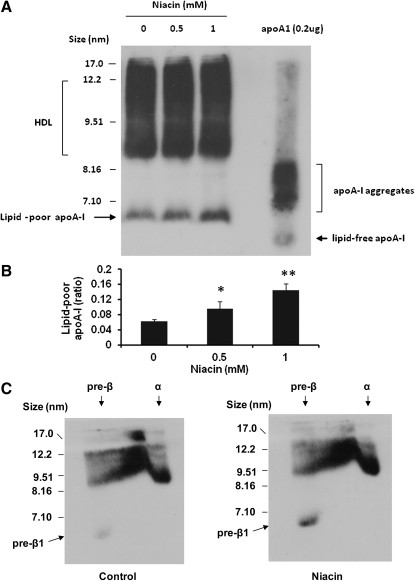
After treatment of HepG2 cells with niacin for 24 h, an aliquot of the culture medium (20 ul) was directly used for lipoprotein fractionation in native gradient PAGE (3-30%) and then transferred to PVDF membranes for immuno-blotting with anti-human apoA-I antibody. Niacin treatment increased the formation of lipid-poor apoA-I particles ( ∼ 2.4-fold) that contain a single apoA-I molecule with molecular size <7.1nm but larger than a single lipid-free apoA-I molecule, as compared with the purified apoA-I control (Fig. 2A , B) . Larger HDL particles in sizes of >7.1 nm appear to be discrete bands, concentrated at the sizes of 8.6–9.4 nm that are larger than the purified apoA-I aggregates containing more than 2–4 apoA-I molecules (23, 36). These findings suggest that niacin increased the early lipidation of apoA-I, leading to the formation of HDL particles.
Fig. 2.
Niacin increased the formation of lipid-poor apoA-I particles and pre β 1 migrating HDL particles. A: Native gradient PAGE analysis of culture medium from HepG2 cells. 0.2 μ g of purified apoA-I was used for positive control. B: Densitometric quantitation of the lipid-poor apoA-I versus total HDL particles from A; *P < 0.05; **P < 0.01. C: 10 μ g of medium protein was analyzed by 2D electrophoresis and immunoblotting with apoA-I antibody. Representatives of at least three experiments are shown.
Two-dimensional gel analysis demonstrated that niacin increased the pre β 1 migrating HDL ( ∼ 2.9-fold), which corresponds to lipid-poor apoA-I particles observed in native PAGE gel (Fig. 2C) . Also, niacin treatment increased the formation of α -migrating HDL particles by about 21% (5181 ± 300 vs. 4281 ± 126 scanning arbitrary units; n = 3, P < 0.05).
Niacin stimulated phospholipid and cholesterol efflux from HepG2 cells
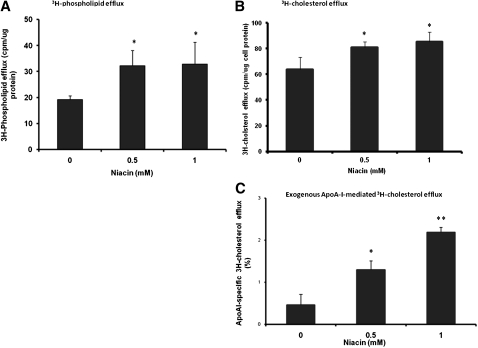
Lipid-poor apoA-I or pre β 1-HDL is mainly composed of one apoA-I molecule with asmall amount of phospholipids originating from cells (23). To investigate mechanisms for niacin-induced lipidation of apoA-I, we first tested the effect of niacin on cellular lipid efflux. Pretreatment of HepG2 cells with niacin increased the efflux of metabolically labeled 3H-choline/phospholipids and 3H-cholesterol (Fig. 3A , B) . Also, niacin dose-dependently increased the exogenous apoA-I-specific 3H-cholesterol efflux (subtracted the efflux value in the absence of the added apoA-I) from HepG2 cells (Fig.3C) .
Fig. 3.
Niacin stimulated phospholipids (A) and cholesterol efflux (B, C) from HepG2 cells. A, B: HepG2 cells were metabolically labeled with 1 uCi/ml of 3H-choline or 2 uCi/ml of 3H-cholesterol in DMEM containing 5 mg/ml BSA free of fatty acids for 48 h in the presence of various amount of niacin as indicated. The medium was removed at the end of incubation. Cells were washed with PBS two times and incubated with the same medium in the presence of various amount of niacin containing 15 μ g/ml of recombinant human apoA-I for 4 h. ApoA-I-containing particles from the medium were immuno-precipitated and quantified by β -counting. C: Exogenous ApoA-I-specific cholesterol efflux was calculated by subtracting the efflux value of experiments that contained no apoA-I from the value that contained apoA-I. Efflux activity is expressed as the percent of cpm in medium over the total (medium plus cells). Data are expressed as mean ± SD of three experiments; *P < 0.05; **P < 0.01.
Niacin increased expression of ABCA1
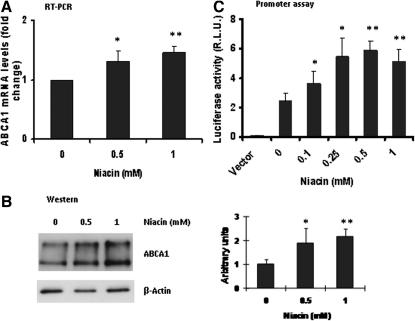
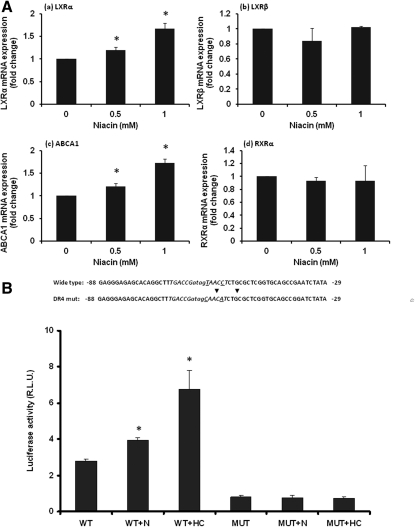
The membrane ABCA1 plays an essential role in HDL biogenesis, and the ABCA1-mediated lipid efflux from cells is a critical step for lipidation of apoA-I in early formation of HDL particles (36–40). As shown in , niacin increased ABCA1 mRNA and ABCA1 protein expression. To further examine the niacin effect on ABCA1, we constructed a luciferase reporter plasmid containing the promoter sequence of human ABCA1 gene and transfected into HepG2 cells to measure the promoter activity. Results showed that niacin increased transcription of ABCA1 gene in a dose-dependent manner (Fig. 4C) . These data suggest that niacin increased ABCA1 expression through its gene transcription.
Fig. 4.
Niacin increased expression of ABCA1. A: Real-time RT-PCR analysis of ABCA1 mRNA expression. B: Immunoblotting analysis of HepG2 cell proteins treated with niacin. Shown right is densitometric quantitation of three blots with fold changes compared with control. C: Dual luciferase reporter assay for human ABCA1 promoter. Data are expressed as mean ± SD of three experiments; *P < 0.05; **P < 0.01.
Niacin-stimulated ABCA1 transcription and function is dependent on DR4
ABCA1 gene expression is influenced by a number of factors but mainly regulated through the conserved consensus cis-acting element DR4 [direct repeats of the motif AGGTCA or TGACCT (antisense), separated by four nucleotides], a binding site for nuclear receptor LXR α in the proximal promoter region of the ABCA1 gene (TGACCGatagTAACCT) (41, 42). LXR α , stimulated by oxycholesterols such as 22-(R) hydroxycholesterol (HC), binds to the DR4 site as hetero-dimer with its partner protein RXR to increase the transcription of ABCA1 gene (Fig. 5). We examined expression of these nuclear receptors. Niacin treatment did not change RXR α nor another LXR isoform LXR β mRNA expression, but increased LXR α parallel with increased ABCA1 mRNA expression (Fig. 5A). Promoter reporter assay showed that niacin, like the LXR agonist 22HC, significantly stimulated ABCA1 promoter activity. When DR4 sequence was mutated, the ABCA1 promoter activity was dramatically decreased. Moreover, compared with the wild-type, the mutated DR4 eliminated both niacin- and 22HC-induced ABCA1 promoter activity (Fig. 5B).
Fig. 5.
Niacin-stimulated ABCA1 transcription is dependent on DR4. A: RT-qPCR. HepG2 cells were incubated with niacin for 48 h and total RNA were extracted for RT-qPCR as described in Materials and Methods. * P < 0.01. B: Promoter assay. pGL3-ABCA1 (WT) or pGL3-ABCA1 containing mutated DR4 (MUT) were transfected into HepG2 cells for dual luciferase assay in the presence or absence of 0.5 mM niacin (N) and 10 μ M 22-(R) hydroxycholesterol (HC). Data are expressed as mean ± SD of three experiments; *P < 0.01.
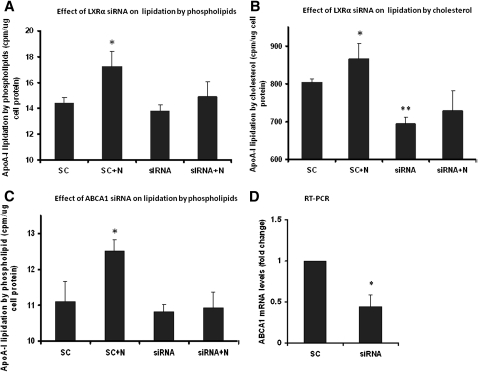
Further, knocking down LXR α by small RNA interference decreased ABCA1 expression by 35% (RT-PCR fold change: 1.0 ± 0 vs. 0.65 ± 0.05, P < 0.01) and eliminated niacin-induced lipidation of apoA-I by cholesterol and phospholipids (Fig. 6A , B) whereas apoA-I expression was not altered (data not shown). Directly knocking down ABCA1 by RNA interference reduced ABCA1 mRNA expression (Fig. 6D) and abolished niacin-induced apoA-I lipidation by phospholipids (Fig. 6C).
Fig. 6.
Niacin-stimulated ABCA1 function is mediated through LXR α . Knocking down LXR α by siRNA abolished niacin-induced apoA-I lipidation by phospolipids (A) and cholesterol (B). Knocking down ABCA1 eliminated niacin-induced apoA-I lipidation by phospholipids (C) and reduced ABCA1 expression (D). SC: Scrambled control siRNA; N: 0.5 mM niacin. Data are expressed as mean ± SD of three experiments; *P < 0.05; **P < 0.01.
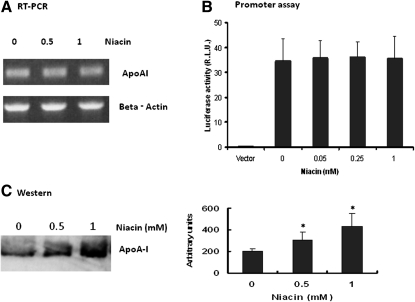
Niacin did not affect apoA-I gene expression but increased apoA-I accumulation in the culture medium
ApoA-I is a main acceptor for the cellular lipids during the formation of HDL particles. We then tested the effect of niacin on expression of apoA-I in our experimental conditions. RT-PCR and the luciferase reporter data showed that niacin did not change apoA-I mRNA expression and apoA-I promoter activity (Fig. 7A , B). These data confirmed that niacin has no effect on apoA-I gene expression in our cell culture conditions, consistent with previous reports that niacin did not affect apoA-I biosynthesis in HepG2 cells (19). However, Western blotting demonstrated that niacin significantly increased apoA-I protein accumulation in cell culture medium (Fig. 7C) indicating that niacin may have reduced the removal from medium of basal secretion of apoA-I and confirming our previous observation (19).
Fig. 7.
Niacin did not affect apoA-I gene expression but increased apoA-I secretion/accumulation in culture medium. A: RT-PCR assay using β actin as reference. B: Dual-luciferase assay for ApoA-I promoter in HepG2 cells. Data are expressed as mean ± SD of 3 experiments. C: Immuno-blotting analysis of apoA-I from HepG2 cell culture medium. Shown right is densitometric quantitation of three blots; *P < 0.01.
DISCUSSION
In this study, we show that niacin increases the lipidation of apoA-I containing particles with phospholipid and cholesterol in HepG2 cell culture medium. Native gradient polyacrylamide gel and immuno-blotting analysis showed that niacin increased the generation of lipid poor apoA-I particles and specifically the formation of the pre β 1 migrating HDL particles (Fig. 2). These lipid-poor/pre- β 1 HDL particles, composed of a single apoA-I molecule and a small amount of phospholipids, are much more stable than lipid free apoA-I, and they are initial forms of HDL with significant biological activities (23–25). In the liver, they actively accept cellular cholesterol/phospholipids to form nascent discoidal HDL particles. In the circulation, the nascent HDL particles get enriched with cholesterol esters by the action of LCAT activity to generate spherical, α -migrating mature HDL particles. Our findings suggest that niacin plays an important role in early formation of HDL particles by increasing the lipidation of secreted apoA-I.
It has been well documented that most of the phospholipids transferred to newly secreted apoA-I are derived from the ABCA1-promoted lipid efflux activity at the cell surface in the early biosynthesis of HDL (23–27). To investigate mechanisms for niacin-induced lipidation of apoA-I, we tested its effect on cellular lipid efflux from HepG2 cells. Using exogenously added apoA-I as acceptor, we found that niacin increased the efflux of phospholipid and cholesterol from HepG2 cells (Fig. 3). However niacin treatment does not alter biosynthesis of cholesterol and phospholipids (35). Significantly, niacin increased ABCA1 expression without alteration of apoA-I gene expression. These findings suggest niacin increased lipidation of basal freshly synthesized/secreted apoA-I promoted by ABCA1-mediated lipid efflux. Such a mechanism would theoretically stabilize and prevent its rapid and premature clearance in vivo soon after secretion.
Regulation of ABCA1 expression is complex at both transcriptional and posttranscriptional levels (41–43). Evidence from in vitro and in vivo studies has shown that ABCA1 expression is mainly regulated through the conserved consensus cis-acting element DR4, a binding site for nuclear receptor LXR α in the proximal promoter region of the ABCA1 gene (41, 42), although an additional DR4 element has also been found in the first intron of the human/mouse ABCA1 gene (34). In this study, using an ABCA1 promoter reporter assay, we directly tested niacin's effect on ABCA1 transcription in HepG2 cells. Niacin stimulated ABCA1 promoter activity whereas mutated DR4 eliminated niacin-induced stimulatory effects (Fig. 5). Furthermore, knocking down the expression of LXR α by RNA interference suppressed niacin-induced lipidation of apoA-I (Fig. 6). These findings indicate that DR4 is required for niacin-induced biosynthesis of HDL in HepG2 cells.
Although evidence of the effect of niacin on ABCA1 expression has been shown in macrophages (44, 45), the detailed effects of niacin on the ABCA1-mediated cholesterol efflux to apoA-I in hepatocytes, the major cells contributing to plasma HDL levels, are not known. In macrophages, by activating the prostaglandin synthesis pathway through its receptor HM74 and HM74a, niacin induces synthesis of PGD2 followed by 15-deoxy-delta 12,14-prostaglandin J2, the most potent endogenous ligand of PPAR γ , to increase PPAR γ -dependent expression of genes including ABCA1 (45). In adipocytes, niacin through binding and activating its receptor HM74/HM74a inhibits lipolysis by reducing intracellular cAMP and release of free fatty acids into the circulation (46). However, the absence or very limited expression of niacin receptors found in the liver (47) and HepG2 cells (data not shown) raised questions as to whether similar mechanisms for niacin action observed in macrophages or other tissues exist in the liver. This study does not answer the question of how niacin increases ABCA1 transcription. We and others (44) have found that niacin increases cellular free cholesterol and decreases cholesterol ester (data not shown). Increased levels of free cholesterol result in enhanced oxysterol production that increases LXR α expression, activating transcription of ABCA1 gene through binding to DR4 site, thus resulting in increased cholesterol cellular efflux. The present data that niacin increases LXR α expression support this possibility (Fig. 5A). Whether modulation of enzymes such as ACAT and/or cholesteryl ester hydrolases that regulate free cellular cholesterol are involved in niacin action requires further investigation.
There is a possibility that increased LXR expression by niacin could also lead to stimulating its target genes that are involved in biosynthesis of triglycerides in the liver. However, niacin has also been found to inhibit DGAT2, a key enzyme for triglyceride biosynthesis, and increase apoB100 degradation (35, 48). These effects would lead to decreased synthesis and secretion of VLDL resulting in a lower plasma triglyceride (VLDL-TG) level, which has been observed in patients clinically. It may be possible that niacin-mediated inhibition of DGAT2 may override other regulatory events involved in triglyceride synthesis.
In summary, the findings reported in this paper indicate that niacin increases early hepatic HDL biogenesis by stimulating phospholipids/cholesterol efflux through a DR4-dependent transcription of ABCA1 gene. Niacin-mediated increased apoA-I lipidation and biogenesis of nascent HDL (known for its functional properties) as reported in this study, may serve as a significant mechanism by which niacin stabilizes liver generated HDL particles, thus preventing premature HDL catabolism upon their secretion in vivo.
Footnotes
Abbreviations:
- CHD
- coronary heart disease
- 2D
- two-dimentional gel electrophoresis
- DR4
- direct repeat 4
- HepG2
- human hepatoblstoma cell line
- LP-AI
- HDL particles containing only apoA-I
- LP-AI+AII
- HDL particles containing both apoA-I and apoA-II
- LXR α
- liver X receptor alpha
- PVDF
- polyvinylidene fluoride
- siRNA
- small interfering RNA
- TBE
- tris/borate/ethylenediaminetetraacetic acid buffer
This study was supported by the Southern California Institute for Research and Education.
REFERENCES
- 1.Meyers C. D., Kashyap M. L. 2005. Pharmacologic augmentation of high-density lipoproteins. Curr. Opin. Cardiol. 20: 307–312 [DOI] [PubMed] [Google Scholar]
- 2.Brown G., Albers J. J., Fisher L. D., Schaefer S. M., Lin J. T., Kaplan C., Zhao X. Q., Bisson B. D., Fitzpatrick V. F., Dodge H. T. 1990. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N. Engl. J. Med. 323: 1289–1298 [DOI] [PubMed] [Google Scholar]
- 3.Brown B. G., Zhao X. Q., Chait A., Fisher L. D., Cheung M. C., Morse J. S., Dowdy A. A., Marino E. K., Bolson E. L., Alaupovic P., et al. 2001. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345: 1583–1592 [DOI] [PubMed] [Google Scholar]
- 4.Rader D. J. 2007. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat. Clin. Pract. Cardiovasc. Med. 4: 102–109 [DOI] [PubMed] [Google Scholar]
- 5.Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. 1991. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88: 2039–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganji S. H., Qin S., Zhang L. H., Kamanna V. S., Kashyap M. L. 2009. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 202: 68–75 [DOI] [PubMed] [Google Scholar]
- 7.Cockerill G. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. 1995. HDL inhibits cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 15: 1987–1994 [DOI] [PubMed] [Google Scholar]
- 8.Moore R.E., M. Navab, J. S. Millar, F. Zimetti, S. Hama, G. H. Rothblat, and D. J. Rader 2005. Increased atherosclerosis in mice lacking apolipoprotein AI attributes to both impaired reverse cholesterol transport and increased inflammation. Circ. Res. 97: 763–771 [DOI] [PubMed] [Google Scholar]
- 9.Wong N. D., Malik S., Kashyap M. L.2005. Dyslipidemia. In: Preventive Cardiology. N. D. Wong, H. R. Black, J. M. Gardin, editors. McGraw Hill Co., Inc., New York. 183–211.
- 10.Kamanna V. S., Kashyap M. L. 2008. Mechanism of action of niacin. Am. J. Cardiol. 101: 20B–26B [DOI] [PubMed] [Google Scholar]
- 11.Carlson L. A. 2006. Nicotinic acid and other therapies for raising high-density lipoprotein. Curr. Opin. Cardiol. 21: 336–344 [DOI] [PubMed] [Google Scholar]
- 12.Meyers C. D., Kamanna V. S., Kashyap M. L. 2004. Niacin therapy in atherosclerosis. Curr. Opin. Lipidol. 15: 659–665 [DOI] [PubMed] [Google Scholar]
- 13.Bruckert E., Labreuche J., Amarenco P. 2010. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 210: 353–361 [DOI] [PubMed] [Google Scholar]
- 14. AIM-HIGH Investigators, W. E. Boden, J. L. Probstfield, T. Anderson, B. R. Chaitman, P. Desvignes-Nickens, K. Koprowicz, R. McBride, K. Teo, and W. Weintraub 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267 [DOI] [PubMed] [Google Scholar]
- 15.Sakai T., Kamanna V. S., Kashyap M. L. 2001. Niacin, but not gemfibrozil, selectively increases LP-AI, a cardioprotective subfraction of HDL, in patients with low HDL cholesterol. Arterioscler. Thromb. Vasc. Biol. 21: 1783–1789 [DOI] [PubMed] [Google Scholar]
- 16.Grundy S. M., Mok H. Y., Zech L., Berman M. 1981. Influence of nicotinic acid on metabolism of cholesterol and triglycerides in man. J. Lipid Res. 22: 24–36 [PubMed] [Google Scholar]
- 17.Shepherd J., Packard C. J., Patsch J. R., Gotto A. M., Jr, Taunton O. D. 1979. Effects of nicotinic acid therapy on plasma high density lipoprotein subfraction and composition and on apolipoprotein A metabolism. J. Clin. Invest. 63: 858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamon-Fava S., Diffenderfer M. R., Barrett P. H., Buchsbaum A., Nyaku M., Horvath K. V., Asztalos B. F., Otokozawa S., Ai M., Matthan N. R., et al. 2008. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler. Thromb. Vasc. Biol. 28: 1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin F. Y., Kamanna V. S., Kashyap M. L. 1997. Niacin decreases removal of high density lipoprotein AI but not cholesterol ester by HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 17: 2020–2028 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L. H., Kamanna V. S., Zhang C. M., Kashyap M. L. 2008. Niacin inhibits surface expression of ATP synthase beta chain in HepG2 cells: implications for raising HDL. J. Lipid Res. 49: 1195–1201 [DOI] [PubMed] [Google Scholar]
- 21.Glass C. K., Pittman R. C., Keller G. A., Steinberg D. 1983. Tissue sites of degradation of apoprotein A-I in the rat. J. Biol. Chem. 258: 7161–7167 [PubMed] [Google Scholar]
- 22.Saku K., Mendoza S. G., Laver M., Hynd B. A., Gartside P. S., Kashyap M. L. 1988. High-density lipoprotein apolipoprotein AI and AII turnover in moderate and severe proteinuria. Nephron. 50: 112–115 [DOI] [PubMed] [Google Scholar]
- 23.Chau P., Nakamura Y., Fielding C. J., Fielding P. E. 2006. Mechanism of prebeta-HDL formation and activation. Biochemistry. 45: 3981–3987 [DOI] [PubMed] [Google Scholar]
- 24.Lee J. Y., Parks J. S. 2005. ATP-binding cassette transporter AI and its role in HDL formation. Curr. Opin. Lipidol. 16: 19–25 [DOI] [PubMed] [Google Scholar]
- 25.Rothblat G. H., Phillips M. C. 2010. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 21: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulya A., Lee J. Y., Gebre A. K., Boudyguina E. Y., Chung S. K., Smith T. L., Colvin P. L., Jiang X. C., Parks J. S. 2008. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J. Lipid Res. 49: 2390–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345 [DOI] [PubMed] [Google Scholar]
- 29.Bodzioch M., Orsó E., Klucken J., Langmann T., Böttcher A., Diederich W., Drobnik W., Barlage S., Büchler C., Porsch-Özcürümez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351 [DOI] [PubMed] [Google Scholar]
- 30.Rust S., Rosier M., Funke H., Amoura Z., Piette J-C., Deleuze J-F., Brewer H. B., Jr, Duverger N., Denèfle P., Assmann G. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355 [DOI] [PubMed] [Google Scholar]
- 31.Clee S. M., Kastelein J. J., van Dam M., Marcil M., Roomp K., Zwarts K. Y., Collins J. A., Roelants R., Tamasawa N., Stulc T., et al. 2000. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 106: 1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon R. M., González M. A., Adams M. H., Tolbert D. S., Leu J. H., Cefali E. A. 2007. Effect of the rate of niacin administration on the plasma and urine pharmacokinetics of niacin and its metabolites. J. Clin. Pharmacol. 47: 681–688 [DOI] [PubMed] [Google Scholar]
- 33.Zhang L. H., Kamanna V. S., Ganji S. H., Xiong X. M., Kashyap M. L. 2010. Pioglitazone increases apolipoprotein A-I production by directly enhancing PPRE-dependent transcription in HepG2 cells. J. Lipid Res. 51: 2211–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singaraja R. R., Bocher V., James E. R., Clee S. M., Zhang L. H., Leavitt B. R., Tan B., Brooks-Wilson A., Kwok A., Bissada N., et al. 2001. Human ABCA1 BAC transgenic mice show increased high density lipoprotein cholesterol and ApoAI-dependent efflux stimulated by an internal promoter containing liver X receptor response elements in intron 1. J. Biol. Chem. 276: 33969–33979 [DOI] [PubMed] [Google Scholar]
- 35.Jin F. Y., Kamanna V. S., Kashyap M. L. 1999. Niacin accelerates intracellular ApoB degradation by inhibiting triacylglycerol synthesis in human hepatoblastoma (HepG2) cells. Arterioscler. Thromb. Vasc. Biol. 19: 1051–1059 [DOI] [PubMed] [Google Scholar]
- 36.Duong P. T., Weibel G. L., Lund-Katz S., Rothblat G. H., Phillips M. C. 2008. Characterization and properties of pre beta-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-I. J. Lipid Res. 49: 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rye K. A., Barter P. J. 2004. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24: 421–428 [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama S. 2005. Assembly of high density lipoprotein by the ABCA1/apolipoprotein pathway. Curr. Opin. Lipidol. 16: 269–279 [DOI] [PubMed] [Google Scholar]
- 39.Zheng H., Kiss R. S., Franklin V., Wang M. D., Haidar B., Marcel Y. L. 2005. ApoA-I lipidation in primary mouse hepatocytes: separate controls for phospholipid and cholesterol transfers. J. Biol. Chem. 280: 21612–21621 [DOI] [PubMed] [Google Scholar]
- 40.Brewer H. B., Jr, Remaley A. T., Neufeld E. B., Basso F., Joyce C. 2004. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 24: 1755–1760 [DOI] [PubMed] [Google Scholar]
- 41.Venkateswaran A., Laffitte B. A., Joseph S. B., Mak P. A., Wilpitz D. C., Edwards P. A., Tontonoz P. 2000. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. USA. 97: 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chinetti G., Lestavel S., Bocher V., Remaley A. T., Neve B., Torra I. P., Teissier E., Minnich A., Jaye M., Duverger N., et al. 2001. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 7: 53–58 [DOI] [PubMed] [Google Scholar]
- 43.Fernández-Hernando C., Suárez Y., Rayner K. J., Moore K. J. 2011. MicroRNAs in lipid metabolism. Curr. Opin. Lipidol. 22: 86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubic T., Trottmann M., Lorenz R. L. 2004. Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem. Pharmacol. 67: 411–419 [DOI] [PubMed] [Google Scholar]
- 45.Knowles H. J., te Poele R. H., Workman P., Harris A. L. 2006. Niacin induces PPARgamma expression and transcriptional activation in macrophages via HM74 and HM74a-mediated induction of prostaglandin synthesis pathways. Biochem. Pharmacol. 71: 646–656 [DOI] [PubMed] [Google Scholar]
- 46.Wu Z. H., Zhao S. P. 2009. Niacin promotes cholesterol efflux through stimulation of the PPARgamma-LXRalpha-ABCA1 pathway in 3T3–L1 adipocytes. Pharmacology. 84: 282–287 [DOI] [PubMed] [Google Scholar]
- 47.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., Pfeffer K., Offermanns S. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9: 352–355 [DOI] [PubMed] [Google Scholar]
- 48.Ganji S. H., Tavintharan S., Zhu D., Xing Y., Kamanna V. S., Kashyap M. L. 2004. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 45: 1835–1845 [DOI] [PubMed] [Google Scholar]