Abstract
The low-grade inflammation observed in obesity has been associated with a high-fat diet, though this relation is not fully understood. Bacterial endotoxin, produced by gut microbiota, may be the linking factor. However, this has not been confirmed in obese patients. To study the relationship between a high-fat diet and bacterial endotoxin, we analyzed postprandial endotoxemia in morbidly obese patients after a fat overload. The endotoxin levels were determined in serum and the chylomicron fraction at baseline and 3 h after a fat overload in 40 morbidly obese patients and their levels related with the degree of insulin resistance and postprandial hypertriglyceridemia. The morbidly obese patients with the highest postprandial hypertriglyceridemia showed a significant increase in lipopolysaccharide (LPS) levels in serum and the chylomicron fraction after the fat overload. Postprandial chylomicron LPS levels correlated positively with the difference between postprandial triglycerides and baseline triglycerides. There were no significant correlations between C-reactive protein (CRP) and LPS levels. The main variables contributing to serum LPS levels after fat overload were baseline and postprandial triglyceride levels but not glucose or insulin resistance. Additionally, superoxide dismutase activity decreased significantly after the fat overload. Postprandial LPS increase after a fat overload is related to postprandial hypertriglyceridemia but not to degree of insulin resistance in morbidly obese patients.
Keywords: chylomicrons, insulin resistance, nutrition, triglycerides, metabolic endotoxemia, obesity
Obesity is an increasingly prevalent health problem, very often accompanied by other diseases, the most common being insulin resistance, type 2 diabetes mellitus, and cardiovascular complications (1, 2). Furthermore, an association has been reported between obesity and both oxidative stress and increased inflammation (3, 4). Obesity facilitates the development of a low-grade inflammatory state, characterized by increased plasma levels of proinflammatory cytokines (4). However, the factors that trigger this low-grade inflammation in obesity are unclear. Postprandial lipidemia has recently emerged as a potential candidate because the ingestion of a high-fat meal leads to the systemic increase of a wide range of inflammatory mediators (5–7) and an increase in oxidative stress markers (8). However, the cause of these postprandial events that occur in association with the postprandial triglyceride response remains poorly understood. A possible link is bacterial endotoxin [lipopolysaccharide (LPS)], a component of the Gram-negative bacteria cell wall that is present in large quantities in the human gut (9). Endotoxins circulate in the plasma of healthy human subjects at low concentrations (known as metabolic endotoxemia), and an elevated concentration of circulating LPS has been associated with a higher risk for atherosclerosis (10). There is evidence that metabolic plasma LPS levels are modulated by food content: the higher the fat content, the higher the concentration of plasma LPS (11). Small amounts of LPS are absorbed from the gut in healthy animals (12), and there is evidence that chylomicrons likely also transport significant amounts of absorbed gut LPS (13–15). In concordance with these data, some studies have shown that a high-fat meal leads to an increase in postprandial endotoxemia (7, 14).
Obesity tends to be accompanied by the consumption of a high-fat diet, and interestingly, the proportion of Gram-negative bacteria in microflora is higher in obese subjects than in lean subjects (16, 17). Thus, these conditions would enhance the translocation of endogenous LPS from the gut during fat absorption, which would lead to the low-grade inflammation observed in these patients (7, 18). However, no studies have yet examined metabolic endotoxemia in obese patients. This is of particular interest, as metabolic endotoxemia could be involved in the development of obesity-related comorbidities, as it has been associated with the development of insulin resistance in mice (18).
Thus, the aim of this study was to analyze postprandial endotoxemia in morbidly obese patients after a fat overload and to determine its relationship with postprandial hypertriglyceridemia and insulin resistance.
MATERIAL AND METHODS
Subjects and study design
A total of 40 morbidly obese patients [body mass index (BMI) >40 kg/m2] were selected from our database according to their degree of insulin resistance (HOMA-IR): 20 morbidly obese patients with HOMA-IR < 5 and 20 morbidly obese patients with HOMA-IR > 8. Those patients with intermediate values of HOMA-IR (HOMA-IR > 5 or < 8) were excluded. The cut-off point for the HOMA-IR was taken from previous studies carried out in a healthy population with no carbohydrate metabolism disorders (2). Patients were excluded if they had cardiovascular disease, arthritis, acute inflammatory disease, infectious disease, renal disease, were receiving treatment for hyperlipidemia or diabetes or were taking medications that could influence gastric emptying or the absorption time. All the patients were recruited by endocrinologists and gave informed consent to the study, which was approved by the Ethics Committee of Virgen de la Victoria Clinical University Hospital, Malaga, Spain.
After an overnight fast, all 40 participants underwent a 50 g fat overload with a preparation (patent No. P201030776). Only water was permitted during the process and no physical exercise was undertaken. The preparation of 100 ml contained 50 g fat, of which 10 g were saturated, 29.46 g were monounsaturated, and 10.625 g were polyunsaturated. Each 100 ml contained <1 g lauric acid, <1 g myristic acid, 4.8 g palmitic acid, 1.4 g stearic acid, 27.7 g oleic acid, 9.6 g linoleic acid, 1.4 g behenic acid and 0.5 g lignoceric acid. This test was previously validated in another study by our group (19). All the participants followed the same diet on the day prior to fat overload.
The 40 patients were classified according to their degree of insulin resistance (HOMA-IR, calculated as described below) and the Delta triglycerides (ΔTG), measured as the difference between postprandial triglycerides and baseline triglycerides, as follows: Group 1 (HOMA-IR ≤ 5 and ΔTG < 80 mg/dl), Group 2 (HOMA-IR > 8 and ΔTG < 80 mg/dl), Group 3 (HOMA-IR ≤ 5 and ΔTG > 80 mg/dl) and Group 4 (HOMA-IR > 8 and ΔTG > 80 mg/dl). The cut-off point for the triglyceride difference was determined by the median.
Biochemical analyses
At baseline and 3 h after the high-fat meal, blood samples were obtained from the antecubital vein and placed in vacutainer tubes (BD vacutainer™, London, UK). The serum was separated by centrifugation for 10 min at 4,000 rpm and frozen at −80°C until analysis. Data recorded for all subjects were age, weight, height (to calculate the BMI, calculated as the weight in kg divided by the height in square meters), and waist and hip circumferences (to calculate the waist-to-hip ratio, calculated as the waist circumference divided by the hip circumference). Serum glucose, uric acid, cholesterol, triglycerides, HDL cholesterol, free-fatty acids, C-reactive protein (CRP) and transaminases were measured in a Dimension autoanalyzer (Dade Behring Inc., Deerfield, IL) by enzymatic methods (Randox Laboratories Ltd., UK). Insulin levels were quantified by radioimmunoassay supplied by BioSource S.A. (Nivelles, Belgium). The LDL cholesterol was calculated from the Friedewald equation. The insulin resistance was calculated from the homeostasis model assessment of insulin resistance (HOMA-IR) with the formula: insulin resistance = [fasting serum insulin (μU/ml) × fasting blood glucose (mmol/L)]/22.5 (20). Leptin was analyzed by ELISA kits (DSL, Webster, TX). Adiponectin was analyzed by ELISA kits (DRG Diagnostics GmbH, Germany). Superoxide dismutase (SOD) activity was measured in plasma using commercial kits (Cayman Chemical, Ann Arbor, MI).
Chylomicrons were separated from serum by ultracentrifugation at 30,000 rpm for 30 min at room temperature (Beckman TLA 100.3). The top layer was carefully isolated and resuspended in endotoxin-free saline solution to the initial volume.
LAL assays
Serum LPS and chylomicron LPS concentrations were measured by endotoxin assay, based on a Limulus amebocyte extract with a chromogenic Limulus amebocyte lysate (LAL) assay (QCL-1000, Lonza Group Ltd.). Samples were diluted in pyrogen-free water and heated at 70°C for 10 min to inactivate endotoxin-neutralizing agents that inhibit the activity of endotoxin in the LAL assay. Then, Pyrosperse reagent (Lonza Group Ltd.), a metallo-modified polyanionic dispersant, was added to the test samples at a ratio of 1/200 (v/v) before LAL testing to minimize interference in the reaction. Internal control of recovery calculation was included in the assessment. All samples were tested in duplicate and results were accepted when the intra-assay CV was less than 10%. The endotoxin content was expressed as endotoxin units (EU) per ml. Exhaustive care was taken to avoid environmental endotoxin contamination and all material used for sample preparation and the test was pyrogen-free.
Statistical analysis
Study groups were compared by ANOVA and Bonferroni's posthoc tests at both baseline and in the postprandial state. Analysis of the effects of the fat overload on the biological variables was done by Wilcoxon test. Spearman correlation analyses were done to study the associations between variables. Multiple linear regression analysis was performed to evaluate which variables contributed more to LPS levels. In all cases, the rejection level for a null hypothesis was an alpha = 0.05 for two tails. Calculations were performed with SPSS software (version 15.0; SPSS Iberica, Madrid, Spain).
RESULTS
The anthropometric and biochemical variables of the morbidly obese patients are summarized in Table 1.
TABLE 1.
Anthropometric and biochemical variables of the study groups
| Group 1 (n = 10) | Group 2 (n = 10) | Group 3 (n = 10) | Group 4 (n = 10) | All (n = 40) | |
| Age (years) | 40.00 ± 11.58a | 38.71 ± 8.63a | 43.14 ± 6.568a | 42.00 ± 6.00a | 41.41 ± 9.36 |
| Weight (Kg) | 129.77 ± 27.50a, b | 152.64 ± 23.90b, c | 118.96 ± 11.93a | 156.95 ± 11.29c | 139.38 ± 25.48 |
| BMI (kg/m ) | 51.69 ± 9.16a, b | 59.85 ± 8.86a | 46,66 ± 6.54b | 55,72 ± 9.56a, b | 53.70 ± 9.49 |
| Waist (cm) | 130.05 ± 10.63a | 147.29 ± 14.64b | 123.00 ± 5.66a | 152.00 ± 7.80b | 137.80 ± 15.24 |
| Hip (cm) | 147.40 ± 12.62a | 155.14 ± 10.24a | 145.20 ± 11.28a | 149.17 ± 16.52a | 149.32 ± 12.61 |
| Waist/Hip ratio | 0.88 ± 0.08a, b | 0.95 ± 0.04b, c | 0.85 ± 0.05a | 1.03 ± 0.10c | 0.92 ± 0.09 |
| Glucose (mg/dl) | 93.00 ± 16.32a | 110.43 ± 26.25a | 96.20 ± 6.94a | 103.33 ± 11.22a | 100.14 ± 17.99 |
| Insulin (µUI/ml) | 15.86 ± 5.54a | 39.77 ± 9.74b | 12.38 ± 2.33a | 42.52 ± 8.21b | 26.93 ± 15.03 |
| HOMA-IR | 3.59 ± 1.15a | 10.54 ± 2.01b | 2.94 ± 0.64a | 10.79 ± 2.19b | 6.76 ± 4.00 |
| Uric acid (mg/dl) | 5.85 ± 1.60a, b | 6.33 ± 1.37a | 5.27 ± 1.07a, b | 7.20 ± 1.98b | 6.16 ± 1.61 |
| Chol (mg/dl) | 211.40 ± 31.18a | 187.57 ± 44.02a | 183.00 ± 37.50a | 183.50 ± 27.85a | 194.39 ± 35.73 |
| FFA 0 h (mmol/L) | 0.52 ± 0.20a | 0.55 ± 0.21a | 0.42 ± 0.16a | 0.49 ± 0.17a | 0.51 ± 0.19 |
| FFA 3 h (mmol/L) | 0.74 ± 0.27a | 0.74 ± 0.20a | 0.77 ± 0.26a | 0.83 ± 0.25a | 0.76 ± 0.25 |
| TG 0 h (mg/dl) | 133.97 ± 77.15a, b | 215.29 ± 105.69a | 98.93 ± 46.93b | 110.40 ± 19.80a | 142.99 ± 82.74 |
| TG 3 h (mg/dl) | 166.75 ± 70.17a | 251.00 ± 106.27a | 205.21 ± 43.95a | 224.29 ± 29.87a | 207.01 ± 75.97 |
| ΔTG (mg/dl) | 32.78 ± 22.99a | 35.71 ± 22.82a | 106.28 ± 29.32b | 113.88 ± 31.06b | 64.02 ± 45.28 |
| HDL-C (mg/dl) | 48.20 ± 5.43a | 41.14 ± 6.94a, b | 45.80 ± 11.08a, b | 37.83 ± 5.84b | 43.79 ± 7.92 |
| LDL (mg/dl) | 140.71 ± 22.43a | 98.80 ± 24.92b | 105.71 ± 22.31a, b | 123.95 ± 19.24a, b | 120.30 ± 26.16 |
| GOT (units/L) | 26.30 ± 25.95a | 19.86 ± 7.47a | 20.00 ± 7.87a | 34.67 ± 23.50a | 25.36 ± 19.52 |
| GGT (units/L) | 27.30 ± 11.73a | 26.43 ± 11.34a | 26.00 ± 9.14a | 51.33 ± 29.66b | 32.00 ± 18.86 |
| Leptin (ng/ml) | 61.17 ± 32.94a | 58.05 ± 23.28a | 52.63 ± 29.28a | 71.47 ± 25.87a | 60.71 ± 29.39 |
| Adiponectin (ng/ml) | 9.35 ± 3.25a | 6.85 ± 3.33a | 9.49 ± 3.12a | 7.49 ± 4.01a | 8.52 ± 3.49 |
| CRP 0 h (mg/dl) | 6.15 ± 6.67a, b | 10.09 ± 10.70a | 3.98 ± 3.94b | 7.42 ± 6.03a, b | 7.03 ± 7.60 |
| CRP 3 h (mg/dl) | 5.11 ± 3.93a | 8.43 ± 9.67a | 3.86 ± 2.94a | 7.38 ± 5.64a | 6.11 ± 6.13 |
Values are presented as means ± SD. Different letters indicate significant differences (P < 0.05) between the study groups (ANOVA, Bonferoni test). Group 1: Patients with HOMA-IR ≤ 5 and ΔTG < 80 mg/dl; Group 2: patients with HOMA-IR > 8 and ΔTG < 80 mg/dl; Group 3: patients with HOMA-IR ≤ 5 and ΔTG > 80 mg/dl; Group 4: patients with HOMA-IR > 8 and ΔTG > 80 mg/dl. BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; Chol, cholesterol; FFA, free fatty acids; TG, triglycerides; ΔTG, difference between postprandial and baseline triglyceride levels ; GOT, glutamic oxalacetic transaminase; GGT, γ-glutamyltransferase; CRP, C-reactive protein.
Plasma CRP levels were significantly higher in group 2 than in group 3, but there was no significant difference between postprandial CRP levels (Table 1).
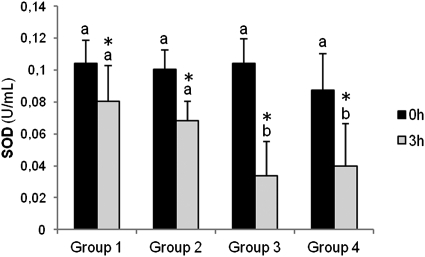
No differences were seen between the study groups in baseline plasma SOD activity, but after fat overload, this was significantly lower in the groups with ΔTG>80 mg/dl. After the fat overload, there was a significant drop in SOD activity in all the groups, significantly more pronounced in the groups with ΔTG>80 mg/dl (Fig. 1).
Fig. 1.
Baseline and postprandial plasma superoxide dismutase activity in the four study groups. Results are presented as means ± SD. Different letters indicate significant differences between the study groups (ANOVA, Bonferroni test). *P < 0.05 between baseline and postprandial values. Group 1: patients with HOMA-IR ≤ 5 and ΔTG < 80 mg/dl; Group 2: patients with HOMA-IR > 8 and ΔTG < 80 mg/dl; Group 3: patients with HOMA-IR ≤ 5 and ΔTG > 80 mg/dl; Group 4: patients with HOMA-IR > 8 and ΔTG > 80 mg/dl. SOD, superoxide dismutase.
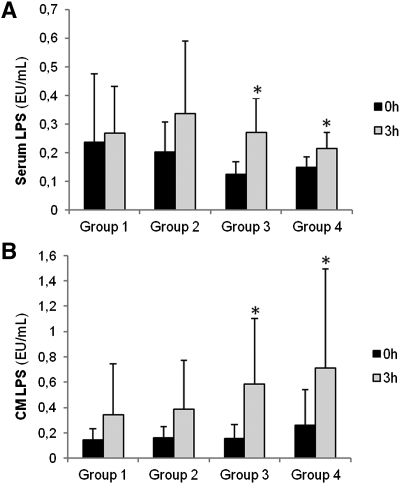
Although groups 3 and 4 appear to have lower serum LPS levels, no significant differences were found between groups at 0 h or 3 h in either serum or chylomicron LPS levels; only the groups with the highest ΔTG had a significant increase over baseline after fat overload in both serum and chylomicron LPS levels (Fig. 2).
Fig. 2.
Baseline and postprandial serum and chylomicron LPS levels in the four study groups. Results are presented as means ± SD. * P < 0.05 between baseline and postprandial values; Group 1: patients with HOMA-IR ≤ 5 and ΔTG < 80 mg/dl; Group 2: patients with HOMA-IR > 8 and ΔTG < 80 mg/dl; Group 3: patients with HOMA-IR ≤ 5 and ΔTG > 80 mg/dl; Group 4: patients with HOMA-IR > 8 and ΔTG > 80 mg/dl. LPS, lipopolysaccharide; CM, chylomicron.
Plasma LPS levels at 0 h correlated positively with triglyceride levels at 0 h. In addition, plasma LPS levels at 3 h correlated positively with triglyceride levels at both 0 h and 3 h, and with plasma glucose levels. Chylomicron LPS levels at 3 h as well as Delta chylomicron LPS (ΔCM LPS; measured as the difference between postprandial chylomicron LPS and baseline chylomicron LPS levels) showed a positive correlation with the ΔTG (Table 2). Inflammatory (CRP) and hormonal (adiponectin and leptin) variables as well as anthropometric variables (BMI, waist, and age) showed no significant correlations with LPS levels (data not shown).
TABLE 2.
Correlations between baseline and postprandial LPS levels in all the study subjects
| TG 0 h | TG 3 h | ΔTG | Insulin | HOMA-IR | Glucose | |
| LPS S 0 h | 0.480a | 0.374 | −0.257 | −0.060 | 0.008 | 0.283 |
| LPS S 3 h | 0.852b | 0.837b | −0.180 | −0.069 | 0.063 | 0.442a |
| ΔLPS S | 0.416a | 0.489a | 0.042 | −0.016 | 0.056 | 0.188 |
| LPS CM 0 h | −0.048 | 0.170 | 0.371 | 0.098 | 0.071 | −0.022 |
| LPS CM 3 h | −0.045 | 0.192 | 0.401a | −0.009 | −0.032 | −0.008 |
| ΔLPS QM | −0.041 | 0.192 | 0.394a | −0.053 | −0.072 | −0.001 |
Correlations were determined by Spearman's correlation coefficient test. LPS, lipopolysaccharide; S, serum; CM, chylomicron; TG, triglycerides; ΔTG, difference between postprandial and baseline triglyceride levels; HOMA-IR, Homeostasis model assessment of insulin resistance.
P < 0.05.
P < 0.01.
We carried out a multiple regression analysis with those factors associated with serum LPS levels at 0 h and 3 h. We considered as independent variables age, sex, BMI, variables with significant differences between the study groups in univariate analysis [waist circumference, insulin, HOMA-IR, triglyceride levels, ΔTG, uric acid, HDL-C, LDL-C, gamma-glutamyl transferase (GGT), and CRP], and variables that correlated significantly with LPS levels (Table 3). This analysis confirmed that the baseline triglyceride level was the best variable to predict the baseline serum LPS level. In addition, triglyceride levels at 0 h and 3 h were significantly and independently associated with LPS levels after fat overload.
TABLE 3.
Multiple linear regression analysis with serum LPS levels at both 0 h and 3 h after the fat overload as the dependent variables, in all the study subjects
| Serum LPS 0 h (R = 0.480; R = 0.230) | |||
| β | P | 95% CI | |
| TG 0 h | 0.480 | 0.013 | 0.000–0.001 |
| Serum LPS 3 h (R = 0.885; R = 0.783) | |||
| β | P | 95% CI | |
| TG 0 h | 0.483 | 0.015 | −0.001–0.003 |
| TG 3 h | 0.407 | 0.033 | 0.000–0.002 |
TG, triglycerides. Independent variables: Triglycerides 0 h, Triglycerides 3 h, ΔTG (difference between postprandial and baseline triglyceride levels), HOMA-IR, insulin, glucose, body mass index, C-reactive protein, age, sex, waist circumference, uric acid, HDL-C, LDL-C and GGT.
A multiple regression analysis was also done with chylomicron LPS levels at 0 h and 3 h as dependent variables and the same independent variables as described above. The ΔTG was the only variable significantly and independently associated with chylomicron LPS at 3 h (Table 4). In contrast, no significant association was found with chylomicron LPS levels at 0 h.
TABLE 4.
Multiple linear regression analysis with chylomicron LPS levels at 3 h after the fat overload as the dependent variable, in all the study subjects
| Chylomicron LPS 3 h (R = 0.401; R = 0.161) | |||
| β | P | 95% CI | |
| ΔTG | 0.401 | 0.047 | 0.000-0.009 |
TG, triglycerides. Independent variables: Triglycerides 0 h, Triglycerides 3 h, ΔTG, (difference between postprandial and baseline triglyceride levels), HOMA-IR, insulin, glucose, body mass index, C-reactive protein, age, sex, waist circumference, uric acid, HDL-C, LDL-C and GGT.
DISCUSSION
The results of this study show that a fat overload leads to increased LPS levels related with chylomicrons. This increase was associated with postprandial hypertriglyceridemia but not with insulin resistance in morbidly obese patients.
The degree of metabolic endotoxemia is related to fat ingestion and some authors have suggested that it might be responsible, at least in part, for the low-grade inflammation observed in obese patients (7, 18). However, to date, all studies dealing with endotoxemia and fat intake have been carried out in healthy lean subjects (7, 14). Thus, ours is the first study to examine this hypothesis in morbidly obese patients and to attempt to clarify the relationship between metabolic endotoxemia, hypertriglyceridemia, and insulin resistance in obesity.
The intestinal microflora is considered a source of circulating LPS (9). In fact, small amounts of LPS are absorbed from the gut in healthy animals (12) and bioactived LPS is detectable in low amounts in the blood of healthy human subjects, even in the apparent absence of infections (21, 22). Chylomicrons have been associated with metabolic endotoxemia. Both animal and in vitro studies have demonstrated that chylomicron formation promotes LPS absorption (13). A recent study has also shown human chylomicrons can be postprandial carriers of LPS in healthy humans (14).
Our study agrees with the idea of chylomicron LPS transport because the patients with higher increases in triglyceride levels over baseline displayed higher levels of chylomicron LPS after the fat overload. Concordantly with the idea that chylomicrons promote LPS absorption, a high-fat meal leads to increased endotoxemia in healthy humans (7, 14). In consequence, it has been hypothesized that endogenous LPS levels could be responsible for the low-grade inflammation observed in obese subjects who have a high fat intake.
Obesity is now considered to be a condition that facilitates the development of a low-grade inflammatory state, characterized by increased plasma levels of proinflammatory cytokines such as tumor necrosis factor α, interleukins (IL), and cytokine-like proteins known as adipokines (4). It has been reported that patients with morbid obesity have a greater postprandial response to fat overload, and the postprandial response is associated with a greater increase in oxidative stress and inflammation (8, 23). Bacterial endotoxin is increasingly being considered as a potential inflammatory mediator of obesity, diabetes, and atherosclerosis (10, 17, 24, 25). Laugerette et al. (14) showed that healthy subjects following a mixed meal containing lipids from different food products undergo a transient increase in endotoxemia associated with raised inflammation biomarkers such as sCD14 and an early peak of IL-6. Others have reported postprandial endotoxemia in healthy humans after a fat load (50 g of butter on toast) but failed to observe postprandial inflammation (7). We found no relationship between CRP levels and LPS levels in our patients. In fact, CRP seems to be more related to obesity or insulin resistance. This agrees with previous studies finding no relationship between CRP and endotoxemia or postprandial response (7). This is probably because CRP is a marker of long-term inflammation rather than short-term inflammation (which is the situation we studied in the postprandial state 3 h after fat load), and this may also explain the differences found in CRP levels according to the degree of insulin resistance but not to postprandial response. Clarifying the relationship between obesity and metabolic endotoxemia will require further studies exploring this association in obese patients. However, we did find that patients with high triglyceride increases after fat overload showed a decrease in antioxidant defenses because they had lower postprandial SOD activity than the patient groups with lower triglyceride increases after fat overload. Thus, these results show for the first time a possible link between oxidative stress and metabolic endotoxemia.
Studies in mice showed a link between postprandial endotoxemia and postprandial hypertriglyceridemia when comparing the groups of mice fed with different diets (11, 18). Our study shows that the postprandial triglyceride level was more influential than the degree of insulin resistance in postprandial LPS levels and that serum LPS rose significantly after fat overload only in morbidly obese patients displaying the highest triglyceride increase after a fatty meal. It is worth noting, though, that despite the lack of a significant difference between groups in fasting LPS levels, the serum LPS increase might be driven by lower baseline LPS levels in groups 3 and 4. Interestingly, we found that the baseline triglyceride level was the variable that best predicted the baseline LPS level in serum. This shows a clear relationship between triglyceride metabolism and endotoxemia, though further studies will be necessary to elucidate what other mechanisms related to triglyceride metabolism, apart from chylomicrons, could be determining fasting endotoxemia.
The specific mechanisms leading to insulin resistance have been partially characterized and have revealed an incomplete picture of a complex cross-talk integrating metabolic, nutritional, and inflammatory signaling pathways, eventually leading to the development of obesity-induced insulin resistance (26). Among the various nutritional factors involved in the development of insulin resistance, postprandial hypertriglyceridemia-associated increased endotoxemia could be responsible for a higher oxidative stress increase and the degree of inflammation that influences the insulin signaling pathways. Cani et al. (18) showed that LPS-infused mice had higher glucose and insulin levels than control mice, suggesting that LPS could initiate insulin resistance and the development of diabetes. However, here we show that in morbidly obese patients, LPS levels are associated with triglyceride levels but not the degree of insulin resistance.
A noteworthy finding is that chylomicron LPS values were slightly higher than serum values, a result previously reported by other authors (13, 14). It would be expected that chylomicron LPS levels were lower than (or at most the same as) serum LPS levels. It has been previously reported that certain proteins in serum could interfere with LAL enzymatic reaction (27) in spite of using heating to eliminate serum complement factor or dispersant agents. Thus, a plausible explanation for these discrepancies between serum and chylomicron LPS levels could be that LAL reaction-interfering serum molecules were not isolated in the chylomicron fraction during ultracentrifugation and they stayed in the remaining fraction.
In conclusion, LPS levels rose after a fat overload in morbidly obese persons and those patients with a high postprandial triglyceride increase showed a higher increase in chylomicron LPS levels after the fat overload as well as higher oxidative stress.
Acknowledgments
The authors thank all the subjects for their collaboration, and IMABIS. We also gratefully acknowledge the help of Ian Johnstone for his expertise in preparing this manuscript and Juan Alcaide-Torres for his technical contribution.
Footnotes
Abbreviations:
- BMI
- body mass index
- CM
- chylomicron
- CRP
- C-reactive protein
- GGT
- gamma-glutamyl transferase
- HOMA-IR
- homeostasis model assessment of insulin resistance
- IL
- interleukin
- LAL
- Limulus amebocyte lysate
- LPS
- lipopolysaccharide
- SOD
- superoxide dismutase
- TNFα
- tumor necrosis factor alpha
- ΔTG
- Delta triglycerides
This study was funded by the Fondo de Investigación Sanitaria “Centros de Investigación En Red” (CIBER, CB06/03/0018) of the “Instituto de Salud Carlos III”, FIS PS09/00997, FIS 08/1655 and CP07/00095 of the “Instituto de Salud Carlos III”, Madrid, Spain. SAS 08/325 and SAS 10/0696 Consejería de Salud, Junta de Andalucía. M.C.P. was a recipient of a FPU grant from Education Ministry, Madrid, Spain [AP2009-4537]. The authors have no financial or other contractual agreements that might cause conflicts of interest or be perceived as causing conflicts of interest.
REFERENCES
- 1.Reaven G. 2005. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight / obese individuals. Diab. Vasc. Dis. Res. 2: 105–112 [DOI] [PubMed] [Google Scholar]
- 2.Barbarroja N., López-Pedrera R., Mayas M. D., García-Fuentes E., Garrido-Sánchez L., Macías-González M., El Bekay R., Vidal-Puig A., Tinahones F. J. 2010. The obese healthy paradox: is inflammation the answer? Biochem. J. 430: 141–149 [DOI] [PubMed] [Google Scholar]
- 3.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114: 1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature. 444: 860–867 [DOI] [PubMed] [Google Scholar]
- 5.van Oostrom A. J., Rabelink T. J., Verseyden C., Sijmonsma T. P., Plokker H. W., De Jaegere P. P., Castro-Cabezas M. 2004. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis. 177: 175–182 [DOI] [PubMed] [Google Scholar]
- 6.Aljada A., Mohanty P., Ghanim H., Abdo T., Tripathy D., Chaudhuri A., Dandona P. 2004. Increase in intranuclear nuclear factor κB and decrease in inhibitor κB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am. J. Clin. Nutr. 79: 682–690 [DOI] [PubMed] [Google Scholar]
- 7.Erridge C., Attina T., Spickett C. M., Webb D. J. 2007. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 86: 1286–1292 [DOI] [PubMed] [Google Scholar]
- 8.Cardona F., Tunez I., Tasset I., Murri M., Tinahones F. J. 2008. Similar increase in oxidative stress after fat overload in persons with baseline hypertriglyceridemia with or without the metabolic syndrome. Clin. Biochem. 41: 701–705 [DOI] [PubMed] [Google Scholar]
- 9.Berg R. D. 1996. The indigenous gastrointestinal microflora. Trends Microbiol. 4: 430–435 [DOI] [PubMed] [Google Scholar]
- 10.Wiedermann C. J., Kiechl S., Dunzendorfer S., Schratzberger P., Egger G., Oberhollenzer F., Willeit J. 1999. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J. Am. Coll. Cardiol. 34: 1975–1981 [DOI] [PubMed] [Google Scholar]
- 11.Amar J., Burcelin R., Ruidavets J. B., Cani P. D., Fauvel J., Alessi M. C., Chamontin B., Ferriéres J. 2008. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 87: 1219–1223 [DOI] [PubMed] [Google Scholar]
- 12.Ravin H. A., Rowley D., Jenkins C., Fine J. 1960. On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. J. Exp. Med. 112: 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoshal S., Witta J., Zhong J., de Villiersa W., Eckhardt E. 2009. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 50: 90–97 [DOI] [PubMed] [Google Scholar]
- 14.Laugerette F., Vors C., Géloën A., Chauvin M. A., Soulage C., Lambert-Porcheron S., Peretti N., Alligier M., Burcelin R., Laville M., et al. 2011. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 22: 53–59 [DOI] [PubMed] [Google Scholar]
- 15.Vreugdenhil A. C., Rousseau C. H., Hartung T., Greve J. W., van't Veer C., Buurman W. A. 2003. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immunol. 170: 1399–1405 [DOI] [PubMed] [Google Scholar]
- 16.Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. 2006. Microbial ecology: human gut microbes associated with obesity. Nature. 444: 1022–1023 [DOI] [PubMed] [Google Scholar]
- 17.Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. 2006. An obesity associated gut microbiome with increased capacity for energy harvest. Nature. 444: 1027–1031 [DOI] [PubMed] [Google Scholar]
- 18.Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., et al. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 56: 1761–1772 [DOI] [PubMed] [Google Scholar]
- 19.Cardona F., Tinahones F. J. Composition useful for the detection of postprandial hypertriglyceridemia. Spain patent with license number P201030776.
- 20.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 21.Brenchley J. M., Price D. A., Schacker T. W., Asher T. E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., et al. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12: 1365–1371 [DOI] [PubMed] [Google Scholar]
- 22.Nádházi Z., Takats A., Offenmüller K., Bertók L. 2002. Plasma endotoxin level of healthy donors. Acta Microbiol. Immunol. Hung. 49: 151–157 [DOI] [PubMed] [Google Scholar]
- 23.Cardona F., Tunez I., Tasset I., Montilla P., Collantes E., Tinahones F. J. 2008. Fat overload aggravates oxidative stress in patients with the metabolic syndrome. Eur. J. Clin. Invest. 38: 510–515 [DOI] [PubMed] [Google Scholar]
- 24.Greaves D. R., Channon K. M. 2002. Inflammation and immune responses in atherosclerosis. Trends Immunol. 23: 535–541 [DOI] [PubMed] [Google Scholar]
- 25.Stoll L. L., Denning G. M., Weintraub N. L. 2004. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24: 2227–2236 [DOI] [PubMed] [Google Scholar]
- 26.Greenberg A. S., Obin M. S. 2006. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 83: 461S–465S [DOI] [PubMed] [Google Scholar]
- 27.Hurley J. C. 1995. Endotoxemia: methods of detection and clinical correlates. Clin. Microbiol. Rev. 8: 268–292 [DOI] [PMC free article] [PubMed] [Google Scholar]