Abstract
The extent of hypercholesterolemia varies considerably in patients with familial hypercholesterolemia (FH). We hypothesized that the variability of the FH phenotype might be partly explained by variation in proprotein convertase subtilisin kexin type 9 (PCSK9) activity. Individuals between 18 and 53 years of age who had been tested for a pathogenic LDLR or APOB mutation were eligible. Mutation carriers with a LDL-C level below the 75th percentile (called “FH low”) were selected, as well as those with LDL-C above the 90th percentile (called “FH high”). Relatives who tested negative for the mutation were the “controls.” PCSK9 plasma levels were assessed in 267 individuals who did not receive cholesterol-lowering treatment at the time of the study. Mean PCSK9 plasma levels (95% CI) were lower in the FH-low group compared with the FH-high group [152 (137–167) ng/ml vs. 186 (165–207) ng/ml, P = 0.010] and the control group [177 (164–190) ng/ml, P = 0.013]. Mean PCSK9 levels did not statistically differ between the FH-high and control groups (P = 0.50). Plasma PCSK9 levels are positively associated with LDL-C levels in FH patients and might contribute to the phenotypic severity in this disorder. Therefore, the results of pharmaceutical inhibition of PCSK9 in FH patients are eagerly awaited.
Keywords: proprotein convertase subtilisin kexin type 9, atherosclerosis, low-density lipoprotein cholesterol, gene expression
Familial hypercholesterolemia (FH, MIM #143890) is a frequent autosomal codominant disorder of lipoprotein metabolism. It is clinically characterized by elevated levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), the presence of tendon xanthomas, and premature atherosclerosis. Defects in genes that code for proteins involved in the hepatic clearance of LDL-C underlie this hereditary disorder (1). In fact, more than a 1,000 different mutations in the genes coding for the LDL-receptor (LDLR, MIM +606945), apolipoprotein B (APOB, MIM +107730), and proprotein convertase subtilisin/kexin type 9 (PCSK9, MIM +607786) are now known to cause FH (1–3). If left untreated, the risk of cardiovascular disease is severely increased (4), but the prognosis of FH can be improved substantially with cholesterol-lowering treatment (5).
The identification of a mutation that underlies FH in a particular kindred enables genetic testing of family members for the presence of the same mutation and makes it possible to initiate effective medical management before the cardiovascular consequences of FH become clinically manifest (4). This notion has led to the implementation of a nationwide genetic cascade screening program for FH in the Netherlands, and since 1994, approximately 20,000 individuals with FH have been found and treated (6).
However, molecularly diagnosed FH patients do not always exhibit a hypercholesterolemic phenotype. In fact, 15% of the heterozygous mutation carriers identified by our national screening program show pretreatment LDL-C levels below the 75th percentile for age and gender (7). The reasons why some individuals with a confirmed FH genotype lack the hypercholesterolemia phenotype are largely unknown.
We hypothesized that such nonpenetrance of an FH mutation could, in part, be explained by variation in PCSK9 activity. PCSK9 is a natural inhibitor of the LDLR: it binds to the hepatic LDLR and thereby directs it toward lysosomal degradation rather than to recycling to the cell membrane (8, 9). The presence of a specific gain-of-function mutation in PCSK9 aggravates the hypercholesterolemia phenotype exerted by a concurrent pathogenic LDLR mutation (10, 11). Conversely, low activity of PCSK9 could lead to increased presence of LDLR at the hepatic cell surface and, consequently, to increased clearance of plasma LDL-C. This would theoretically reduce the extent of cholesterol elevation caused by an LDLR mutation.
To test this hypothesis, we measured plasma PCSK9 levels in individuals who underwent DNA testing for genetic FH (12).
METHODS
Study population and design
The study population derived from participants of a previous single center cross-sectional study, described in detail elsewhere (12). In short, we recruited individuals from the database of the national screening program for autosomal dominant hypercholesterolemia. Men and women between 18 and 55 years of age were eligible for the original study if they were genetically tested for the specific pathogenic (13) LDLR or APOB mutation residing within their family between January 2007 and January 2010 and if they had a known lipid profile. Individuals were excluded if they were unable to participate within 18 months after the genetic test. Individuals using cholesterol-lowering medication before genetic testing and probands, who were primarily clinically diagnosed, were excluded. Individuals from whom we failed to obtain sufficient plasma, required for PCSK9 measurements, were also excluded.
Individuals who had been identified with a pathogenic mutation were categorized based on their untreated LDL-C level at genetic diagnosis. Mutation carriers with a LDL-C level below the age- and sex-specific 75th percentile were referred to as “FH low”; those with LDL-C above the 90th percentile were referred to as “FH high.” A third group, consisting of first-degree relatives negative for the familial LDLR or APOB mutation, was referred to as “control.”
The selected individuals who consented made a single study visit to the Academic Medical Center in Amsterdam within 18 months after the genetic test. The study was approved by the local Ethics Committee.
Biochemical analyses and cIMT assessment
Blood samples were obtained for analysis of lipid measures and spare plasma between 8 AM and 10 AM after an overnight fast. These samples were collected in 7 ml EDTA Vacutainer® (K3E 15% 0.084 ml; BD Vacutainer Systems, Plymouth, UK) venous blood collection tubes using standard phlebotomy practices. Immediately after collection, tubes were gently inverted five times, and then centrifuged at 1,500–2,000 g for 15 min. The supernatant plasma was centrifuged again in similar fashion. The plasma was transferred into 2 ml freezer vials in 0.5 ml aliquots. The samples were frozen at −80°C and shipped on dry ice. PCSK9 concentrations were measured in triplicate using the CY-8079 ELISA kit (Cyclex, Nagano, Japan), according to the manufacturer's protocol.
The medical history was recorded and physical examination performed according to a standardized procedure (13). Carotid arteries were examined with ultrasound to assess intima-media thickness (cIMT), using methodology previously described in detail (14).
Statistical analysis
Differences in demographic and clinical characteristics among the three predefined groups (FH low, FH high, and control) were evaluated using linear or logistic regression analysis. Linear regression analysis was applied to evaluate the association between PCSK9 and patient characteristics, LDL-C, or cIMT, and to assess differences in plasma PCSK9 levels among the three predefined groups. Multivariable regression models were applied to adjust for potential confounders. Inclusion in a final model was determined by backward stepwise elimination.
All analyses were performed using the generalized estimating equations (GEE) method to account for correlations within families. The exchangeable correlation structure was used for these models. The main study outcome pertains to the individuals who remained untreated until the study visit. For transparency, we also analyzed the entire population of participants, including individuals who initiated statin treatment after genetic diagnosis (see the supplementary data).
Variables with a skewed distribution were log-transformed before statistical analyses. A P-value < 0.05 was considered statistically significant. Data were analyzed with SPSS for Windows 16.0.2 (Chicago, IL).
RESULTS
Study population
Among the screened population, 2,016 individuals met inclusion criteria for the original study. Recruitment was discontinued when a sufficient number of individuals with and without genetic FH were enrolled. A total of 421 individuals provided written informed consent to participate in the original study. Of these 421 individuals, 378 were included for the subanalysis of PCSK9 levels: 13 individuals without FH were excluded because they were older than 53 years, and for 30 individuals, we did not have spare plasma to measure PCSK9. The median period between genetic testing was 11 (IQR: 8–14) months.
As expected, carriers were more often treated with statins after diagnosis than were noncarriers, and those from the FH-high group had initiated statin treatment more often than the individuals from the FH-low group (see supplementary Table I). In total, 267 individuals (71%) were still untreated at the time of the study visit. Clinical characteristics of the untreated participants, subdivided into the three groups, are summarized in Table 1. LDL-C levels were comparable between the individuals from the FH-low and control groups, whereas levels were significantly higher in the FH-high group. Accordingly, the mean IMT of the three segments of the left and right carotid arteries was greater in the FH-high group than in the FH-low group and the control group.
TABLE 1.
Characteristics of the untreated study participants
| Control | FH Low | FH High | Control vs. FH Low | Control vs. FH High | FH Low vs. FH High | |
| n = 112 | n = 94 | n = 61 | P | P | P | |
| Males [number (%)] | 54 (48) | 37 (39) | 25 (40) | 0.20 | 0.36 | 0.84 |
| Age (years) | 40.7 ± 8.1 | 36.7 ± 8.4 | 33.9 ± 8.6 | <0.001 | <0.001 | 0.054 |
| Hypertension [number (%)] | 10 (9) | 5 (5) | 4 (7) | 0.33 | 0.63 | 0.72 |
| Diabetes [number (%)] | 0 (0) | 0 (0) | 1 (2) | — | 0.17 | 0.21 |
| Current smoker [number (%)] | 23 (21) | 16 (17) | 18 (30) | 0.52 | 0.19 | 0.066 |
| Body mass index (kg/m ) | 25.6 ± 4.1 | 25.6 ± 5.3 | 24.3 ± 4.8 | 0.95 | 0.098 | 0.097 |
| Systolic blood pressure (mmHg) | 128 ± 15 | 124 ± 13 | 122 ± 14 | 0.038 | 0.014 | 0.54 |
| Lipid profile (mmol/l) | ||||||
| At genetic FH test | ||||||
| pLDL (IQR) | 40 (19–65) | 45 (20–63) | 97 (95–98) | 0.89 | <0.001 | <0.001 |
| At study visit | ||||||
| TC | 5.3 ± 1.1 | 5.4 ± 1.1 | 7.1 ± 1.2 | 0.24 | <0.001 | <0.001 |
| LDL-C | 3.3 ± 0.9 | 3.5 ± 1.0 | 5.3 ± 1.1 | 0.11 | <0.001 | <0.001 |
| HDL-C | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 0.65 | 0.83 | 0.55 |
| Triglycerides (IQR) | 0.9 (0.6–1.4) | 0.7 (0.5–1.1) | 0.7 (0.5–1.1) | 0.024 | 0.012 | 0.69 |
| Mean cIMTa (SE) (mm) | 0.63 ± 0.008 | 0.62 ± 0.009 | 0.67 ± 0.013 | 0.38 | 0.009 | 0.001 |
pLDL, percentile LDL-C for age and gender.
Adjusted for age, gender, body mass index, systolic blood pressure, and smoking.
Plasma PCSK9 levels in the predefined groups and the association with LDL-C
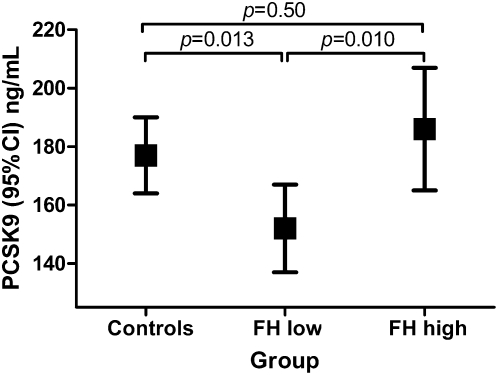
Because of the substantial effect of statin treatment on PCSK9 levels (supplementary Table II), our main study outcome was based on the untreated individuals. The untreated individuals from the FH-low group (n = 94) had significantly lower mean PCSK9 levels [152 (137–167) ng/ml] compared with untreated individuals from the FH-high group [n = 61, 186 (165–207) ng/ml, P = 0.010] and controls [n = 112, 177 (164–190) ng/ml, P = 0.013] (Fig. 1). As supplementary Table II shows, PCSK9 levels are associated with several patient characteristics, which were not equally represented among the three predefined groups. Therefore, we adjusted for these characteristics (age, sex, body mass index, and systolic blood pressure) by means of a multiple linear regression analysis.
Fig. 1.
Mean PCSK9 levels in the three groups of untreated participants. The groups were categorized based on genetic FH mutation status and LDL-cholesterol level: the group where mutation was absent (control) and for FH heterozygotes, the untreated LDL-cholesterol percentile, either below the 75th percentile (FH low) or above the 90th percentile (FH high) for age and gender. Data were based on a model adjusted for age, sex, body mass index, and systolic blood pressure.
On treatment, mean PCSK9 levels (95% CI) were significantly lower for the 16 individuals from the FH-low group compared with the 92 FH patients from the FH-high group [167 (135–199) ng/ml vs. 219 (201–238) ng/ml, P = 0.006]. For the entire cohort of both treated and untreated individuals (for characteristics, see supplementary Table I), PCSK9 plasma levels were again significantly lower in the FH-low group compared with the FH-high group (P = 0.001) and the control group (P = 0.004), and levels did not statistically differ between the FH-high and control groups (P = 0.52): mean PCSK9 plasma levels (95% CI) were 183 (169–197) ng/ml, 154 (141–168) ng/ml, and 204 (189–219) ng/ml for the control, FH-low, and FH-high groups, respectively (supplementary Fig. I).
We also associated plasma PCSK9 levels with LDL-C levels at the study visit for the 155 untreated individuals with genetic FH. The multivariable analysis revealed that the mean percentile LDL-C for each mutation and PCSK9 levels were the only two variables that remained independently associated with LDL-C levels after backward elimination (Table 2). A separate analysis for association between PCSK9 and LDL-C plasma levels between the different mutation classes was performed. However, this did not lead to additional insight, due to the few number of individuals in most mutation classes (data not shown). Thus, low LDL-C levels in untreated individuals with genetic FH were primarily observed in those who carried a LDLR or APOB gene mutation that is generally associated with mild hypercholesterolemia and/or in those who had low plasma levels of PCSK9.
TABLE 2.
Association between LDL-C levels at the study visit and clinical characteristics in the 155 untreated participants with genetic FH
| Univariate |
Multivariable |
|||||
| B | 95% CI | P | B | 95% CI | P | |
| Age (years) | 0.006 | −0.019 to 0.031 | 0.65 | — | — | — |
| Gender (male) | −0.013 | −0.448 to 0.421 | 0.95 | — | — | — |
| Body mass index (kg/m2) | −0.008 | −0.049 to 0.034 | 0.71 | — | — | — |
| Mean pLDL-specific mutationa | 0.045 | 0.033 to 0.056 | <0.001 | 0.042 | 0.030 to 0.053 | <0.001 |
| PCSK9 (pg/ml) | 4.56 | 1.93 to 7.19 | 0.001 | 3.11 | 0.78 to 5.40 | 0.009 |
B, unstandardized coefficient of regression model; CI, confidence interval.
Average percentile LDL-C for age and sex for all untreated individuals carrying a specific FH or FDB mutation in order to estimate the severity of that specific mutation, as previously described in detail (see Ref. 13).
Association between plasma PCSK9 levels and cIMT in control individuals
Table 3 depicts the association between mean cIMT and clinical characteristics in the 112 untreated individuals without FH. In the multivariable regression analysis, PCSK9 levels remained statistically significantly associated with cIMT after backward elimination. In contrast, the individual components of the lipid profile did not remain statistically significantly associated in the multivariable model. Thus, plasma PCSK9 levels were positively associated with cIMT even after adjustment for the lipid profile and other traditional cardiovascular risk factors.
TABLE 3.
Association between cIMT and clinical characteristics for untreated controls
| Univariate |
Multivariable |
|||||
| B | 95% CI | P | B | 95% CI | P | |
| Age (years) | 0.008 | 0.006 to 0.010 | <0.001 | 0.007 | 0.005 to 0.009 | <0.001 |
| Gender (male) | 0.057 | 0.014 to 0.100 | 0.010 | 0.062 | 0.027 to 0.098 | 0.001 |
| Body mass index (kg/m2) | 0.009 | 0.004 to 0.014 | 0.001 | 0.005 | 0.000 to 0.009 | 0.039 |
| Systolic blood pressure (mmHg) | 0.003 | 0.001 to 0.004 | 0.001 | — | — | — |
| Tobacco use (pack years) | 0.003 | 0.001 to 0.005 | 0.008 | — | — | — |
| LDL-cholesterol (mmol/l) | 0.044 | 0.022 to 0.067 | <0.001 | — | — | — |
| HDL-cholesterol (mmol/l) | −0.026 | −0.120 to –0.008 | 0.026 | — | — | — |
| Triglyceridesa | 0.043 | 0.003 to 0.082 | 0.036 | — | — | — |
| PCSK9 (pg/ml) | 0.46 | 0.17 to 0.75 | 0.002 | 0.28 | 0.034 – 0.53 | 0.026 |
B, unstandardized coefficient of regression model; CI, confidence interval.
Log-transformed before analysis.
DISCUSSION
In the present study, PCSK9 levels were measured in a cross-sectional study of individuals who had genetic FH, with or without severely elevated LDL-C levels, and controls. Our findings demonstrate that PCSK9 levels were significantly lower in normocholesterolemic FH patients than in the two other groups. Moreover, PCSK9 levels were closely associated with LDL-C levels across all groups. Consequently, a reasonable assumption would be that low plasma PCSK9 activity might lead to lower LDL-C levels in heterozygous FH.
To our knowledge, this study is the first to compare plasma levels of PCSK9 between FH patients with and FH patients without severely elevated LDL-C levels. However, several groups have reported on the effect of genetic variation in the PCSK9 gene on the phenotype of FH. (10, 11, 15) Abifadel and colleagues showed that individuals who coinherited pathogenic mutations in both PCSK9 and LDLR had higher LDL-C levels than did their relatives with either mutation alone (10). Conversely, Strom and colleagues studied the effect of a loss-of-function PCSK9 mutation, R46L, in FH (15). Of 1,130 FH patients screened, they identified the R46L mutation in 30 individuals, who had 6% lower TC levels than did those without the R46L mutation. These results of the association between genetic variation in PCSK9 and LDL-C levels support our findings, as we observed that FH patients with low levels of PCSK9 also have low LDL-C levels.
A crucial question is what causes this variation in PCSK9 levels. Loss-of-function mutations in PCSK9 might be a likely explanation (15). We recently genotyped PCSK9 in a cohort of 77 heterozygous FH patients who were selected for low LDL-C levels. Just like Strom and colleagues (15), we found the R46L variant in PCSK9 in one (1.3%) of those patients (16). Thus, genetic variation in PCSK9 does contribute to a variable FH phenotype, but the explained percentage remains disappointingly low (10, 15). In addition to genetic variation, other factors may affect plasma levels of PCSK9, of which only a fraction has been identified (17).
We also showed that high PCSK9 levels are associated with more pronounced carotid atherosclerosis, apparently independent of lipid levels. Moreover, we recently showed that plasma levels of PCSK9 were positively associated with recurrent coronary events in patients with stable coronary heart disease treated with a low dose atorvastatin in a nested case control study in the Treating to New Targets trial (Huijgen et al., unpublished observations). These findings, combined with the fact that decreased PCSK9 activity is associated with lower LDL-C levels and a reduced risk of coronary heart disease (18), support the inhibition of PCSK9 as a target of great significance. In fact, several agents are already being investigated in humans (19–24).
Several limitations of our study merit discussion. First, this is an observational study, and therefore, a causal relationship between low plasma PCSK9 levels and lack of a hypercholesterolemia phenotype cannot be proved. Second, a substantial number of participants initiated statin treatment between the genetic FH diagnosis and study visit. Because statin treatment results in increased PCSK9 levels, this hinders the interpretation of our findings (25–27). Nevertheless, we could demonstrate that PCSK9 levels were lower in FH patients with low LDL-C levels than in those with hypercholesterolemia, both in treated and untreated individuals. This finding supports the notion that the differences in plasma PCSK9 levels between groups are not solely due to the effect of statin treatment. Last, our cohort of FH patients consisted of carriers of a myriad of pathogenic LDLR and APOB mutations. As a consequence, we were unable to perform meaningful statistics on the effect of specific types of LDLR mutations. The PCSK9 plasma levels remained a predictor of plasma LDL-C levels, however, after adjustment for the mean percentile of LDL-C induced by specific mutations. Thus, the severity of the FH mutation cannot be the only explanation for the association that we observed between plasma PCSK9 levels and LDL-C levels. In line with our findings, plasma PCSK9 levels were observed to be strongly and positively associated with LDL-C levels in a cohort of 260 nontreated FH heterozygotes from South Africa carrying one single LDLR mutation (Lambert et al., unpublished data).
In conclusion, plasma PCSK9 levels likely contribute to low LDL-C levels in FH heterozygotes. Therefore, the results of pharmaceutical inhibition of PCSK9 in FH patients are eagerly awaited.
Supplementary Material
Acknowledgments
The authors thank all study participants, as well as Francine Petrides for her excellent technical assistance.
Footnotes
Abbreviations:
- FH
- familial hypercholesterolemia
- HDL-C
- HDL-cholesterol
- cIMT
- carotid intima-media thickness
- IQR
- borders of quartiles
- LDL-C
- LDL-cholesterol
- PCSK9
- proprotein convertase subtilisin kexin type 9
- TC
- total cholesterol
This work was funded by National Health and Medical Research Council of Australia Project Grant 101867 (to G. L.). J. J. P. K. is involved with several pharmaceutical companies in the field of lipid lowering, including Amgen, Sanofi-Aventis, and Regeneron Pharmaceuticals, all of which are involved in development of PCSK9 inhibiting agents.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and two tables.
REFERENCES
- 1.Goldstein J. L., Hobbs H. H., Brown M. S. 2001. Familial hypercholesterolemia. In The Metabolic and Molecular Bases of Inherited Disease. 8th edition. C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle, eds. McGraw-Hill, New York. 2863–2913 [Google Scholar]
- 2.Innerarity T. L., Weisgraber K. H., Arnold K. S., Mahley R. W., Krauss R. M., Vega G. L., Grundy S. M. 1987. Familial defective apolipoprotein B-100: low density lipoproteins with abnormal receptor binding. Proc. Natl. Acad. Sci. USA. 84: 6919–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34: 154–156 [DOI] [PubMed] [Google Scholar]
- 4.Huijgen R., Vissers M. N., Defesche J. C., Lansberg P. J., Kastelein J. J., Hutten B. A. 2008. Familial hypercholesterolemia: current treatment and advances in management. Expert Rev. Cardiovasc. Ther. 6: 567–581 [DOI] [PubMed] [Google Scholar]
- 5.Versmissen J., Oosterveer D. M., Yazdanpanah M., Defesche J. C., Basart D. C., Liem A. H., Heeringa J., Witteman J. C., Lansberg P. J., Kastelein J. J., et al. 2008. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 337: a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umans-Eckenhausen M. A., Defesche J. C., Sijbrands E. J., Scheerder R. L., Kastelein J. J. 2001. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 357: 165–168 [DOI] [PubMed] [Google Scholar]
- 7.Huijgen R., Kindt I., Verhoeven S. B., Sijbrands E. J., Vissers M. N., Kastelein J. J., Hutten B. A. 2010. Two years after molecular diagnosis of familial hypercholesterolemia: majority on cholesterol-lowering treatment but a minority reaches treatment goal. PLoS ONE. 5: e9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert G., Charlton F., Rye K. A., Piper D. E. 2009. Molecular basis of PCSK9 function. Atherosclerosis. 203: 1–7 [DOI] [PubMed] [Google Scholar]
- 9.Abifadel M., Rabes J. P., Devillers M., Munnich A., Erlich D., Junien C., Varret M., Boileau C. 2009. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum. Mutat. 30: 520–529 [DOI] [PubMed] [Google Scholar]
- 10.Abifadel M., Rabes J. P., Jambart S., Halaby G., Gannage-Yared M. H., Sarkis A., Beaino G., Varret M., Salem N., Corbani S., et al. 2009. The molecular basis of familial hypercholesterolemia in Lebanon: spectrum of LDLR mutations and role of PCSK9 as a modifier gene. Hum. Mutat. 30: E682–E691 [DOI] [PubMed] [Google Scholar]
- 11.Pisciotta L., Priore O. C., Cefalu A. B., Noto D., Bellocchio A., Fresa R., Cantafora A., Patel D., Averna M., Tarugi P., et al. 2006. Additive effect of mutations in LDLR and PCSK9 genes on the phenotype of familial hypercholesterolemia. Atherosclerosis. 186: 433–440 [DOI] [PubMed] [Google Scholar]
- 12.Huijgen R., Vissers M. N., Kindt I., Trip M. D., de Groot E., Kastelein J. J., Hutten B. A. 2011. Assessment of carotid atherosclerosis in normocholesterolemic individuals with proven mutations in the low-density lipoprotein receptor or apolipoprotein B genes. Circ. Cardiovasc. Genet. 4: 413–417 [DOI] [PubMed] [Google Scholar]
- 13.Huijgen R., Kindt I., Fouchier S. W., Defesche J. C., Hutten B. A., Kastelein J. J., Vissers M. N. 2010. Functionality of sequence variants in the genes coding for the low-density lipoprotein receptor and apolipoprotein B in individuals with inherited hypercholesterolemia. Hum. Mutat. 31: 752–760 [DOI] [PubMed] [Google Scholar]
- 14.de Groot E., Hovingh G. K., Wiegman A., Duriez P., Smit A. J., Fruchart J. C., Kastelein J. J. 2004. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 109 (Suppl. 1): III33–III38 [DOI] [PubMed] [Google Scholar]
- 15.Strøm T. B., Holla O. L., Cameron J., Berge K. E., Leren T. P. 2010. Loss-of-function mutation R46L in the PCSK9 gene has little impact on the levels of total serum cholesterol in familial hypercholesterolemia heterozygotes. Clin. Chim. Acta. 411: 229–233 [DOI] [PubMed] [Google Scholar]
- 16.Huijgen R., Sjouke B., Vis K., de Randamie J. S., Defesche J. C., Kastelein J. J., Hovingh G. K., Fouchier S. W. 2012. Genetic variation in APOB, PCSK9, and ANGPTL3 in carriers of pathogenic autosomal dominant hypercholesterolemic mutations with unexpected low LDL-C levels. Hum. Mutat. 33: 448–455 [DOI] [PubMed] [Google Scholar]
- 17.Baass A., Dubuc G., Tremblay M., Delvin E. E., O'Loughlin J., Levy E., Davignon J., Lambert M. 2009. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin. Chem. 55: 1637–1645 [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. C., Boerwinkle E., Mosley T. H., Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272 [DOI] [PubMed] [Google Scholar]
- 19.Ni Y. G., Condra J. H., Orsatti L., Shen X., Di Marco S., Pandit S., Bottomley M. J., Ruggeri L., Cummings R. T., Cubbon R. M., et al. 2010. A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake. J. Biol. Chem. 285: 12882–12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N., Fisker N., Asselin M. C., Lindholm M., Rosenbohm C., Orum H., Elmen J., Seidah N. G., Straarup E. M. 2010. A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo. PLoS ONE. 5: e10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham M. J., Lemonidis K. M., Whipple C. P., Subramaniam A., Monia B. P., Crooke S. T., Crooke R. M. 2007. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 48: 763–767 [DOI] [PubMed] [Google Scholar]
- 22.Frank-Kamenetsky M., Grefhorst A., Anderson N. N., Racie T. S., Bramlage B., Akinc A., Butler D., Charisse K., Dorkin R., Fan Y., et al. 2008. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA. 105: 11915–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duff C. J., Scott M. J., Kirby I. T., Hutchinson S. E., Martin S. L., Hooper N. M. 2009. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem. J. 419: 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan J. C., Piper D. E., Cao Q., Liu D., King C., Wang W., Tang J., Liu Q., Higbee J., Xia Z., et al. 2009. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA. 106: 9820–9825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welder G., Zineh I., Pacanowski M. A., Troutt J. S., Cao G., Konrad R. J. 2010. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J. Lipid Res. 51: 2714–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubuc G., Chamberland A., Wassef H., Davignon J., Seidah N. G., Bernier L., Prat A. 2004. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 24: 1454–1459 [DOI] [PubMed] [Google Scholar]
- 27.Careskey H. E., Davis R. A., Alborn W. E., Troutt J. S., Cao G., Konrad R. J. 2008. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 49: 394–398 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.