Abstract
All cells in the intestinal villus of the rat are capable of synthesizing protein from amino acid precursors (l-leucine). Moreover, polyribosomes from both crypts and villi are equally able to incorporate l-leucine into protein. Unlike other tissues, e.g. liver, there is no diurnal variation of protein synthesis in the intestine of the unfed rat, whether leucine is administered intraluminally or intravenously.
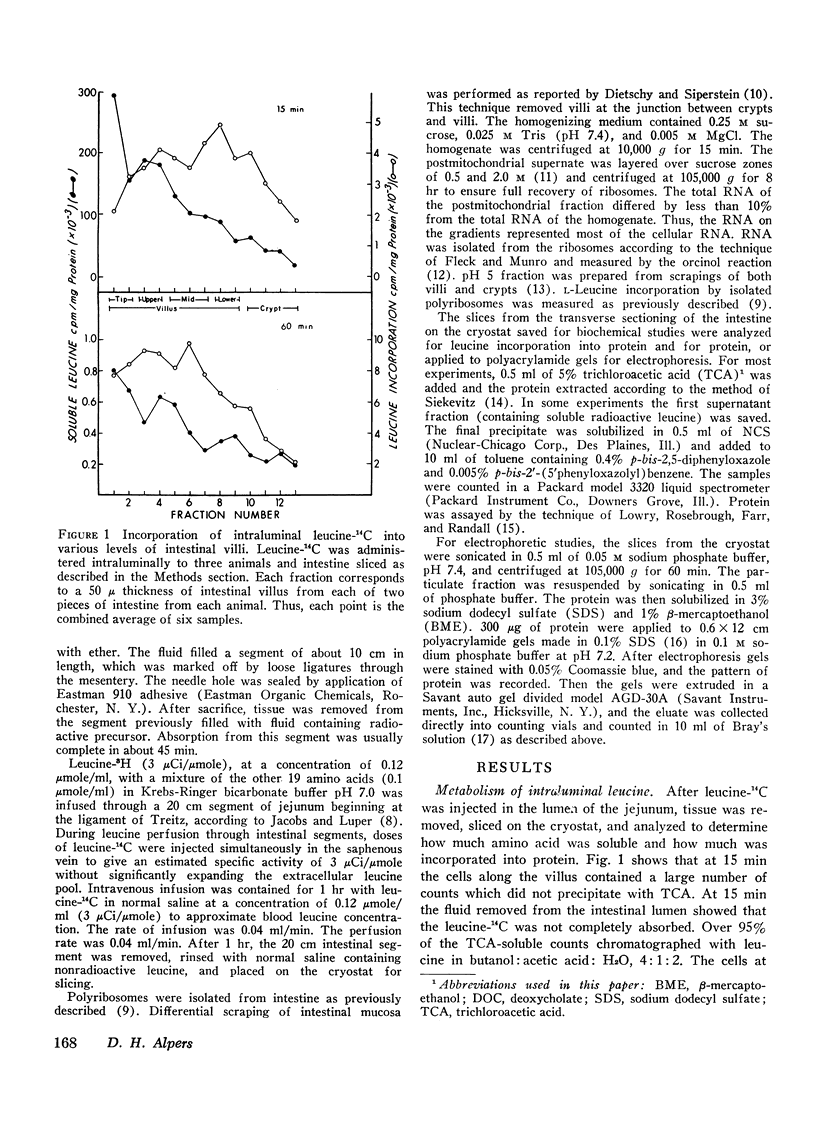
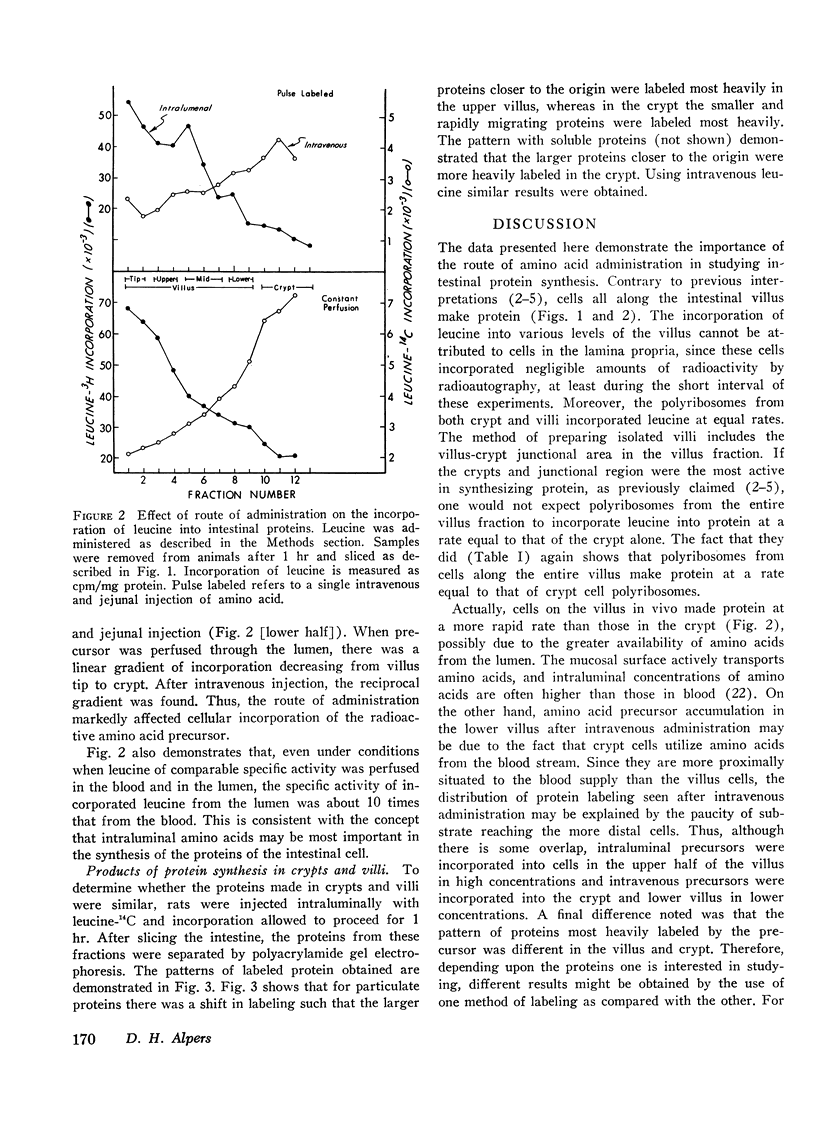
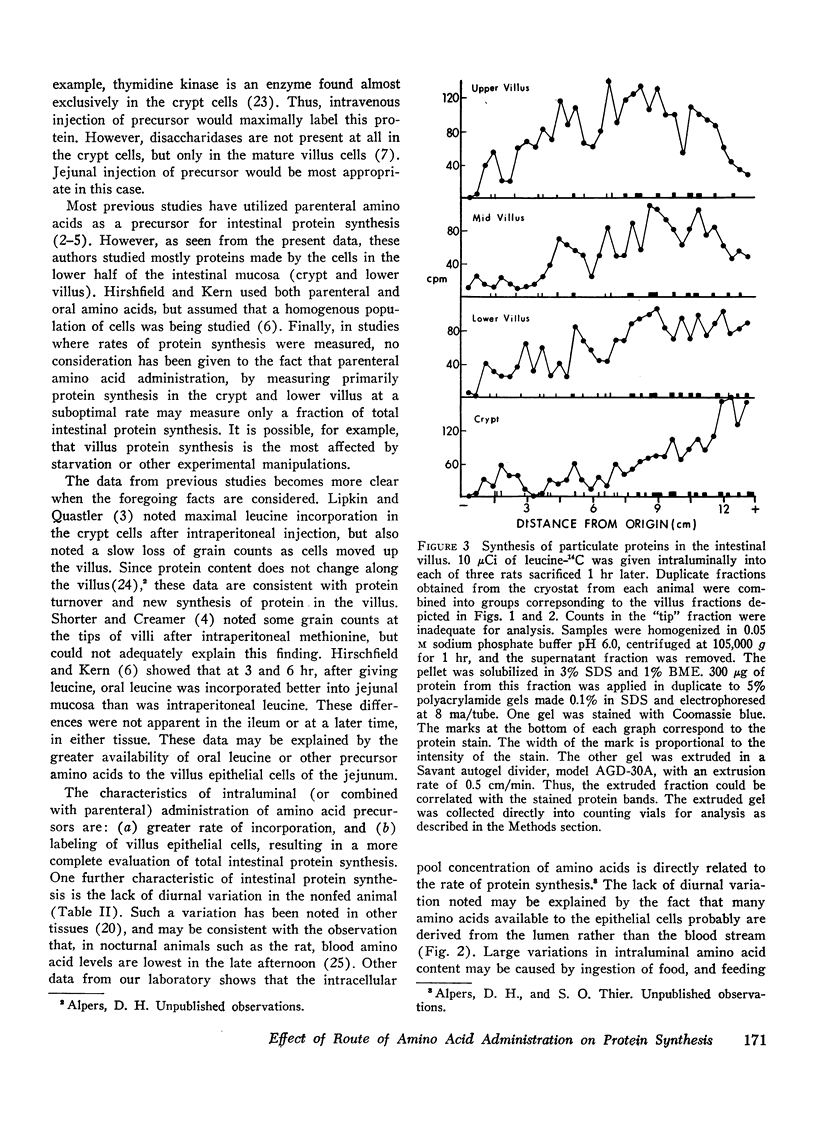
The route of administration of precursor (l-leucine) is important in determining which part of the villus incorporates the label into protein. After intravenous administration, protein from cells near the villus-crypt junction is most heavily labeled, whereas after intraluminal administration protein from cells near the villus tip is most heavily labeled. The pattern of proteins most heavily labeled by radioactive precursor is different in the villus when compared with proteins from the crypt cells. Smaller molecular weight membrane-bound proteins are preferentially labeled in the crypt cells, whereas on the villus the pattern of labeling is more evenly distributed among the various proteins. Moreover, intraluminal leucine is utilized for protein synthesis to a greater extent than that in the blood, when the concentration in both compartments is similar. Thus, intraluminal and intravenous injections of labeled precursor are not equivalent. Both routes should be considered in data for experiments measuring intestinal protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Isselbacher K. J. Protein synthesis by rat intestinal mucosa. The role of ribonuclease. J Biol Chem. 1967 Dec 10;242(23):5617–5622. [PubMed] [Google Scholar]
- DAWSON R., HOLDSWORTH E. S. An investigation into protein digestion with 14C-labelled protein. 1. The general pattern of 14C incorporation in body tissues and fluids of the rat up to 3 h after feeding. Br J Nutr. 1962;16:13–25. doi: 10.1079/bjn19620002. [DOI] [PubMed] [Google Scholar]
- Das B. C., Gray G. M. Protein synthesis in small intestine: localization and correlation with DNA synthesis and sucrase activity. Biochim Biophys Acta. 1969 Nov 19;195(1):255–257. doi: 10.1016/0005-2787(69)90625-x. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Feigin R. D., Beisel W. R., Wannemacher R. W., Jr Rhythmicity of plasma amino acids and relation to dietary intake. Am J Clin Nutr. 1971 Mar;24(3):329–341. doi: 10.1093/ajcn/24.3.329. [DOI] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfield J. S., Kern F., Jr Protein starvation and the small intestine. 3. Incorporation of orally and intraperitoneally administered 1-leucine 4,5-3H into intestinal mucosal protein of protein-deprived rats. J Clin Invest. 1969 Jul;48(7):1224–1229. doi: 10.1172/JCI106086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imondi A. R., Balis M. E., Lipkin M. Changes in enzyme levels accompanying differentiation of intestinal epithelial cells. Exp Cell Res. 1969 Dec;58(2):323–330. doi: 10.1016/0014-4827(69)90512-6. [DOI] [PubMed] [Google Scholar]
- JACOBS F. A., LUPER M. Intestinal absorption by perfusion in situ. J Appl Physiol. 1957 Jul;11(1):136–138. doi: 10.1152/jappl.1957.11.1.136. [DOI] [PubMed] [Google Scholar]
- James W. P., Alpers D. H., Gerber J. E., Isselbacher K. J. The turnover of disaccharidases and brush border proteins in rat intestine. Biochim Biophys Acta. 1971 Feb 23;230(2):194–203. doi: 10.1016/0304-4165(71)90204-2. [DOI] [PubMed] [Google Scholar]
- KELLER E. B., ZAMECNIK P. C. The effect of guanosine diphosphate and triphosphate on the incorporation of labeled amino acids into proteins. J Biol Chem. 1956 Jul;221(1):45–59. [PubMed] [Google Scholar]
- Koldovský O., Herbst J. J., Burke J., Sunshine P. RNA and DNA in intestinal mucosa during development of normal and cortisone-treated rats. Growth. 1970 Dec;34(4):359–367. [PubMed] [Google Scholar]
- LIPKIN M., QUASTLER H. Studies of protein metabolism in intestinal epithelial cells. J Clin Invest. 1962 Mar;41:646–653. doi: 10.1172/JCI104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nasset E. S. Role of the digestive system in protein metabolism. Fed Proc. 1965 Jul-Aug;24(4):953–958. [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- PALAY S. L., KARLIN L. J. An electron microscopic study of the intestinal villus. I. The fasting animal. J Biophys Biochem Cytol. 1959 May 25;5(3):363–372. doi: 10.1083/jcb.5.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosensweig N. S., Herman R. H., Stifel R. B. Dietary regulation of small intestinal enzyme activity in man. Am J Clin Nutr. 1971 Jan;24(1):65–69. doi: 10.1093/ajcn/24.1.65. [DOI] [PubMed] [Google Scholar]
- SHORTER R. G., CREAMER B. Ribonucleic acid and protein metabolism in the gut. I. Observations in gastro-intestinal cells with rapid turnover. Gut. 1962 Jun;3:118–128. doi: 10.1136/gut.3.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P. Uptake of radioactive alanine in vitro into the proteins of rat liver fractions. J Biol Chem. 1952 Apr;195(2):549–565. [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]


